Abstract
Background
The expression of retinal CaMKII is up-regulated in the retina of the rdta mouse in which rod photoreceptors are genetically ablated. As ionotropic glutamate receptors are known substrates of CAMKII, this study set out to determine if the protein levels of ionotropic glutamate receptors in the rdta mouse retina are also affected.
Results
The NMDA receptor subunits (NR1, NR2A/B) and the GluR1; AMPA receptor subunit (GluR1) were examined in immunolabeled western blots. The results demonstrate that the amounts of NR1 and NR2A/B receptor subunits are significantly increased in crude synaptic membrane fractions isolated from retinae of the rdta mice when compared to their normal, littermate controls. The GluR1 receptor subunit and its phosphorylation are simultaneously increased in retinae of the rdta mice.
Conclusions
These data indicate that the NMDA receptors and AMPA (GluR1) receptors are altered in the retinae of rdta mice that lack rod photoreceptors. Because the rods are lost at an early stage in development, it is likely that these results are indicative of synaptic reorganization in the retina.
Background
Glutamate is believed to be the major excitatory neurotransmitter in the retina [1,2], as it is in the rest of the central nervous system. Glutamate receptors are characterized by their sensitivity to specific glutamate analogues and by specific features of the glutamate-elicited currents. Ionotropic glutamate receptors mediate fast synaptic transmission between neurons because the receptors and the ion channel form one complex. Two types of ionotropic glutamate receptors have been classified: (1) NMDA receptors, which bind glutamate and the glutamate analogue N-methyl-D-aspartate (NMDA); (2) non-NMDA receptors, which are stimulated by kainate, AA-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and quisqualate, but not NMDA. Glutamate binding to non-NMDA receptors opens ion channels more permeable to sodium (Na+) and potassium (K+) than calcium (Ca2+). In contrast to the non-NMDA receptors, the high conductance channel associated with the NMDA receptors is permeable to Ca2+ as well as to Na+ and K+. Also NMDA-gated currents typically have slower kinetics than kainate- and AMPA-gated channels [3].
NMDA receptors are heteromeric ion channels composed of one NR1 subunit with combination of other NR2 subunits [4,5,6,7]. The NMDAR1 (NR1) and the NMDAR2 (NR2A-D) have been cloned [4,5,6,8,9]. It has been shown that NR1 possesses all the properties characteristic of the NMDA receptor channel complex [4,7,9,10,11,12] whereas the NR2 subunit has no independent channel activity in its homomeric structure but potentiates the NMDA receptor activity when expressed in combination with the NR1 subunit [4,13,14]. The expression of NR1 and the composition of the NR2 subunits are developmentally regulated. Based on the electrophysiological data, excitatory synaptic transmission is mediated in large part by NMDA receptors in immature neurons [15,16,17,18,19] whereas the activity of non-NMDA receptors is increased following synaptic maturation [19]. NMDA receptors containing NR2B subunits are present in the neonatal forebrain, and over the course of development, they are replaced or supplemented with NR2A-containing receptors [13,20]. Dynamic and environmentally driven changes in the NMDA receptor expression have been shown in the visual cortex [21,22] and LGN [23] where the expression is altered by visual activity. For example, dark rearing from birth attenuates the developmental increase in NR2A in the visual cortex [24,25].
In contrast to previous observations that the AMPA receptor expression is relatively constant in glutamate sensitive neurons [26], recent studies have shown that the expression of AMPA receptor subunits, especially GluR1, the major functional subunit of the AMPA receptor [11] is regulated developmentally at regional, cellular, and synaptic levels [27,28,29,30]. It has been shown that the AMPA receptors can move in and out of synapses as synaptic connections are strengthened and weakened [31,32,33]. These dynamic changes were blocked by NMDA receptor antagonists [33]. However, the molecular basis for this activity-induced change in AMPA receptors remains unknown.
In the present study, the prospect of a visual-regulated expression and composition of NMDA receptor subunits, NR1 and NR2A/B in the retina is tested by using the rdta transgenic mouse in which the rod photoreceptors have been genetically ablated. The expression of GluR1 is also examined because it is the major functional subunit of the AMPA receptors. This receptor subunit is a substrate of calcium/calmodulin-dependent protein kinase II (CaMKII) which is up-regulated in the retina of the rdta mice [34].
