Abstract
Osteoarthritis is a common joint disease for which there are currently no disease-modifying drugs available. Degradation of the cartilage extracellular matrix is a central feature of the disease and is widely though to be mediated by proteinases that degrade structural components of the matrix, primarily aggrecan and collagen. Studies on transgenic mice have confirmed the central role of Adamalysin with Thrombospondin Motifs 5 (ADAMTS-5) in aggrecan degradation, and the collagenolytic matrix metalloproteinase MMP-13 in collagen degradation. This review discusses recent advances in current understanding of the mechanisms regulating expression of these key enzymes, as well as reviewing the roles of other proteinases in cartilage destruction.
Keywords: osteoarthritis, proteinase, cartilage, aggrecanase, collagenase
2. Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease affecting millions of people worldwide [1]. The disease is a leading cause of disability in the elderly, causing pain, stiffness and loss of function in articulating joints. OA is characterised by changes in the anatomy of load-bearing joints that lead to degradation of articular cartilage, inflammation of the synovium (synovitis), changes to subchondral bone and growth of new bone and cartilage (osteophytes) at the joint edge (see Figure 1)[2, 3]. The causes of OA are not fully understood, but mechanical factors such as joint injury and obesity are thought to be primary initiators of disease, with other risk factors such as age, gender and genetics contributing to disease development and progression [3, 4]. There are currently no disease-modifying OA drugs available, and treatment is limited to symptomatic relief or surgical replacement of affected joints. There is thus considerable interest in developing effective treatments that can halt or reverse the progression of the disease.
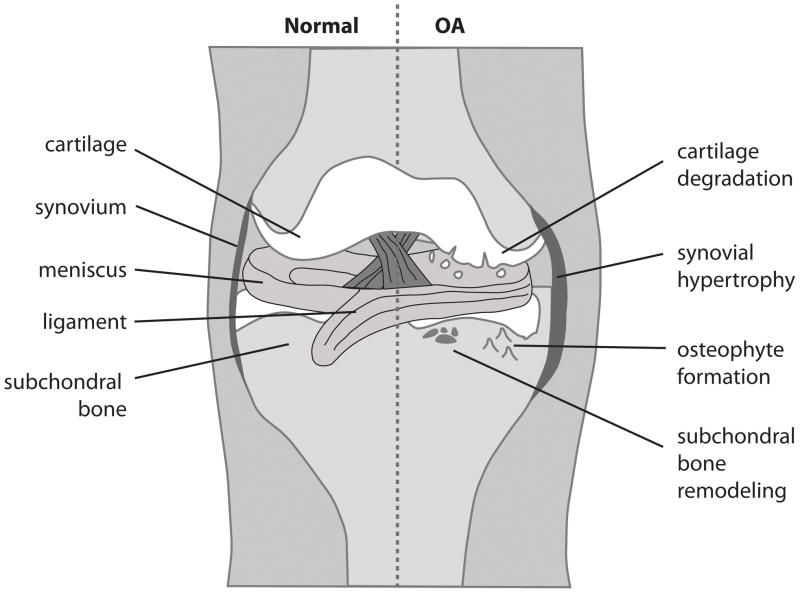
Figure 1. Cartoon representation of normal and osteoarthritic joint.
OA is characterised by changes to various tissues within synovial joints. The cartilage matrix is degraded by collagenases and aggrecanases, leading to fibrillation and subsequent loss of the articulating cartilage surface. Synovial fibroblasts undergo hypertrophy and inflammatory cells infiltrate the synovium. Bone remodelling leads to the formation of osteophytes at the cartilage/bone interface and subchondral bone sclerosis.
Loss of cartilage is central to the aetiology of OA. Cartilage is composed of one cell type, the chondrocytes, which are surrounded by a large volume of extracellular matrix (ECM). The matrix can be divided into zones based on their distance from the chondrocyte and matrix composition (see [4] for review). The pericellular matrix is localized immediately adjacent to the cell and is enriched with perlecan, type VI collagen and various regulatory molecules and growth factors that modulate chondrocyte function. The zone next to the pericellular matrix is the territorial matrix and further removed is the interterritorial matrix whose major components are collagen II and aggrecan. Collagen provides the tissue with tensile strength, while aggrecan is the major cartilage proteoglycan, drawing water into the matrix and allowing it to resist compression. Degradation of collagen and aggrecan is central to OA pathology, although degradation of less abundant molecules that participate in matrix organisation is also likely to contribute to disease progression [4]. This review describes the current understanding of which proteinases are responsible for aggrecan and collagen degradation in OA, and discusses recent advances in understanding the factors regulating their expression and activity. Other proteinases with potential roles in OA pathology are also highlighted.
3. Aggrecan-degrading enzymes
Aggrecan is a large proteoglycan containing numerous chondroitin sulfate and keratan sulfate glycosaminoglycan moieties, which are central to the function of the molecule as they draw water into the cartilage matrix, giving it the ability to withstand compression. Aggrecan is sensitive to proteolysis at numerous sites along its length. Cleavage of aggrecan in the interglobular domain (IGD) between the N-terminal G1 and G2 globular domains is thought to be of greatest pathological importance, as this releases the glycosaminoglycan-bearing region of aggrecan from the cartilage matrix and so abrogates the function of the molecule.
Degradation of aggrecan is an early event in the development of OA and a considerable amount of research has been done to identify the enzyme(s) responsible. Early work of Thomas [5] showed that rabbit ears collapsed after intravenous injection of papain, with the ear cartilage reversibly losing its metachromatic staining. This demonstrated that cartilage proteoglycans, of which aggrecan is now known to be the most abundant, are susceptible to proteolytic degradation. The same effect was observed upon injection of rabbits with large doses of vitamin A [6], which was thought to cause release of endogenous cartilage-degrading acidic proteinases from lysosomes [7]. Lysosomal cathepsins were demonstrated to be present in cartilage and to be able to degrade cartilage proteoglycans at acidic pHs [8–10]. Cathepsin D was considered to be the major cathepsin in cartilage, as cathepsin D-like activity increased 3-fold in OA cartilage [10] and antibodies against cathepsin D inhibited proteoglycan and cartilage degradation at pH 5.0 [11]. However, OA cartilage has a neutral pH [10] and Woessner [9] showed that while pepstatin and chloroquine inhibited proteoglycan degradation at pH 5, they had no effect on degradation at pH 7.2. This important observation indicated that degradation of cartilage proteoglycans at physiological pH was unlikely to be mediated by cathepsins, but rather by an unidentified neutral proteinase.
