Summary
Patellofemoral pain is characterized by pain behind the kneecap and is often thought to be due to high stress at the patellofemoral joint. While we cannot measure bone stress in vivo, we can visualize bone metabolic activity using 18F NaF PET/CT, which may be related to bone stress. Our goals were to use 18F NaF PET/CT to evaluate whether subjects with patellofemoral pan exhibit elevated bone metabolic activity and to determine whether bone metabolic activity correlates with pain intensity. We examined 20 subjects diagnosed with patellofemoral pain. All subjects received an 18F NaF PET/CT scan of their knees. Uptake of 18F NaF in the patella and trochlea was quantified by computing the standardized uptake value and normalizing by the background tracer uptake in bone. We detected increased tracer uptake in 85% of the painful knees examined. We found that the painful knees exhibited increased tracer uptake compared to the pain-free knees of four subjects with unilateral pain (p=0.0006). We also found a correlation between increasing tracer uptake and increasing pain intensity (r2 = 0.55; p = 0.0005). The implication of these results is that patellofemoral pain may be related to bone metabolic activity at the patellofemoral joint.
Keywords: patellofemoral pain, 18F NaF PET/CT, bone metabolic activity
Introduction
Patellofemoral pain syndrome is often characterized by dull, achy pain that is exacerbated by activities such as running, squatting, and stair climbing. Patellofemoral pain accounts for a large fraction of knee disorders seen in sports medicine clinics1, but treatment is challenging because the underlying causes of pain are unclear2. Often, patients with patellofemoral pain do not appear to have structural damage to the bones or cartilage at the joint3, making diagnosis from imaging challenging. Elevated stress in the subchondral bone of the patellofemoral joint is hypothesized to be one cause of pain4; however, stress cannot be measured in vivo. Bone remodels in response to applied joint loads5; therefore, elevated subchondral bone stress may result in increased bone remodeling activity. Bone remodeling activity may also be related to nerve growth and the development of pain6.
Methods to directly image bone metabolic activity can provide insight into the relationship between bone stress and pain. Technetium-99m hydroxymethylene diphosphonate (Tc-99m MDP) bone scintigraphy previously revealed increased bone turnover at the knee in patients with patellofemoral pain7 and knee osteoarthritis8,9; however, the poor spatial resolution of this technique makes it difficult to localize the specific regions of tracer uptake. 18F NaF PET/CT is an alternative to Tc-99m MDP bone scintigraphy that may provide additional insight due to its ability to more specifically localize regions of elevated bone metabolic activity. In this imaging technique, 18F fluoride ions, which have a high affinity for bone mineral10, are injected intravenously. The 18F fluoride ions exchange with hydroxl ions in bone crystal to become naturally incorporated into cortical bone11. Incorporation of the 18F fluoride ion in bone is due to the activity of osteoblasts and osteoclasts during bone remodeling; therefore, processes that increase bone remodeling or bone metabolic activity will result in increased uptake of the tracer11. The advantages of 18F NaF PET/CT over bone scintigraphy are improved spatial resolution, more accurate anatomical localization of tracer uptake using co-registered CT, larger ratio of bone uptake to soft-tissue uptake, and faster study times12.
Characterizing bone metabolic activity using 18F NaF PET/CT in patients with patellofemoral pain has not previously been performed. This information may aid in the diagnosis and treatment of this disorder; therefore, the goals of this study were: to determine whether subjects with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint and evaluate whether the location of elevated bone metabolic activity corresponds to the specific location of pain; and to assess whether the level of bone metabolic activity correlates with reported pain intensity.
Materials and Methods
We studied the knees of 20 subjects (12 male: 29 ± 5 yrs, 1.8 ± 0.1m, 80 ± 15kg; 8 female: 32 ± 6 yrs, 1.6 ± 0.04m, 61 ± 6kg) diagnosed with chronic patellofemoral pain by an experienced sports medicine physician. Subjects experienced pain for a minimum of 3 mos. Subjects were included if they experienced reproducible anterior knee pain during at least two of the following activities: stair ascent/descent, kneeling, squatting, prolonged sitting, or isometric quadriceps contraction. Subjects were excluded if they met any of the following criteria: knee ligament instability, patellar tendonitis, joint line tenderness or knee effusion, previous knee injuries or surgery, or patellar dislocation. Both knees of every subject were imaged although only the more painful knee at the time of scanning was analyzed. Due to the radiation exposure of PET/CT scanning, pain-free subjects were not recruited. Four subjects were diagnosed with unilateral pain, and their pain-free knees were used as controls. During the physical exam, the physician determined the region of the joint most sensitive to palpation. This was recorded for comparison with the location of tracer uptake. Furthermore, subjects rated the maximum pain experienced during the past year on a verbal rating scale from 0 to 10, where 0 represents no pain and 10 represents the worst pain imaginable. Prior to participating in the study, all patients were informed about the nature of the study and provided consent as approved by the University Institutional Review Board.