Results
Expression of β-actin is increased in the retinal synaptic membrane fraction isolated from the rdta transgenic mouse
No differences could be detected in the levels of β-actin in retinal homogenates isolated from rdta mice and their littermate controls, and therefore, this molecule was previously used as an internal reference [34]. In this study, β-actin was assessed in the synaptic membrane fraction (SPM) (Figure 1A). A 90% increase of the antibody binding to β-actin was observed in western blots of the SPM fraction of the retinae from the rdta mice relative to their littermate controls (Figure 1B). Therefore the significant increase in the membrane-associated pool seen here must represent an insignificant fraction of the homogenate-associated pool observed in the previous study. Experimentally induced changes in β-actin levels associated with forebrain synapses have been reported recently [35], and the increases observed here are most likely due to changes in the cytoskeletal structure of synapses in the retinae of the rdta mice. This observation requires further investigation.
Figure 1.
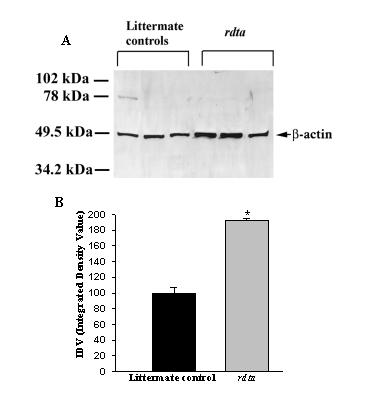
Western blot analysis of the immunolabeling of β-actin in the SPM fraction of the mouse retina. (A) Immunoblots of 6 μg of retinal SPM protein from control animals and the rdta mice were incubated with anti-β-actin antibody. The immunolabeling densities were calculated as the integrated density values (IDV) and plotted in B. (B) The graph presents the changes in the antibody binding to β-actin in three littermate control and three rdta mice. The average density calculated as IDV for the littermate controls was taken as 100%. The immunolabeling of β-actin in the SPM fraction is significantly increased in the rdta retina. Values are means ± S.E.M (n = 3), P < 0.05, one way ANOVA with Bonferroni correction.
NR1 expression and NR2A/B composition are altered in the rdta retina
To determine if the expression of the NMDA receptor and/or its composition was altered as a result of rod photoreceptor ablation, immunoblots were analyzed for the NR1 (Figure 2A), the NR2A (Figure 2B) and the NR2B (Figure 2C) subunits. The immunolabeled NR1, NR2A and NR2B subunits were increased by approximately 123%, 62% and 100% (Figure 2D) respectively; in the membrane fractions isolated from the retinae of the rdta mice.
Figure 2.
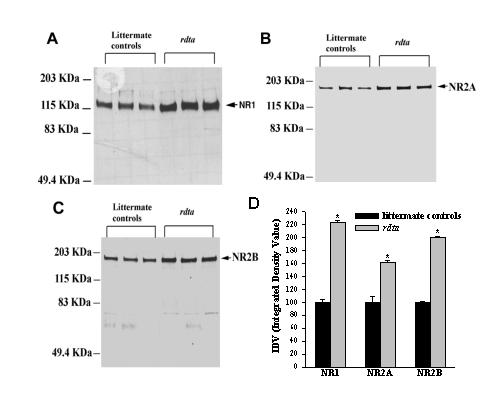
Western blots analyses of NMDA receptor subunits in the mouse retina. (A),(B) and (C) Immunoblots of 6 μg of retinal SPM fraction proteins from controls and the rdta mice were first incubated with anti-NR1 antibody (A), and subsequently reprobed with anti-NR2A (B) and NR2B (C) antibodies, respectively. The immunolabeling densities were calculated as the integrated density values (IDV), and plotted in D. (D) The graph presents the changes in the antibody binding to NR1, NR2A and NR2B in littermate controls and the rdta mice. The average density calculated as IDV for the littermate controls was taken as 100. Values are means ± S.E.M (n = 3), P < 0.05, one way ANOVA with Bonferroni correction.
Phosphorylation of GluR1 is increased in the synaptic membrane fraction isolated from the retinae of rdta mice
The level of GluR1 protein was also examined in immunoblots (Figure 3A). The protein level of the GluR1 subunit was 2.3 times greater in the rdta mice than in the wild type mice (Figure 3B).
Figure 3.
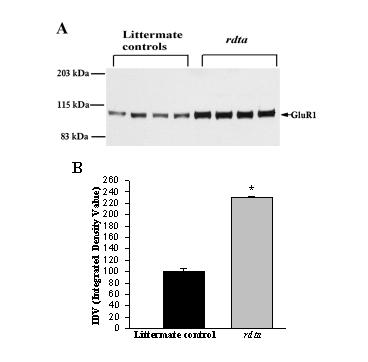
Western blot analysis of GluR1 in the SPM fraction of the mouse retina. (A) Immunoblots of 6 μg of retinal SPM protein from control animals and the rdta mice were incubated with anti-GluR1 antibody. The immunolabeling densities were calculated as the integrated density values (IDV) and plotted in B. (B) The graph presents the changes in the antibody binding to GluR1 in the littermate control and rdta mice. The average density calculated as IDV for the littermate controls was taken as 100%. The immunolabeling of GluR1 in the SPM fraction is significantly increased in the rdta retina. Values are means ± S.E.M (n = 3), P < 0.05, one way ANOVA with Bonferroni correction.