Metalloproteinases found in articular cartilage and bone were subsequently shown to be capable of degrading proteoglycans at neutral pH [12, 13]. Matrix metalloproteinase 3 (MMP-3) was isolated from human articular cartilage [14] and found to cleave the Asn341~Phe342 bond (where ~ indicates the cleavage site) in the aggrecan IGD [15]. Several other MMPs, including MMP-1, -2, -7, -8, -9 and -13, were later found to be able to cleave the same site, as well as other sites towards the C-terminus of the molecule [16–18]. MMPs were thus thought to be the primary aggrecan-degrading enzymes in OA until a landmark study by Sandy and colleagues [19] revealed that the majority of aggrecan fragments present in the synovial fluid of OA patients were cleaved not at the MMP-sensitive Asn341~Phe342 bond, but at the Glu373~Ala374 bond in the IGD. This novel cleavage site was also shown to be the primary site of aggrecan fragmentation in cytokine-stimulated chondrocyte and cartilage explant cultures [20, 21]. Hydrolysis at this site in chondrocyte and cartilage explant cultures was not blocked by TIMP-1, TIMP-2 or synthetic MMP inhibitors [22, 23], indicating that an MMP could not be responsible for the ‘aggrecanase’ activity.
The first ‘aggrecanase’ was purified from IL-1-stimulated bovine nasal cartilage by researchers at DuPont Pharmaceuticals in 1999 [24]. The enzyme was named aggrecanase 1, or A Disintegrin And Metalloproteinase with Thrombospondin motifs 4 (ADAMTS-4) based on its homology to the previously identified enzyme ADAMTS-1 [25]. Shortly thereafter, a homologous enzyme was cloned from murine and bovine cartilage and named aggrecanase 2 or ADAMTS-5 (initially coined ADAMTS-11)[26, 27]. The ADAMTSs are zinc-dependent metalloproteinases of the metzincin family [28](Figure 2). They have numerous ancillary domains that modulate their substrate specificity and activity [29, 30]. ADAMTS-1, -8, -9, -15, -16 and -18, can also degrade aggrecan in vitro [31–35], but ADAMTS-5 is the most active ‘aggrecanase’ in vitro, followed by ADAMTS-4 [30]. ADAMTS-4 and ADAMTS-5 are thus considered to be the major enzymes responsible for pathological cleavage of aggrecan at the Glu373-Ala374 bond in the IGD [23, 36–38].
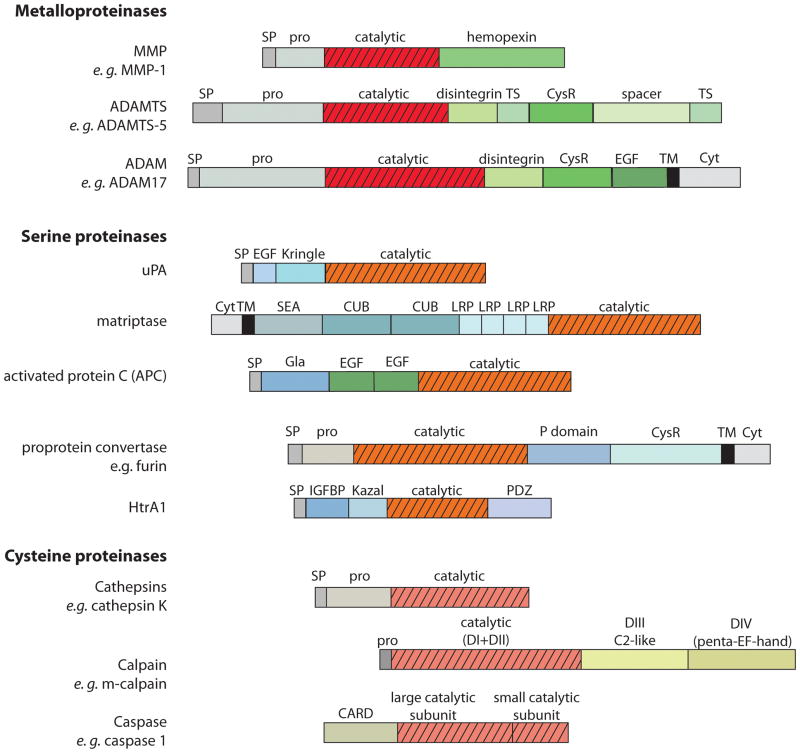
Figure 2. Schematic representation of domain structure of proteinases involved in OA cartilage destruction.
The MMPs, ADAMTSs and ADAMs all contain an N-terminal signal peptide (SP), followed by a pro-domain (pro) and a metalloproteinase catalytic domain. The MMPs then contain a hemopexin ancillary domain [233], while the ADAMTSs contain disintegrin, thrombospondin (TS), cysteine-rich (CysR) and spacer ancillary domains [234]. The ADAMs contain C-terminal disintegrin, CysR, epidermal growth factor-like (EGF-like), transmembrane (TM) and cytoplasmic (Cyt) domains [235].