All subjects received an 18F NaF PET/CT scan of both knees. To minimize the effects of recent physical activity and blood flow on tracer uptake, subjects were seated for 30 mins prior to tracer injection. Subjects then received 5-10mCi of 18F NaF intravenously (0.08mCi/kg) and remained seated until the PET/CT scan. This is an equivalent dose of 5-10mSv and is approximately 1.5 to 3 yrs of background radiation. The PET/CT scan was acquired an average of 69 ± 23 mins following tracer injection. A GE Discovery LS PET/CT scanner (GE Healthcare, Milwaukee, WI) was used. Subjects were scanned in a supine position with their legs strapped together to minimize movement between the CT and PET scans. This improves registration between the two scans. Non-contrast CT images of both knees of the subjects were obtained immediately prior to the PET scan using the following parameters: SFOV: 50cm, matrix size: 512×512, slice thickness: 4.25mm, 140kVp, 90mAs. The PET images were acquired using the following parameters: SFOV: 55cm, matrix: 128×128, slice thickness: 4.25mm, 5 min acquisition/bed position, 1 bed position, ordered subsets expectation maximization (OSEM) iterative reconstruction. The resolution of PET images from this type of scanner is 7 to 8mm (full width at half maximum)13.
Uptake of the 18F NaF tracer was quantified by computing the standardized uptake value (SUV):
The background tracer uptake in bone was computed for each subject. Since all subjects were free of tibiofemoral abnormalities, we defined the average SUV of an axial slice through the tibial epiphysis just proximal to the tibial tuberosity to be representative of typical bone background uptake. We then defined a region of increased tracer uptake to be any group of two or more adjacent pixels that were greater than twice the background tracer uptake. We computed the mean and peak SUV of the regions of tracer uptake. In cases where subjects did not exhibit increased tracer uptake, we computed the mean and peak of the entire patella. To account for variations in uptake between patients, we normalized all SUV values by the background bone SUV for each subject. A Student's t-test was used to assess the significance of differences in tracer uptake between the painful and pain-free knees. A Kappa test was used to evaluate the relationship between the location of pain and the location of tracer uptake, and a Pearson's correlation test was used to evaluate the correlation between pain intensity and tracer intensity.
Results
We detected regions of increased tracer uptake (Fig. 1), indicative of elevated bone metabolic activity in 17 of the 20 painful knees analyzed. The average volume of the regions of increased tracer uptake was 1cm3 (range 0.05 to 3cm3). Regions of increased uptake were found in 15 patellae and four femoral trochleae (two subjects exhibited increased uptake in both the patella and trochlea). None of the four pain-free knees exhibited regions of increased uptake.
Figure 1.
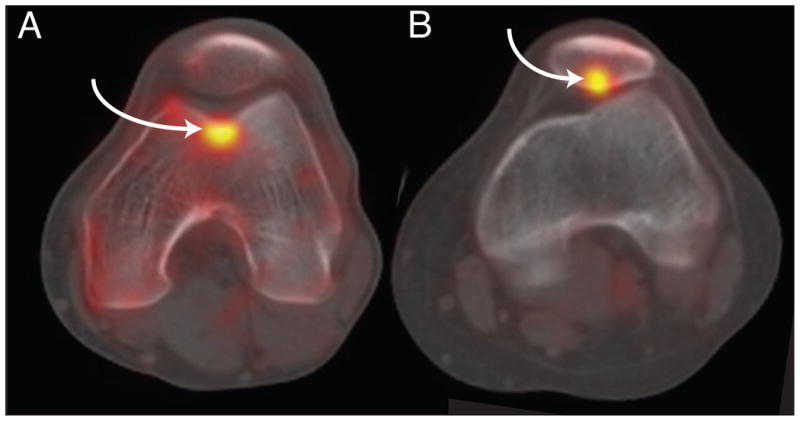
A) Sample 18F NaF PET/CT image of the patellofemoral joint with a region of increased tracer uptake in the trochlea. B) Sample 18F NaF PET/CT image of the patellofemoral joint with a region of increased tracer uptake in the patella. Regions of increased tracer uptake are indicated by the arrows.