Because GluR1 is a known substrate of CaMKII in vitro and in vivo [34,36,37,38,39,40] and because CaMKII expression/activity is up-regulated in the retinae of the rdta mice [34], GluR1 phosphorylation might also be increased in the rodless retina. To test this notion, the SPM proteins isolated from the retinae of the rdta and their littermate controls mice were back-phosphorylated in the presence of Ca2+/calmodulin and γ-32P-ATP (Figure 4A). The bands densities in the rdta mice were low; and a long film exposure was necessary to detect the signal (Figure 4A). The relative position of γ-32P-ATP labeled GluR1 as indicated was confirmed by immunoblotting with GluR1 antibodies (Figure 4B). It was found that γ-32P-ATP labeling of GluR1 by this procedure was greater in the retinae of control mice than in the retina of the rdta mice (Figure 4A), whereas the expression of GluR1 was increased in the rdta mice relative to their littermate controls (Figure 4B). These data indicate that GluR1 phosphorylation in vivo was enhanced in the rdta retinae relative to their littermate controls. This result was confirmed using an additional technique. GluR1 was immunoprecipitated with anti-GluR1 antibody and then subjected to in vitro phosphorylation (Figure 4C). Together, these data indicate that in vivo, the amount of GluR1 and the phosphorylation of GluR1 are simultaneously enhanced in retinal synaptic fractions isolated from the retinae of rdta mice.
Figure 4.
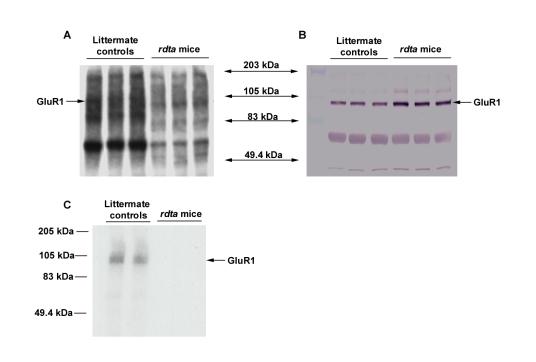
Back -phosphorylation of GluR1 in the retina of the rdta mice and their littermate controls. (A) Ten micrograms of SPM protein from the retinae of the rdta mice and their littermate controls were back-phosphorylated by endogenous CaMKII in the presence of Ca2+/CaM. The samples were then electrophoresed by SDS-PAGE and subsequently transferred to nitrocellular membrane. The phosphorylated bands were visualized by autoradiography. The in vitro phosphorylation was higher in the littermate controls than in the rdta mice. (B) The same blots were then incubated with anti-GluR1 antibody and visualized by alkaline phosphatase conjugated secondary antibody and NBI/BICP substrate. The molecular weight of GluR1 is indicated. The density for GluR1 was elevated in the rdta mice relative to the littermate controls. (C) GluR1s were immunoprecipitated after the back-phosphorylation and subjected to SDS-PAGE. The gel was dried and the image was visualized by autoradiography. These data indicate that the phosphorylation of GluR1 is increased in vivo in the retina of the rdta mice relative to their littermate controls.
Discussion
To our knowledge, this is the first study to demonstrate that the protein levels of NMDA and AMPA receptors are altered in the retina when the rod photoreceptors are absent. Western blots analyses demonstrate that the amounts of NR1, NR2A/B and β-actin are significantly increased in the retinae of rdta mice compared to their littermate controls. Furthermore, the amount of GluR1 expression and its phosphorylation are simultaneously increased in the retinae of the rdta mice. As the retinal thickness of the rdta animals is reduced by as much as 30% relative to littermate control animals, the estimated increases in synaptic relevant molecules as a percentage of total retinal protein could be over-estimated by at least this amount. Even accounting for such a correction the changes reported here remain significant. A previous study using similar western blot demonstrations of an elevation in calcium/calmodulin kinase II protein, a molecule intimately related to those studied here, was shown to be at least qualitatively correct using an independent immunohistochemical assessment [34].