The serine proteinases involved in OA cartilage destruction are more structurally diverse than the metalloproteinases. uPA consists of an N-terminal SP, followed by an EGF-like domain, a Kringle domain and a C-terminal catalytic domain [236]. Matriptase 1 is a type II transmembrane protein, with an N-terminal cytoplasmic domain followed by a TM region [190]. This is followed by a sea urchin sperm protein/enteropeptidase/agrin (SEA) domain, 2 complement C1r/C1s, Uegf, Bmp1 (CUB) domains, 4 low-density lipoprotein receptor-related protein (LRP) domains and a C-terminal catalytic domain [190]. APC consists of a γ-carboxyglutamate (Gla) domain, followed by 2 EGF-domains and a trypsin-like serine proteinase domain [237]. Proprotein convertases such as furin are subtilisin-like serine proteinases consisting of an N-terminal SP and pro-domain, followed by a serine proteinase catalytic domain, a conserved regulatory P domain and a CysR domain [238]. Some of the proprotein convertases (e. g. furin) are type I transmembrane proteins and contain a C-terminal TM and cytoplasmic domain, while others (e. g. PACE4) lack these domains and are soluble [238]. HtrA1 consists of an N-terminal SP, followed by an insulin growth factor binding protein (IGFBP) domain, Kazal proteinase inhibitor (KI) domain, a trypsin-like serine proteinase domain and a C-terminal PDZ domain [199].
The cathepsins have comparatively simple structures, consisting of an SP, pro-domain and catalytic domain with no additional ancillary domains [239]. Classical calpains such as m-calpain consist of 4 domains, with domain I (D1) and domain II (DII) forming the catalytic domain, and domains III and IV (DIII and DIV) regulating catalytic activity and stability [240]. Domain III is C2-like, and domain IV contains 5 EF-hand repeats. Caspases have all contain a large and a small catalytic subunit. N-terminal to this is either an N-terminal caspase recruitment domain (CARD) domain (e. g. in caspase 1, 2, 4 and 5) or an N-terminal death effector domain (DED) (e. g. in caspase 8, 10) [241].
The pathological importance of ADAMTSs to the development of OA was demonstrated by the finding that Adamts5−/− mice develop less severe cartilage damage in a murine surgical model of OA and in an antigen-induced arthritis model [39, 40]. Similarly, transgenic mice with a knock-in mutation of aggrecan preventing ‘aggrecanase’ cleavage of the Glu373-Ala374 bond also develop less severe OA in the surgical OA and antigen-induced arthritis models [38]. Adamts1−/− and Adamts4−/− mice are not similarly protected [41, 42], indicating that ADAMTS-5 is the primary aggrecanase in mice. There is some evidence that ADAMTS-4 may contribute to cartilage degradation in other species, including humans [43–45]. ADAMTS-4 and ADAMTS-5 are thus attractive targets for the development of novel OA therapies, and several synthetic ADAMTS-4 and ADAMTS-5 inhibitors are in the early stages of development [46–49]. One of these inhibitors has recently been shown to block aggrecan degradation in a rat surgical OA model [48].
Cleavage of aggrecan at the Glu373~Ala374 bond is thus a signature of pathological aggrecan loss in OA cartilage. Aggrecan cleavage at the MMP-sensitive Asn341~Phe342 bond is also detectable in OA cartilage [50], and may occur later in the progression of disease. MMPs are also thought to contribute to C-terminal ‘trimming’ of aggrecan, which is considered non-pathological as it does not cause release of the majority of the glycosaminoglycan region of the molecule from the cartilage matrix [23, 36, 37].
4. Collagenases
The primary collagen found in the cartilage ECM is type II collagen, which forms a fibrillar network and provides the cartilage matrix with tensile strength. Along with aggrecan breakdown, degradation of collagen is a central feature of OA [51, 52]. The exact order in which cartilage matrix components are degraded during the development of OA is difficult to ascertain, but a number of in vitro studies on cartilage explants suggest that collagen degradation occurs only after aggrecan is lost from the tissue, and that the presence of aggrecan protects the collagen from degradation [53–56]. Furthermore, while aggrecan loss can be reversed, collagen degradation is irreversible, and cartilage cannot be repaired once collagen is lost [53, 55].
Ehrlich et al. [57] first demonstrated the presence of a collagen-degrading enzyme in OA cartilage in 1977. Fibrillar collagens are highly stable molecules that can be degraded by only a few mammalian enzymes, namely cathepsin K and the collagenolytic MMPs: MMP-1, -8, -13 and -14. MMP-13 is thought to be the primary collagenase in OA, with its expression increased in OA cartilage [51, 58–62] and in rodent surgical OA models [63]. Conditional expression of MMP-13 in murine cartilage induces spontaneous cartilage degradation [64], while Mmp13−/− mice are protected in a surgical OA model [65]. MMP-1 also efficiently cleaves type II collagen (R. Visse, Y. Tominaga, M. Wang, H. Nagase, personal communication), but its role in OA cannot be studied using murine models as murine MMP-1 differs considerably from the human enzyme [66].
The catalytic sites of the MMPs are highly homologous, and historically it has been difficult to generate sufficiently selective synthetic inhibitors to target individual MMPs. Previous attempts to treat cancer with MMP inhibitors failed due to lack of specificity of the inhibitors, which gave rise to toxicity and musculoskeletal side-effects [67]. MMP-13 is unusual among the MMPs, in that it has a very deep S1′ subsite. This feature has been exploited to generate highly selective MMP-13 inhibitors able to block collagen degradation in cartilage explants [51, 68, 69] as well as animal OA models [68, 70] without musculoskeletal side effects [68]. Further evaluation of the therapeutic efficacy of these inhibitors is eagerly awaited.
5. Other MMPs and ADAMs
In addition to MMP13, ADAMTS4 and ADAMTS5, mRNA expression of various other MMPs (e.g. MMP28), adamalysins (e.g ADAM12, ADAM15) and ADAMTSs (e.g. ADAMTS16, ADAMTS17) is reportedly increased in OA [61, 71, 72]. ADAM-8 has been suggested to contribute to OA pathogenesis by cleaving fibronectin, generating fragments that stimulate further cartilage catabolism [73]. Single nucleotide polymorphisms in ADAM12 [72] and ADAMTS14 [74] have reported associations with knee OA. The effects of numerous gene mutations and ablations on murine OA have been reviewed by Little and Fosang [75].