Due to the fusion of the PET images onto CT images, we could accurately localize the regions of increased tracer uptake. Seven subjects exhibited increased tracer uptake on the lateral patellar facet, seven exhibited increased tracer uptake on the medial patellar facet, one exhibited increased tracer uptake on the inferior pole of the patella, two exhibited increased uptake in the medial trochlea, one on the lateral trochlea, and one in the center of the trochlea.
Sixteen subjects localized pain to specific regions of the patellofemoral joint during the physical exam: 7 subjects had pain on the lateral facet, 7 had pain on the medial facet, and 2 experienced pain on the inferior pole of the patella. The remaining four subjects did not exhibit tenderness during the palpation assessment, and we were unable to localize pain in these subjects. Additionally, three subjects did not exhibit increased tracer uptake and were not included in the analysis, leaving a total of 14 subjects for the analysis. We found that in 10 of the 14 subjects the region of pain during palpation corresponded to the region of increased tracer uptake, resulting in a moderate correlation between pain location and location of tracer uptake (κ = 0.44).
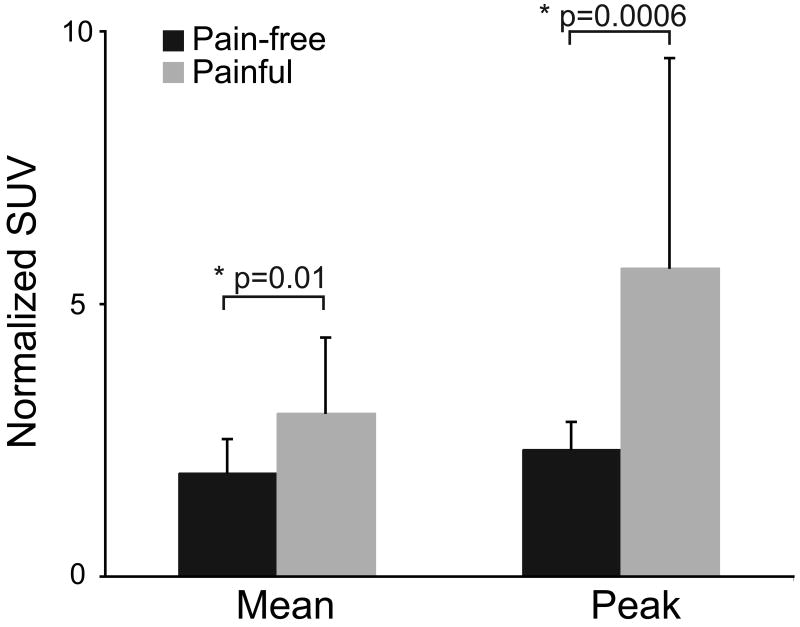
The normalized mean and peak SUV of the region of tracer uptake were greater in the 20 painful knees compared to the four pain-free knees (Fig. 2). The mean normalized SUV was 36% lower in the pain-free knees compared to the painful knees (p = 0.01). The peak normalized SUV was 59% lower in the pain-free knees compared to the painful knees (p = 0.0006).
Figure 2.
Comparison of normalized Standardized Uptake Value between the 20 painful knees and the 4 pain-free knees of the subjects with unilateral pain. The painful knees exhibited increased tracer uptake compared to the pain-free knees. The error bars represent one standard deviation.
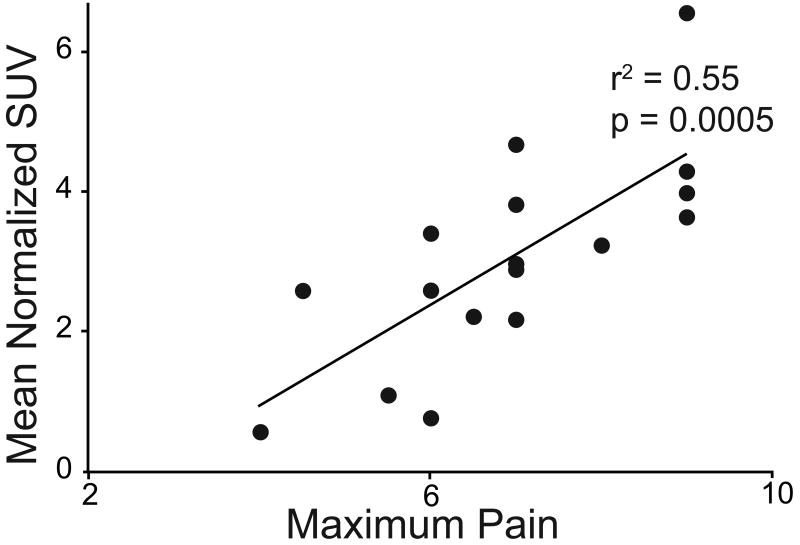
Increasing pain intensity tended to correlate with increasing tracer uptake (Fig. 3). We found a moderate correlation between pain intensity and normalized mean SUV (r2 = 0.55; p = 0.0005). A modest correlation was found between pain intensity and the normalized peak SUV (r2 = 0.29; p = 0.02).