The results presented here are consistent with studies demonstrating activity-based changes in receptors as reported for other parts of the nervous system [21,23,24,25,41]. NR1 expression is not altered in the dark-reared visual cortex [24,25] but contrarily, an intraocular injection of tetrodotoxin (TTX) does increase NR1-antibody binding in layer IV of the cortical column driven by the blocked eye [41]. The latter study supports the notion that neural activity is a regulating factor for NR1 expression. It should not be surprising to find similarities and differences between the experimental paradigms of dark-rearing/visual cortex on the one hand, and photoreceptor-ablation/retina on the other.
Electrophysiological and pharmacological studies have shown that different subunit configurations comprising the receptor confer different functional properties and selectivity to the NMDA receptors [7,11,13,42,43]. For example, the kinetics of the NMDA receptors are regulated by the combinatorial associations of the NR1 and NR2 subunits [44]. Thus, mature NMDA receptors, which contain more NR2A subunit, have a faster decay time than immature receptors which contain more NR2B subunits [20,44]. In the present study, the increase in the protein level of NR2B was much higher than NR2A in the rdta mice. Therefore, the increased expression of NR2B could be indicative of developmental/functional delay due to the lack of rod input.
In the retina, the visual information is transmitted vertically by glutamate containing cells, photoreceptors, bipolar and ganglion cells [45,46,47,48,49] and from the retina to the LGN and visual cortex. The fact that expression of NMDA receptor subunits in the retina can be altered leads us to suggest that the activity-dependent expression of NMDA receptors in the visual cortex or LGN could be co-incident with, or perhaps even dependent on the changes that occur in the retina. The examination of NMDA receptor expression in the LGN and visual cortex of the rdta mouse could prove to be useful for future explorations of this point.
Increases in the amount of protein for ionotropic receptor subunits were observed in the membrane fraction isolated from retinae of the rdta mice. These changes are most likely due to a dynamic structural reorganization in the retina. This is supported, for example, by the co-incident increase in the level of β-actin. While this observation was not expected, it is not necessarily surprising or without precedent. Actin is involved in dynamic structural changes observed in synapses [50]. The level of β-actin, in particular, is known to be altered through the stimulation of kainate receptors [35]. With regard to the retina, both structural and functional plasticity of retinal synapses have been shown to be affected by inhibiting actin turnover with cytochalasin [51].
NMDA-receptor clusters are present within the inner plexiform layer (IPL) of the retina, and the receptor clusters are composed of different subunit combinations: NR1/NR2A, NR1/NR2B and a small number of clusters also contain NR1/NR2A/NR2B [52]. These localizations are observed at the synaptic sites between amacrine and ganglions cells, with very little localization evident at the bipolar cell axon terminals. Thus the changes observed in the present study most likely reflect changes that occur in the inner plexiform layer of the retina.
Changes in NMDA receptor expression are likely to affect neuronal function through regulation of intracellular events [53]. We have previously reported that retinal CaMKII mRNA, protein and enzyme activity (localized in the somas of amacrine cells and ganglion cells and in the inner plexifom layer) but not PKCα (localized in bipolar cells) are increased in the rdta mouse [34]. The NR2 subunits of the NMDA receptor contain serine residues that can be phosphorylated by either CaMKII or PKC [53,54,55]. While stimulation of PKC activity may decrease CaMKII binding to the NMDA receptor complex [56], there is no change in the level of PKC in the rdta mice [34]. Thus, enhanced phosphorylation by CaMKII may be involved in the altered levels of NMDA-receptor subunits observed here, but this remains to be tested. Regulation of neurotransmitter receptor function by protein phosphorylation plays a critical role in the modulation of synaptic transmission and synaptic plasticity [57,58,59,60]. It has been shown that AMPA-receptor phosphorylation is critical for synaptic plasticity in the brain [61] and postsynaptically, GluR1 is mainly regulated through changes in CaMKII phosphorylation [62]. Because CaMKII expression/activity is up-regulated in the rdta retina [34] and because GluR1 is a substrate of CaMKII [36,37,38,39,40], the increased phosphorylation of GluR1 seen here in the retinal synapses of the rdta mice could indicate a synergy between CaMKII and GluR1. Together, these data support the concept that there is an activity-dependent synaptic modification in the retina of the rdta mice. Some types of synaptic plasticity, such as LTP, require activation of the NMDA receptor, postsynaptic Ca2+ ion influx with concomitant activation of CaMKII [63]. The activation of CaMKII then catalyzes the phosphorylation of AMPA-receptors and enhances AMPA receptor responsiveness [37,39,40,60]. The demonstration that GluR1 expression is increased in the retina of the rdta mouse where a rod photoreceptor-mediated visual input is missing is consistent with a previous report in which the level of GluR1 is increased in the deafferented tectum [64,65].