MMP3 is the most strongly expressed MMP in OA cartilage, although its expression decreases in late OA [60, 76]. The enzyme is known to participate in the activation of other MMPs, such as MMP-1 and MMP-13 [77, 78], raising the possibility that it may contribute to OA by activating latent collagenases. The susceptibility of Mmp3−/− mice to OA is, however, unclear. Van Meurs et al. [79] showed that Mmp3−/− mice are protected against collagen loss and aggrecan cleavage at Asn341~Phe342, suggesting that MMP-3 promotes collagenase activation and either direct or indirect MMP-mediated aggrecan cleavage. However, Clements et al. [80] found that Mmp3−/− mice develop more severe surgically induced OA, suggesting that MMP-3 can also serve to protect cartilage in some circumstances.
Mmp9−/− mice are protected in an infectious arthritis model [81] but develop more severe OA in a surgically induced OA model [82]. This difference most likely reflects differences in disease etiology in the two models, as well as differences between mice strains.
Expression profiling studies suggest that MT1-MMP is similarly expressed in normal and OA cartilage [60, 61], although studies on isolated bovine chondrocytes suggest that MT1-MMP expression can be transiently increased by cyclic compression [83]. MT1-MMP is highly expressed in rheumatoid synovial fibroblasts and has been shown to promote invasion of these cells into cartilage [84]. The role of MT1-MMP in OA has not been studied in murine surgical models as MT1-MMP null mice exhibit severe skeletal abnormalities [85].
6. Transcriptional regulation of MMPs and ADAMTSs in OA
Studies on transgenic mice have confirmed the importance of MMP-13 and ADAMTS-5 in the development of OA. As described above, inhibitors targeting these enzymes are in development as potential OA therapies. Additionally, there is considerable interest in understanding the factors that lead to increased activity of these enzymes in OA, with the hope of uncovering therapeutic targets upstream of the effector proteinases. Some of these newly described networks and regulatory mechanisms are discussed below.
6.1. RUNX2
Runt-related transcription factor 2 (RUNX2, also known as core-binding factor 1, or Cbfa1) is a central transcription factor regulating skeleton formation by stimulating osteoblast differentiation [86, 87] and directing the process of endochondral ossification [88] by stimulating expression of genes required for chondrocyte maturation and hypertrophy (e. g. COL10A1, collagen X)[89], degradation of the cartilage matrix (e. g. MMP13) [90, 91] and vascularization of the tissue (e. g. VEGFA)[92]. Chondrocyte hypertrophy, matrix degradation and vascular invasion are also characteristic of OA, leading to the theory that OA may involve aberrant recapitulation of this developmental programme in adult cartilage [93–95].
RUNX2 is not expressed in normal adult cartilage, but its expression and that of several of its target genes increases in early OA [96]. Runx2+/− mice develop less severe cartilage degradation and osteophyte formation in a surgical OA model [97]. Mmp13 is a known RUNX2 target gene [90, 91] and Runx2+/− mice exhibit decreased Mmp13 expression [97]. ADAMTS4 and ADAMTS5 are also thought to be RUNX2 target genes [91, 98, 99], although their expression in Runx2+/− mice has not been reported.
RUNX2 expression can be increased by factors known to promote the development of OA, such as mechanical stimuli [91, 100], hypoxia-inducible factor 2α (HIF-2α) [101] and Indian hedgehog [102]. RUNX-2 may thus be a central transcription factor increasing expression of several OA-promoting genes.
6.2. Inflammation
The role of inflammatory pathways in the aetiology of rheumatoid arthritis is well documented [103]. It is increasingly accepted that inflammation also plays a role in the development of OA [4, 104]. For example, IL-1 is well known to stimulate the expression of MMPs such as MMP1 and MMP13 in OA cartilage [59, 60, 105]. Inflammatory cytokines can also increase chondrocyte expression of ADAMTS4 and ADAMTS5 [106] although some reports indicate that ADAMTS5 expression is largely constitutive (see [28] for review). The central role of IL-1β in murine OA pathology has been demonstrated in numerous studies (see Glasson et al. [82] for review). For example, mice treated with an inhibitor of IL-1β converting enzyme developed less severe joint damage in two arthritis models [107], and ablation of IL-1β has been reported to decrease surgically induced OA [82].
6.2.1. Hypoxia-inducible factor 2α (HIF-2α)
Two recent studies have demonstrated that inflammatory cytokines can stimulate OA cartilage catabolism by inducing nuclear factor- κB (NF-κB)-dependent expression of the transcription factor HIF-2α [94, 108]. IL-1β-induced expression of ADAMTS4, MMP1, MMP3, MMP9, MMP12 and MMP13 in rabbit articular chondrocytes was increased by over-expression of HIF-2α and decreased by HIF-2α siRNA [108]. Ectopic expression of HIF-2α in murine cartilage induces spontaneous cartilage destruction [108], while HIF-2α-deficient mice are resistant to cartilage degradation and osteophyte development in a surgical OA model [94, 108]. HIF-2α has also been shown to regulate developmental endochondral ossification, by inducing expression of genes mediating chondrocyte hypertrophy (e. g. Col10a1), degradation of the cartilage matrix (e.g. Mmp13) and vascular invasion (e. g. Vegfa) [94]. These are known RUNX2 target genes (section 6.1) and HIF-2α has been shown to increase RUNX2 expression [101, 109], suggesting that HIF- 2α may stimulate expression of these genes via RUNX2. However, HIF-2α-dependent expression of Mmp13, Col10a1 and Vegfa is not affected by expression of a dominant negative form of RUNX-2 [101], suggesting that HIF- 2α may also act independently of RUNX-2. HIF- 2α also increased expression of the transcription factor Indian hedgehog [101] that stimulates RUNX-2 expression [102, 110].