Figure 3.
Correlation between normalized mean Standardized Uptake Value and maximum pain felt during the previous year.
Discussion
Our goal was to use 18F NaF PET/CT to determine whether subjects with patellofemoral pain exhibit elevated bone metabolic activity at the patellofemoral joint. We also evaluated whether bone metabolic activity correlates with pain. We hypothesized that pain results from elevated stress at the patellofemoral joint that could lead to elevated bone remodeling activity at the joint. Our results suggest that patellofemoral pain is related to increased 18F fluoride tracer uptake, indicative of elevated bone metabolic activity at the joint.
Previous studies investigating whether patients with patellofemoral pain exhibit increased bone activity using Tc-99m MDP bone scanning revealed diffuse tracer uptake at the knee in 44% of the painful knees examined7. Of the knees with diffuse tracer uptake, over half exhibited increased tracer uptake in the patella7. In our study, we observed increased 18F fluoride uptake in 85% of the knees. This increase in the percentage of subjects with increased uptake may be due to increased sensitivity of 18F PET/CT. In agreement with the previous study, we also found that the majority of the knees exhibited increased uptake in the patella compared to the femur.
While Tc-99m MDP bone scintigraphy is beneficial for identifying diffuse regions of uptake and can differentiate uptake in the patella from uptake in the femur, a significant factor differentiating our study from previous work using bone scintigraphy is that we used registered PET/CT images, allowing for excellent anatomical localization of tracer distribution. This enabled us to accurately identify the specific regions within a bone that exhibited elevated bone metabolic activity.
Increased bone metabolic activity was more often observed in the patella compared to the femur. Among those with increased metabolic activity in the patella, the metabolic activity was located on the posterior surface in 12 of the 15 knees. The increased metabolic activity may be a result of excessive stress in the subchondral bone of the patella. While stress cannot be measured in vivo, previous studies estimated patellofemoral joint stress during activities such as walking, stair climbing, and squatting in patients with patellofemoral pain14-16 and revealed increased joint stress in knees with patellofemoral pain16. Furthermore, octahedral shear stress is greater in patellar cartilage compared to femoral cartilage17, which may be one explanation for our observation that more subjects exhibited increased metabolic activity on the patella compared to the femur. Though these estimates of bone stress used data acquired during weight-bearing activities, they can still provide insight into a potential mechanism of bone metabolic activity measured during the non-weight-bearing conditions of this study.
The presence of increased metabolic activity in the patella may have significant implications. Histological studies demonstrated the presence of sensory nerve fibers containing substance-P, a nociceptive neurotransmitter, in the patella 18, suggesting that this region may be sensitive to mechanical stress. This observation along with our results suggests that the patella is likely to be more affected in patellofemoral pain syndrome and could be the source of pain. Additionally, we saw a moderate correlation between the region of the joint most sensitive to palpation and the location of the elevated bone metabolic activity. We also observed a moderate correlation between the intensity of pain and the intensity of bone metabolic activity, further suggesting a relationship between bone metabolic activity and pain. Treatments aimed at decreasing the stress in the patella may be effective in decreasing bone metabolic activity and alleviating pain.
An additional implication of these results is that 18F NaF PET/CT may be a potential diagnostic tool for evaluating elevated bone stress in patients with patellofemoral pain and identifying specific regions of elevated stress. Future work correlating mechanical stress with bone metabolic activity is needed to confirm the hypothesis that tracer uptake is related to bone stress.