These data indicate that rod photoreceptor-mediated visual input may have a negative effect on GluR1 expression in normal retina during the course of development. However, changes in GluR1 gene expression are not evident in the retinae of rd mice [66] using the in situ hybridization method [67]. Such studies support the possibility that the changes in GluR1 seen here may be due to a post-transcriptional regulation. However, it remains to be tested if the increased amounts of GluR1 observed in this study are due to new protein synthesis or to a translocation from some intracellular compartment to the synaptic membrane [27,29].
In the present study, an increased synaptic expression of the NMDA receptors and GluR1 subunits is associated with changes in CaMKII. It is postulated that CaMKII phosphorylation of GluR1 and NR2A/B may play a role in increasing the presence of these receptors within retinal synapses. Together, these results from the rdta mice demonstrate that it is a useful model system for the study of receptor plasticity in the retina and possibly higher levels of the visual system. The results are supportive of the concept of a plastic retina in which environmental stimuli may induce changes in the structure and chemistry of the retinal synapses, although it is appreciated that the loss of photoreceptors is not quite the same thing as a loss of visual input. This concept can now be tested in the retinae of normal animals using a more physiologically appropriate paradigm.
Materials and Methods
Animal model - the rdta transgenic mouse
All animals experiments were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and with the authorization of the Institutional Animal Care and Use Committee (IACUC).
The rdta mouse has been previously described [68]. Expression of an attenuated diphtheria toxin gene under the control of rhodopsin promoter results in the specific elimination of rod photoreceptors in the retina [34]. The transgene was maintained in the heterozygous state by crossing the rdta mice to C57BL/6J mice.
Age matched littermate control and the rdta transgenic mice were housed under a cyclic regimen of 12 hours light/12 hours dark. Each experimental group contains 4-5 animals. Groups of the rdta and littermate control animals were sacrificed at postnatal day 40 (P40). The animals were euthanized in the light period of the diurnal cycle by injection with sodium pentobarbital (40 mg/ml, 0.5 mg/g body weight). The retinae were used for immunocytochemistry, back phosphorylation assays and immunoprecipitation assays.
Subcellular fractionation
Subcellular fractionation was used to obtain a crude synaptic plasma membrane (SPM) fraction according to a modification [69] of a previously described method [70]. Briefly, retinae were isolated with homogenization buffer (HB, 0.32 mM sucrose, 2 mM Hepes, pH 7.4, 1 mM EGTA, 2 μg/ml leupeptin and 2 μg/mg Aprotinin) and homogenized. The homogenate was centrifuged at 900 g for 10 min to produce a pellet (P1) and a supernatant (S1). P1 was resuspended in HB and centrifuged again at 900 g for 10 min to obtain pellet P1' and supernatant S1'. S1 and S1' were combined and centrifuged at 10,000 g for 20 min to produce pellet P2 and supernatant S2. P2 was resuspended in the lysing buffer (LB, 2 mM Hepes, pH 7.4, 1 mM EGTA, 2 μg/ml leupeptin and 2 μg/mg Aprotinin), and kept on ice for 15 min, homogenized and centrifuged at 19,000 g for 20 min to obtain pellet P3 and supernatant S3. P3 is the fraction containing crude SPM and was resuspended in HB, stored at -80°C prior to use. All procedures were done at 4°C and the protein concentrations of samples were assayed using the Coomassie Blue method. Different amounts of the crude SPM protein, 2, 4, 6, 8, and 12 μg, were used to obtain the standard curves for each antibody. Unless otherwise stated, six micrograms of SPM protein, which is within the linear range of the standard curves (data not shown), was chosen for the comparative study between the rdta and their littermate mice.
Western blotting
The SPM proteins were separated by 7.5% SDS-PAGE gels and subsequently transferred to nitrocellulose membranes. The blots were first blocked with 5% non-fat dry milk in TPBS buffer (PBS with 0.5% Tween 20) at RT for 1 hr, and then incubated with 1:3000 dilution of anti-β-actin (Sigma, St. Louis MO), 0.75 μg/ml of anti-NR1 (Chemicon, Temecula, CA), 1:1000 dilution of anti-NR2A (Chemicon), 0.35 μg/ml of anti-NR2B (Chemicon) and 1 μg/ml of anti-GluR1 (Chemicon) antibodies, respectively, at 4°C overnight. After washing, the secondary antibody conjugated with peroxidase was applied for 1 hr at RT. The bands were visualized using a chemiluminescent detection system (ECL, Amersham Life Science, Arlinton, IL). Each blot was assayed with the four antibodies, stripping the membranes in between each assay.Images of immunoblots were analyzed with a computerized image analysis system (Alpha Innotec Co., San Leandro, CA). The area of each immunolabeled band was calculated as the integrated density value (IDV). SigmaStat and SigmaPlot programs (Jandel Scientific Software, San Rafael, CA) were used to aid the analyses. Comparative studies of protein expression in the retinae of rdta mice and their littermate controls were performed with a minimum of three independent groups of animals.