The role of HIF-2α in the development of human OA is unclear as both increased [94, 108] and decreased [111] HIF-2α expression has been reported in human OA cartilage. Saito et al. [94] reported that a single nucleotide polymorphism that increases HIF-2α expression is associated with knee OA in a Japanese cohort, although Nakajima et al. [112] were unable to replicate this association in a larger patient group. Additionally, In contrast to its hydroxylation-independent catabolic effects in a mouse chondrogenic cell line [94], HIF-2α has been shown to have hypoxia-dependent anabolic effects on human chondrocytes [113].
6.2.2. Histone deacetylases
Histone deacetylases (HDAC) modulate gene expression by increasing histone association with DNA, thus mediating chromatin condensation and inhibiting transcription factor binding [114]. HDACs thus play central roles in numerous physiological and pathological conditions [114]. HDAC can be divided into 3 classes on the basis of sequence homology: 2 classes of classical HDAC and the class III NAD+-dependent sirtuin family.
HDAC inhibitors have been shown to block inflammatory cytokine-induced expression of MMP1, MMP13, ADAMTS4 and ADAMTS 4 and ADAMTS5 in human chondrocytes [115–117]. The protective effect of HDAC inhibitors is further illustrated by the demonstration that they can block inflammatory cytokine-stimulated degradation of both proteoglycans and collagen in bovine cartilage explants [115]. The effect of HDAC inhibitors on surgically induced murine OA has not been reported, but HDAC inhibitors can block collagen-induced arthritis in mice [118]. HDAC1, HDAC2 and HDAC7 are all expressed at elevated levels in human OA cartilage [117, 119].
HDAC inhibitors block both HIF-1α and HIF-2α transcriptional activity [120]. HDAC4, HDAC6 and HDAC7 have been shown to stimulate HIF-1α activity [121, 122], while the HDAC Sirtuin 1 (SIRT1) stimulates HIF-2α transcriptional activity [123]. HDACs may thus promote cartilage catabolism by stimulating HIF-2α activity.
Some studies suggest that HDACs can also serve to protect cartilage. SIRT1 contributes to cartilage homeostasis by inhibiting chondrocyte apoptosis [124] and stimulating expression of cartilage-specific genes [125]. HDAC4 has been shown to interact with and inhibit the activity of RUNX2 [126]. Hdac4−/− mice exhibit a similar cartilage hypertrophy phenotype to RUNX2-over-expressing chondrocytes [88, 126] and over-expression of HDAC4 inhibits chondrocyte hypertrophy [126]. Conversely, HDAC4 expression is reduced by the chondroprotective miR140 [127]. Further studies are required to delineate the roles of individual HDAC in cartilage homeostasis and OA development.
6.3. miR140
MicroRNAs (miRNAs) are non-coding RNA sequences that post-transcriptionally down-regulate gene expression by interacting with the 3′ untranslated region of target mRNAs, leading either to degradation of the mRNA or repression of its expression. miRNAs were first identified in 1993 as regulators of C. elegans larval development [128], and have since been shown to regulate expression of numerous genes, often in a tissue-specific or developmental-stage specific manner.
miR-140 is abundantly expressed in normal cartilage under the control of the master cartilage transcription factor Sox9 [129]. miR-140 expression is reduced in OA cartilage [62, 127, 130] and miR-140−/− mice develop accelerated spontaneous and surgically induced OA, while mice over-expressing miR-140 are resistant to antigen-induced arthritis [129]. Adamts5 has been shown to be a target for miR140, with Adamts5 expression increased in miR-140−/− chondrocytes and decreased in miR-140 over-expressing mice [129].
Iliopoulos et al. [62] identified 16 microRNAs with altered expression in OA cartilage. miR-22 is thought to increase expression of MMP13 [62], while miR-9, miR-27a and miR-27b have been reported to inhibit MMP13 expression in human OA chondrocytes [130–132].
6.4. Mechanical stimuli
Mechanical loading is an important factor in cartilage homeostasis. Both disuse and excessive use of joints can initiate cartilage degradation [133, 134], with mechanical injury shown to increase expression of RUNX2 [91, 100], MMP1, MMP3 [135], MMP13 [136] and ADAMTS5 [136]. Several research groups are investigating the molecular mechanisms by which moderate mechanical load maintains joint function and protects cartilage by suppressing expression of catabolic proteinases.
Recent studies have identified the transcription co-regulator CITED2 (cAMP-responsive element-binding protein/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2) as a novel mediator of mechanical responses in cartilage [137, 138]. Experimental mechanical stimuli have been shown to increase levels of CITED2, which in turn suppresses MMP1 and MMP13 expression by decreasing interaction of the MMP transactivator Ets-1 with its co-activator p300 [137, 138]. Immobilization of rat hind limbs reduces CITED2 expression, leading to increased expression of MMP1 and MMP3, and cartilage degradation [138, 139]. The effects of CITED2 on ADAMTS expression and its role in OA remain to be studied.
Fibroblast growth factor 2 (FGF-2) is thought to act as a transducer of protective mechanical signals from the pericellular matrix to chondrocytes [140]. Fgf−/− mice develop accelerated spontaneous and surgically induced OA, accompanied by an increase in Adamts5 expression and aggrecanase activity [63]. Addition of exogenous FGF-2 to cytokine-stimulated normal human cartilage explants suppresses ADAMTS4 and ADAMTS5 expression and reduces cartilage degradation [106, 116]. The mechanism by which FGF-2 suppresses ADAMTS expression is not currently known [63]. In addition to these protective effects, FGF-2 has also been reported to have catabolic effects (reviewed in [141]), stimulating expression of MMP1, MMP3 and MMP13 in cartilage explants and cultured chondrocytes [116, 135, 142–144]. Factors such as the force of the mechanical load and the pattern of FGF receptor expression may determine whether FGF-2 transmits a protective or a catabolic signal to chondrocytes.