A limitation of our study was that we did not evaluate pain-free controls due to the ionizing radiation involved in PET/CT scanning. We chose to measure bone metabolic activity in the knees of subjects with unilateral pain as an alternative. This limits the number of pain-free knees that we could study, as only four subjects had unilateral pain. Additional subjects are needed for a more complete analysis. Furthermore, the pain-free knee of a subject with unilateral patellofemoral pain may have altered bone metabolic activity patterns compared to a knee of a subject who has never experienced knee pain. Nonetheless, the comparison of pain-free and painful knees provides interesting insights in the absence of true control knees. Another limitation is that the definition of increased tracer uptake (greater than twice the bone background) was chosen arbitrarily, and our results may differ if we choose a different threshold. We did have a nuclear medicine radiologist independently examine the images, and his assessment of regions of increased tracer uptake was in agreement with the regions we identified using our threshold. This provides confidence that the regions identified in this study correspond to clinically meaningful increases in bone metabolic activity.
Despite these limitations, there are a number of important implications of this study. Our results suggest that subjects with patellofemoral pain exhibit elevated bone metabolic activity that may indicate a source of pain in these patients. We found that the majority of subjects exhibited increased bone metabolic activity in the posterior patella, suggesting that this region may play a role in the development of pain. The implications of these results are that treatments to reduce bone metabolic activity by reducing the stress at the patella may be effective in alleviating pain in these subjects.
Acknowledgments
We thank the following nuclear medicine technologists for their help acquiring the PET/CT images: Christine Fuji, Paulo Castaneda, Lincoln Sanders, and Matthew Gabriele. We thank the PET/CT research coordinator, Lindee Burton. We thank Jarrett Rosenberg for help with statistical analysis. Financial support was provided by the NIH (EB0002524, EB005790, U54 GM072970, RO1HD33929, RO1HD046814, R90 DK071508) and the Stanford PET/CT Joint Venture. This material is based upon work supported in part by the Office of Research and Development (Rehabilitation R&D Service grant #A2592R), Department of Veterans Affairs.
References
- 1.Devereaux MD, Lachmann SM. Patello-femoral arthralgia in athletes attending a Sports Injury Clinic. Br J Sports Med. 1984;18:18–21. doi: 10.1136/bjsm.18.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaBella C. Patellofemoral pain syndrome: evaluation and treatment. Primary Care. 2004;31:977–1003. doi: 10.1016/j.pop.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Pihlajamaki HK, Kuikka PI, Leppanen VV, et al. Reliability of clinical findings and magnetic resonance imaging for the diagnosis of chondromalacia patellae. J Bone Joint Surg Am. 92:927–934. doi: 10.2106/JBJS.H.01527. [DOI] [PubMed] [Google Scholar]
- 4.Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30:447–456. doi: 10.1177/03635465020300032501. [DOI] [PubMed] [Google Scholar]
- 5.Carter DR. Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int. 1984;36 1:S19–24. doi: 10.1007/BF02406129. [DOI] [PubMed] [Google Scholar]
- 6.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 7.Naslund JE, Odenbring S, Naslund UB, Lundeberg T. Diffusely increased bone scintigraphic uptake in patellofemoral pain syndrome. Br J Sports Med. 2005;39:162–165. doi: 10.1136/bjsm.2004.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–563. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrae F, Shouls J, Dieppe P, Watt I. Scintigraphic assessment of osteoarthritis of the knee joint. Ann Rheum Dis. 1992;51:938–942. doi: 10.1136/ard.51.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med. 1972;2:31–37. doi: 10.1016/s0001-2998(72)80005-9. [DOI] [PubMed] [Google Scholar]
- 12.Grant FD, Fahey FH, Packard AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 13.Schmidtlein CR, Kirov AS, Nehmeh SA, et al. Validation of GATE Monte Carlo simulations of the GE Advance/Discovery LS PET scanners. Med Phys. 2006;33:198–208. doi: 10.1118/1.2089447. [DOI] [PubMed] [Google Scholar]
- 14.Besier TF, Gold GE, Delp SL, et al. The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. J Orthop Res. 2008;26:1627–1635. doi: 10.1002/jor.20663. [DOI] [PubMed] [Google Scholar]
- 15.Brechter JH, Powers CM. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait Posture. 2002;16:115–123. doi: 10.1016/s0966-6362(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 16.Heino Brechter J, Powers CM. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med Sci Sports Exerc. 2002;34:1582–1593. doi: 10.1097/00005768-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Besier TF, Gold GE, Beaupre GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc. 2005;37:1924–1930. doi: 10.1249/01.mss.0000176686.18683.64. [DOI] [PubMed] [Google Scholar]
- 18.Wojtys EM, Beaman DN, Glover RA, Janda D. Innervation of the human knee joint by substance-P fibers. Arthroscopy. 1990;6:254–263. doi: 10.1016/0749-8063(90)90054-h. [DOI] [PubMed] [Google Scholar]