Back phosphorylation assay for GluR1 in the rdta retina
This experiment was performed with two different procedures. The first procedure is to study the in vitro phosphorylation and to determine the level of 32P-ATP labeling in fluorographs. The second procedure is the use of an antiGluR1 antibody and western blotting to confirm that 32P labeling contains GluR1.
To determine if GluR1 phosphorylation by CaMKII in vivo is altered in the rdta retina, the SPM proteins of the rdta retina and their littermate controls are back phosphorylated by endogenous CaMKII in the presence of Ca2+/CaM (1 mM CaCl2 and 0.02 mg/ml calmodulin). The reaction mixture contains 50 mM PIPES pH 7.5, 0.2 mg/ml BSA, 20 mM MgCl2, 50 μM cold ATP and 0.04 μCi/μl 32P-ATP. The reaction was performed at 37°C for 3 min. Basically, if the result shows that GluR1 has a lower amount of γ-32P-ATP labeling on the film, it indicates that GluR1 has already been phosphorylated in vivo [71].
To confirm that the 32P labeled signal on the film is GluR1, the blots were incubated with anti-GluR1 antibody. The immunolabeled bands were visualized with alkaline-phosphatase conjugated secondary antibody using NBT/BCIP enzyme substrate (Chemicon).
Immunoprecipitation of GluR1
To provide further evidence for the presence of in vivo phosphorylation, a second procedure was used. Immunoprecipitation of GluR1 was performed after the back phosphorylation of retinal SPM proteins (described above) using an immunoprecipitation kit (Protein G; Boehringer Mannheim, Indianapolis, IN). In this case, 40 μg SPM retinal proteins from normal and rdta mice were used. The phosphorylation reaction was stopped by addition of a final concentration of 2 mM EGTA to chelate Ca2+. To block endogenous phosphatase activity, 20 mM NaPPi was added. The SPM proteins were first incubated with 50 μl of protein G-agarose in buffer-1 (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate, 2 μg/ml aprotinin, 5 mM EGTA, 4 mM Pefabloc SC, 1 μM pepstatin and 10 μg/ml leupeptin) at 4°C for 3 hours on a rocking platform to reduce background caused by non-specific absorption of irrelevant cellular proteins. After brief centrifugation, the supernatants were mixed with 1 μg of anti-GluR1 antibody and incubated at 4°C for 1 hour. Five microliter of the protein G-agarose was then added to each sample and incubated at 4°C overnight on a rocking platform. The samples were washed two times with buffer-1, two times with buffer-2 (50 mM Tris-HCl, pH 7.5, 500 mM NaCl, 0.1% Nonidet P40 and 0.05% sodium deoxycholate) and one time with buffer-3 (50 mM Tris-HCl, pH 7.5, 0.1% Nonidet P40 and 0.05% sodium deoxycholate). After removing the last traces of the final wash from the agarose pellet, 30 μl of the sample buffer (0.125 M Tris, 2% SDS, 10% sucrose, 5% 2-mercaptoethanol, and 0.02% bromophenol blue at a pH of 8.0) was added to each tube and the tubes were heated at 95°C for 5 minutes. The supernatants were collected and subjected to a 7.5% SDS-PAGE. The gels were dried by vacuum at 75°C for 1 hour and then exposed to film at -80°C for 2-3 days.
Abbreviations
NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GluR, glutamate receptor; SPM, crude synaptic membrane; CaMKII, calcium/calmodulin-dependent protein kinase II; SDS PAGE, sodium dodecyl sulfate electrophoresis.
Acknowledgments
Acknowledgments
The authors thank Dr. Maureen A McCall for providing the rdta transgenic mouse line. This work was supported by NSF-EPS-9874764, Kentucky Lions Eye Research Foundation and an unrestricted grant from Research to Prevent Blindness, Inc.
Contributor Information
Ling O Liu, Email: 10liu002@gwise.louisville.edu.
Aicha Laabich, Email: a01aab01@athena.louisville.edu.
Andrea Hardison, Email: linda_jerald@mindspring.com.
Nigel GF Cooper, Email: nigelcooper@louisville.edu.