6.5. Extracellular sulfatases
Sulf-1 and Sulf-2 are recently described extracellular sulfatases that remove the 6-O-sulfate group from glucosamine residues of heparan sulfate [145]. The enzymes modulate the activity of various heparin-binding growth factors and chemokines by blocking their binding to heparan sulfate components of the ECM [146]. Cartilage expression of Sulf-1 and Sulf-2 increases with age and in OA [147, 148]. The enzymes appear to be chondroprotective, as Sulf-1−/− or Sulf-2−/− mice develop accelerated spontaneous OA upon aging, and also develop more severe cartilage damage in a surgical OA model [148]. Sulf-null chondrocytes showed a more catabolic phenotype than wild-type chondrocytes, with increased expression of MMP-13 and ADAMTS-5, and decreased expression of aggrecan and type II collagen [148]. Sulf-1−/− or Sulf-2−/− mice showed an increase in FGF-2-dependent Erk1/2 phosphorylation, and a decrease in BMP-7-dependent Smad1/5 phosphorylation, indicating that the Sulfs inhibit FGF-2 activity and promote BMP-7 activity [148]. Similar effects were observed upon treating human chondrocytes with Sulf-1 and Sulf-2 siRNA [148]. BMP-7 is known to have anabolic effects on cartilage [149], while both anabolic and catabolic effects have been reported for FGF-2 [63, 144]. The Sulfs are likely to affect the activity of growth factors and cartilage proteins other than BMP-7 and FGF-2, with their overall effect on cartilage homeostasis determined by the balance of these changes. For example, both the ADAMTSs and their endogenous inhibitor Tissue Inhibitor of Metalloproteinases 3 (TIMP-3) can bind to heparin [35, 150, 151] and the effect of Sulf-1 and Sulf-2 on their activity remains to be determined.
6.6. Syndecan 4
Expression of the transmembrane heparan sulfate proteoglycan syndecan 4 is elevated in human OA cartilage and in rodent OA models [152, 153]. Sdc4−/− mice developed less severe cartilage damage in a surgical OA model, accompanied by a reduction in both proteoglycan loss and aggrecan cleavage at the Glu373~Ala374 ‘aggrecanase’ bond [153]. Mice injected with syndecan 4 blocking antibodies were similarly protected against ADAMTS-5-mediated cartilage damage [153]. ADAMTS-5 interacts with the heparan sulfate chains of syndecan 4 [153], but the molecular mechanisms by which syndecan 4 promotes ADAMTS-5 activity are unclear. Sdc4−/− mice have reduced levels of Mmp3 expression, and Echtermeyer et al. [153] argue that MMP-3 contributes to activation of ADAMTS-5. ADAMTSs are thought to be activated primarily by proprotein convertases [154, 155], and there is currently no evidence for direct activation of ADAMTS-5 by MMP-3 or by other MMPs, or for reduced ADAMTS-5 activation in the Sdc4−/− mice.
Syndecan 1 is also expressed at elevated levels in OA cartilage [152, 156], and has been shown to retain ADAMTS-4 on the surface of human chondrosarcoma cells [157]. Syndecan 4 may increase ADAMTS-5 activity by similarly modulating the localisation of the enzyme, or that of its physiological inhibitor, TIMP-3. It would be interesting to investigate the effect of the sulfatases Sulf-1 and Sulf-2 (section 6.5) on syndecan 4 binding to ADAMTS- 5.
6.7. DDR-2
The discoidin domain receptors DDR-1 and DDR-2 are receptor tyrosine kinases that bind to native collagen types I, II, III, IV and V [158–160]. Binding of collagen to the extracellular domains of the DDRs causes autophosphorylation of their cytoplasmic domains, initiating downstream signalling events including increased expression of the collagenases MMP-1 and MMP-13 [158, 161]. Expression of DDR-2 is increased in human OA [162, 163]. Ddr2+/− mice are protected against spontaneous and surgically induced OA and show reduced expression of MMP-13 [164].
The most abundant collagen in cartilage is type II collagen, which has been shown to phosphorylate DDR-2, albeit less strongly than collagen type I and III [158]. However, type II collagen is localised in the inter-territorial matrix and is not present in the pericellular matrix, so there is unlikely to be direct contact between chondrocytes and type II collagen in healthy cartilage. Xu et al. [164] suggest that damage to the pericellular matrix may occur early in the development of OA, leading to aberrant interaction of chondrocytes with type II collagen and initiation of catabolic signalling. The serine proteinase HtrA1 (section 8.3) is localised in the pericellular matrix [165, 166] and is able to degrade a variety of matrix components, leading Xu et al. to propose that it may play a role in degradation of the pericellular matrix in early OA, initiating catabolic DDR-2 signalling and further MMP-13-mediated degradation of the collagen matrix.
6.8. PAR-2
Expression of protease-activated receptor 2 (PAR-2) is increased in human OA chondrocytes [167, 168] and subchondral bone osteoclasts [169]. The receptor appears to promote cartilage degradation, as PAR-2−/− mice are protected in surgical OA models [170, 171]. Similarly, treatment of wild-type mice with a PAR-2 antagonist or a PAR-2 blocking antibody protects against the development of OA [170].
PAR-2 is a member of a family of seven transmembrane G-protein-coupled receptors that are activated by cleavage of their extracellular domains by serine proteinases, generating a tethered ligand that stimulates receptor activation and downstream signaling. The mechanisms of PAR-2 activation in OA and the downstream signaling consequences remain to be elucidated. PAR-2 can be activated by matriptase 1 (section 8.1, [172]), but other cartilage serine proteinases are also likely to contribute to its activation. PAR-2 activation has been shown to increase expression of MMP-1 and MMP-13 in OA chondrocytes [168], but effects on ADAMTS expression have not been reported.
6.9. Wnt signalling
Wnt signalling is critical in skeletal development, and there has been considerable interest in the role of Wnt signalling in the development of OA [173]. Wnt signalling affects multiple cellular pathways in chondrocytes, but recent studies have indicated that Wnt signalling promotes OA at least in part by increasing expression of cartilage-degrading proteinases. For example, siRNA against the Wnt co-receptor LRP-5 causes a decrease in MMP13 expression [174]. Furthermore, Wnt-induced signalling protein 1 (WISP-1) has been shown to increase expression of MMP3, MMP9 and ADAMTS4, but not ADAMTS5 [175]. Adenoviral expression of WISP-1 in mouse knee joints induced cartilage degradation and aggrecan hydrolysis at both Asn341~Phe342 and Glu373~Ala374, indicators of MMP and aggrecanase cleavage, respectively[175]. WISP-1 expression is increased in human and murine OA cartilage [175], and a single nucleotide polymorphism in WISP-1 is reportedly associated with spinal OA in Japanese women [176].
7. Inhibitors of MMPs and ADAMTSs
The tissue inhibitors of metalloproteinases (TIMPs) are the endogenous inhibitors of the MMPs and some members of the ADAM and ADAMTS families (see [177] for review). The MMPs are strongly inhibited by all four of the mammalian TIMPs (TIMP-1, -2, -3 and -4), with the exception of some of the membrane-type MMPs that are poorly inhibited by TIMP-1. Conversely, ADAMTS-4 and ADAMTS-5 are effectively inhibited only by TIMP-3 [178, 179]. As TIMP-3 can inhibit both MMPs and ADAMTSs, it is a central inhibitor of cartilage degradation. Addition of exogenous TIMP-3, but not TIMP-1 or TIMP-2 blocks cartilage degradation in explant cultures [180], and injection of TIMP-3 blocks cartilage breakdown in a rat surgical model of OA [181]. The chondroprotective role of TIMP-3 is confirmed by the finding that Timp3−/− mice develop increased cartilage degradation upon aging [182] and increased cartilage damage in an antigen-induced arthritis model [183]. While TIMP-3 mRNA levels are not significantly altered in OA [63, 184, 185], levels of TIMP-3 protein are reduced in human OA cartilage [185]. TIMP-3 can be endocytosed and degraded by chondrocytes [186], suggesting that its activity in cartilage may be regulated post-translationally rather than transcriptionally. Agents such as pentosan polysulfate that block TIMP-3 endocytosis are able to increase cartilage levels of TIMP-3 and to inhibit aggrecan degradation [186]. Pentosan further protects cartilage by increasing the affinity of TIMP-3 for ADAMTS-4 and ADAMTS-5 by more than 100-fold [186].
The susceptibility of other Timp-null mice to OA has not been reported. TIMP-2 has no effect on glycosaminoglycan release from bovine, porcine or human cartilage explants, while TIMP-1 has been shown to partially inhibit glycosaminoglycan release from human but not bovine or porcine cartilage [56, 106, 180]. Expression of TIMP-4 is decreased in OA cartilage [61], and a single nucleotide polymorphism in the 3′ untranslated region of TIMP-4 is reportedly associated with OA in a Korean cohort [187].
8. Serine proteinases in OA
8.1. Collagenase activators
The collagenases MMP-1 and MMP-13 are known to be activated by a number of other proteinases, including MMP-3 and the serine proteinase plasmin, which is in turn generated from plasminogen by urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) [77, 78]. Increased expression of MMP-3 [60, 76], tPA [188] and uPA [188] have all been reported in OA. As discussed above (section 4), degradation of aggrecan in cytokine-stimulated bovine and porcine cartilage explant model systems occurs within the first week, while collagen degradation occurs later [23, 53–56]. Collagenolysis can be initiated during the first week of culture by addition of proMMP activators such as MMP-3 or p-aminophenylmercuric acetate [189], indicating that collagenases are expressed during this time, but that they are largely present as inactive zymogen forms and that their activation is the rate-limiting step in cartilage collagenolysis. Collagenase activity can be inhibited by addition of serine proteinase inhibitors, indicating that serine proteinases are primarily responsible for activation of collagenases in cartilage [189].
Milner et al. [172] have recently reported that the type II transmembrane serine proteinase matriptase 1 (or membrane-type serine proteinase 1, MT-SP1, Figure 2)[190] is a novel activator of collagenase activity and is up-regulated in OA cartilage. In addition to activating proMMP-1 and proMMP-3 in vitro [172, 191], addition of recombinant matriptase 1 stimulated collagen breakdown in bovine and human OA cartilage explants [172]. The enhanced collagen degradation could be blocked by the metalloproteinase inhibitor GM6001, suggesting that matriptase 1 acted by increasing MMP-mediated collagenolysis [172]. Matriptase 1 was found to stimulate cartilage expression of MMP1, MMP3 and MMP13 [172], and to activate the collagenase activator uPA [192]. Matriptase 1 thus stimulates cartilage collagenolysis through multiple inter-related mechanisms, making it an attractive target for the development of chondroprotective therapies.
Activated protein C (APC) is known primarily as a serine proteinase of the coagulation cascade, but Jackson et al. [193] showed that APC is also expressed by OA chondrocytes in regions of cartilage fibrillation, although not in normal cartilage. Addition of exogenous APC increased cytokine-stimulated aggrecan and collagen degradation in ovine cartilage explants [193] and collagen degradation in equine explants [194]. The catabolic effects of APC could be partially inhibited by addition of a broad-spectrum MMP inhibitor [193, 194], indicating that APC acts by increasing MMP activity. APC had no effect on expression of a range of MMPs, ADAMTSs or TIMPs, but increased activation of proMMP-2 and proMMP-9, although not proMMP-13 [193, 194].
8.2. ADAMTS activators
The ADAMTSs are activated by members of the family of proprotein convertases (PC), including furin, Paired Basic Amino Acid Cleaving Enzyme 4 (PACE4), PC5/6 and PC7 (Figure 2)[154, 155]. Inhibition of furin-like enzymes inhibits aggrecan and collagen degradation in cartilage explants [195]. PACE4 is thought to be the primary member of the family responsible for aggrecanase activation in cartilage, and its expression is increased in OA [76, 196]. Reducing PACE4 expression with siRNA significantly inhibited aggrecanase activity in cultured human chondrocytes and partially blocked aggrecan degradation in OA cartilage explants [196].