References
- Barnstable CJ. Monoclonal antibodies which recognize different cell types in the retina. Nature. 1980;286:231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Basinger SF. Pattern recognition of amino acid signatures in retinal neurons. J Neurosci. 1995;15:5106–5129. doi: 10.1523/JNEUROSCI.15-07-05106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Katsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Megure H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. Cloning and expression of the 4 subunits of the NMDA receptor channel. FEBS Lett. 1992;313:34–38. doi: 10.1016/0014-5793(92)81178-O. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Karp SJ, Masu M, Eki T, Ozawa K, Nakanishi S. Molecular cloning and chromosomal localization of the key subunit of the human N-methyl-D-aspartate receptor. J Biol Chem. 1993;268:3728–3733. [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Rolden LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–34. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler N, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Wu GY, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decreases in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Fox K, Daw N, Sato H, Czepita D. Dark-rearing delays the loss of NMDA-receptor function in kitten visual cortex. Nature. 1991;350:342–344. doi: 10.1038/350342a0. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Prusky G. Retinal activity regulates developmental switches in functional properties and ifenprodil sensitivity of NMDA receptors in the lateral geniculate nucleus. Dev Brain Res. 1997;101:165–175. doi: 10.1016/S0165-3806(97)00061-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Cooper NGF, Mower GD. Developmental changes in the expression of NMDA receptor subunits (NR1, NR2A, NR2B) in the cat visual cortex and the effects of dark rearing. Mol Brain Res. 2000;78:196–200. doi: 10.1016/S0169-328X(00)00076-0. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear M. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nature Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Barinaga M. New clues to how neurons strengthen their connections. Science. 1999;284:1755–1757. doi: 10.1126/science.284.5421.1755. [DOI] [PubMed] [Google Scholar]
- Archibald K, Molnar E, Henley JM. Differential changes in the subcellular distribution of alpha-amono-3-hydroxy-5-methyl-4-isoxazole propionate and N-methyl-D-asparate receptors in neonate and adult rat cortex. Neurosci Lett. 1999;270:49–52. doi: 10.1016/S0304-3940(99)00466-8. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Jackson-Lewis V, Chen X, Langston JW, Przedborski S. The postnatal development of AMPA receptor subunits in the basal ganglia of the rat. Dev Neurosci. 1998;20:19–33. doi: 10.1159/000017295. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Furuta A, Blackstone CD. AMPA receptor protein in developing rat brain: glutamate receptor-1 expression and localization change at regional, cellular, and subcellular levels with maturation. Neuroscience. 1998;83:917–928. doi: 10.1016/S0306-4522(97)00411-9. [DOI] [PubMed] [Google Scholar]
- Standley S, Wagle N, Baudry M. Developmental changes in subcellular AMPA/GluR receptor populations in rat forebrain. Dev Brain Res. 1998;107:277–283. doi: 10.1016/S0165-3806(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll , Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K and, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Liu OL, Li G, McCall AM, Cooper NGF. Photoreceptor regulated expression of Ca2+/calmodulin-dependent protein kinase II in the mouse retina. Mol Brain Res. 2000;82:150–166. doi: 10.1016/S0169-328X(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Wyneken U, Smalla KH, Marengo JJ, Soto D, de la Cerda A, Tischmeyer W, Grimm R, Boeckers TM, Wolf G. Orrego F, Gundelfinger ED. Kainate-induced seizures alter protein composition and N-methyl-D-aspartate receptor function of rat forebrain postsynaptic densities. Neuroscience. 2001;102:65–74. doi: 10.1016/S0306-4522(00)00469-3. [DOI] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ishida A, Katagiri H, Mishina M, Fujisawa H, Manabe T, Takahashi T. Calcium and calmodulin-dependent phosphorylation of AMPA type glutamate receptor subunits by endogenous protein kinases in the post-synaptic density. Mol Brain Res. 1997;46:338–342. doi: 10.1016/S0169-328X(97)00073-9. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hoppocampal neurons. J Neurosci. 1994;14:1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano S, Chang CK, Shatz CJ. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.neuro.17.1.31. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Res. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Ehinger B, Ottersen OP, Storm-Mathisen J, Dowling JE. Bipolar cells in the turtle retina are strongly immunoreactive for glutamate. Proc Batl Acad Sci USA. 1988;85:8321–8325. doi: 10.1073/pnas.85.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jojich L, Pourcho RG. Glutamate immunoreactivity in the cat retina: a quantitative study. Vis Neurosci. 1996;13:117–133. doi: 10.1017/s0952523800007173. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Fletcher EL. Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J Comp Neurol. 1993;336:174–193. doi: 10.1002/cne.903360203. [DOI] [PubMed] [Google Scholar]
- Van Haesendonck E, Missotten L. Glutamate-like immunoreactivity in the retina of a marine teleost, the dragonet. Neurosci Lett. 1990;111:281–286. doi: 10.1016/0304-3940(90)90275-E. [DOI] [PubMed] [Google Scholar]
- Yang CY, Yazulla S. Glutamate- GABA-, and GAD-immunoreactivities co-localize in bipolar cells of tiger salamander retina. Vis Neurosci. 1994;11:1193–1203. doi: 10.1017/s0952523800006994. [DOI] [PubMed] [Google Scholar]
- Matus A, Brinkhaus H, Wagner U. Actin dynamics in dendritic spines: a form of regulated plasticity at excitatory synapses. Hippocampus. 2000;10:555–560. doi: 10.1002/1098-1063(2000)10:5<555::AID-HIPO5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ter-Margarian A, Djamgoz MB. Cytochalasin inhibits light-dependent synaptic plasticity of horizontal cells in teleost retina. Neurosci Lett. 1992;147:131–135. doi: 10.1016/0304-3940(92)90577-T. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. doi: 10.1002/(SICI)1096-9861(20000424)420:1<98::AID-CNE7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Xu JL, Lin SY, Levine ES, Black IB. NMDA receptor subunits in the postsynaptic density of rat brain: expression and phosphorylation by endogenous protein kinase. Mol Brain Res. 1998;59:215–228. doi: 10.1016/S0169-328X(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Quantification of AMPA receptor surface expression in cultured hippocampal neurons. Neurosc. 1997;78:361–371. doi: 10.1016/S0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Bellone C, Cattabeni F, Di Luca M. Protein kinace C activation modulates α-calmodulin kinase II binding to NR2A subunit of N-Methyl-D-Aspartate receptor complex. J biol chem. 2001;276:7609–7613. doi: 10.1074/jbc.M009922200. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Blackstone CD, Huganir RL. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993;16:147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Blackstone CD, Huganir RL. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 1992;6:2514–2523. [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bi-directional synaptic plasticity. Nature. 2000;405:955–999. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Vinade L, Dosemeci A. Regulation of the phosphorylation state of the AMPA receptor GluR1 subunit in the postsynaptic density. Cell Mol Neurobiol. 2000;20:451–463. doi: 10.1023/A:1007019030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Pires RS, Ferro ES, Britto LRG. Expression of the AMPA-type glutamate receptor subunits in the chick optic tectum changes biphasically after retinal deafferentation. Brain Res. 1998;810:283–287. doi: 10.1016/S0006-8993(98)00937-8. [DOI] [PubMed] [Google Scholar]
- Pires RS, Reboucas NA, Duroisin RM, Britto LR. Retinal lesions induce differential changes in the expression of flip and flop isoforms of the glutamate receptor subunit GluR1 in the chick optic tectum. Mol Brain Res. 2000;76:341–346. doi: 10.1016/S0169-328X(00)00016-4. [DOI] [PubMed] [Google Scholar]
- Bowes C, Li T, Frankel WN, Danciger M, Coffin JM, Applebury ML, Farber DB. Localization of a retroviral element within the rd gene coding for the beta subunit of cGMP phosphodiesterase. Proc Natl Acad Sci USA. 1993;90:2955–2959. doi: 10.1073/pnas.90.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Zhang C, Hamassaki-Britto DE, Britto LRG. Changes in expression of glutamate receptor subunits following photoreceptor degeneration in the rd retina. Neurosci Lett. 1995;183:83–86. doi: 10.1016/0304-3940(94)11120-8. [DOI] [PubMed] [Google Scholar]
- McCall MA, Gregg RG. Kerriman K, Goto Y, Peachey NS, Stanford LR. Morphological and physiological consequences of the selective elimination of rod photoreceptors in transgenic mice. Exp Eye Res. 1996;63:35–50. doi: 10.1006/exer.1996.0089. [DOI] [PubMed] [Google Scholar]
- Laabich A, Cooper NGF. Regulation of calcium/calmodulin-dependent protein kinase II in the adult rat retina is mediated by ionotropic glutamate receptors. Exp Eye Res. 1999;68:703–713. doi: 10.1006/exer.1999.0664. [DOI] [PubMed] [Google Scholar]
- De Robertis E. Ultrastructure and cytochemistry of the synaptic region. The macromolecular components involved in nerve transmission are being studied. Science. 1967;156:907–914. doi: 10.1126/science.156.3777.907. [DOI] [PubMed] [Google Scholar]
- Caputi A, Gardoni F, Cimino M, Pastorino L, Cattabeni F, Luca MD. CaMKII-dependent phosphorylation of NR2A and NR2B is decreased in animals characterized by hippocampal damage and impaired LTP. Eur J Neurosci. 1999;11:141–148. doi: 10.1046/j.1460-9568.1999.00414.x. [DOI] [PubMed] [Google Scholar]