8.3. HtrA1
HtrA (high temperature requirement A) was originally identified as an E. coli heat shock protein and was then shown to be a trypsin-like serine proteinase involved in degradation of misfolded proteins [197, 198]. Homologues were subsequently identified in mammals, with 4 isotypes found in humans [199](Figure 2). Expression of HtrA1 is elevated in OA cartilage [76, 166, 172, 200, 201], and expression of HtrA3 and HtrA4 may also be increased in OA [76]. Few serine proteinases are thought to participate directly in degradation of the cartilage ECM, but HtrA1 degrades a variety of cartilage matrix proteins, including aggrecan, decorin, fibromodulin and fibronectin in vitro [165, 200]. The enzyme has been suggested to degrade the pericellular matrix, a factor proposed to increase catabolic DDR-2 signalling (section 6.7) [164, 166]. Type VI collagen is absent from the pericellular matrix surrounding chondrocytes expressing HtrA1, suggesting that this enzyme may contribute to type VI collagen degradation as well [166]. Type VI collagen is resistant to MMP-1, MMP-2, MMP-3 and MMP-9, but can be degraded in vitro by serine proteinases including elastase, trypsin and cathepsin G [202].
HtrA1 can also degrade aggrecan, cleaving at the VQTV356~357TWPD bond in the IGD, between the MMP and ADAMTS cleavage sites [201]. Aggrecan fragments bearing the VQTV356 neo-epitope are detectable in OA but not in normal cartilage [201]. HtrA1 is unlikely to contribute greatly to pathological aggrecan cleavage however, as VQTV356 fragments are present at 20-fold lower levels in OA cartilage extracts than ADAMTS-generated NITEGE373 fragments [201].
8.4. Other serine proteinases
Fibroblast activation protein α (FAPα) is a type II transmembrane serine proteinase with increased expression in OA [203]. However, the substrates and function of the enzyme in cartilage are unknown.
Complement 1s is able to degrade insulin-like growth factor binding protein 5 (IGFBP5) in vitro [204]. Complement 1s inhibitors have been shown to reduce proteolysis of IGFBP5 in a canine OA model, leading to an increase in concentrations of insulin-like growth factor 1 (IGF1) and reduced cartilage damage [205].
9. Cysteine proteinases
9.1. Cathepsins
The papain-like cysteine proteinase cathepsin K is the only enzyme other than the collagenolytic MMPs that can hydrolyse native triple helical type I and type II collagen [206]. Chondrocyte expression of cathepsin K is increased in OA [207, 208] and the enzyme has been proposed to play a role in degradation of collagen in the cartilage matrix and in subchondral bone [209, 210].
Cathepsin K is highly expressed in osteoclasts, and studies on null mice and patients with genetic mutations indicate that cathepsin K is important for physiological bone development and remodelling [211, 212]. Such a developmental bone phenotype prevents use of the null mice to determine the role of cathepsin K in OA development. No conditional cartilage knockout of cathepsin K has been reported to date. Mice over-expressing cathepsin K develop increased spontaneous cartilage damage upon aging [209], although these mice also have a developmental bone phenotype that may hamper interpretation of the results [213]. Cathepsin K inhibitors reduce collagen breakdown in OA cartilage explants [214] and animal OA models [215]. Taken together, these studies suggest that cathepsin K contributes to the development of OA. However, Takahashi et al. [208] suggest that synovial cathepsin K can also protect cartilage, as siRNA down-regulation of the proteinase in synovium increased expression of MMP13 and accelerated cartilage degradation in a rabbit surgical OA model.
Cathepsin K is primarily localized intracellularly within lysosomes, but can also be secreted from synovial fibroblasts [210]. In vitro, the enzyme is active against collagen between pH 4.0 and 6.5 [206, 216]. pH values as low as pH 5.5 have been reported for OA cartilage [207], suggesting that the pH within arthritic joints may permit extracellular cathepsin K to retain collagenolytic activity. Additionally, cathepsin K retains some collagenolytic activity at neutral pH. In vitro, cathepsin K has been shown to form an oligomeric complex with chondroitin-4-sulfate, increasing the stability and collagenolytic activity of the enzyme [217, 218]. Cathepsin K can also cleave aggrecan at multiple sites in the G1 domain and CS2 region, as well as at one site in the IGD region [216], generating chondroitin sulfate-containing fragments that can interact with cathepsin K and stimulate its collagenolytic activity [216].
Expression of other cathepsins, including cathepsin B, D and S, is increased in OA cartilage, synovium and synovial fluid [10, 210, 219]. Cathepsins B and D can also cleave aggrecan in vitro [9, 16] but aggrecan degradation at neutral pH cannot be blocked by cathepsin inhibitors [220], suggesting that these enzymes do not contribute to pathological aggrecan degradation in vivo. Cathepsin B has recently been shown to degrade the HDAC Sirt1 (section 6.2.2) [221].
9.2. Calpains
Expression of the calcium-dependent cysteine proteinases μ-calpain (calpain 1) and m-calpain (calpain 2) is increased in OA cartilage [76]. The enzymes are expressed by chondrocytes and synovial fibroblasts [222, 223], and while they are intracellular enzymes, they have been detected in synovial fluid [224, 225]. They have a neutral pH optimum, so may be active extracellularly [226].
m-Calpain has been shown to cleave aggrecan in vitro at a number of sites in the IGD, KS and CS1 regions [222, 227, 228]. Cleavage in the KS region has been studied in the greatest detail, using an antibody recognising the C-terminal neoepitope PGVA709 [227, 228]. Cleavage at this site would cause release of the majority of the GAG-bearing region of aggrecan from the cartilage matrix, as is the case with MMP and ADAMTS cleavage in the IGD. This neoepitope has been detected in bovine and human cartilage [222, 227], but G1-PGVA709 fragments are present in OA cartilage at levels 18-fold lower than MMP-generated G1-DIPEN341 fragments and 63-fold lower than ADAMTS-generated G1-NITEGE373 fragments [228]. This suggests that a calpains play a minor role in pathological aggrecan cleavage in vivo. Fragments corresponding to m-calpain cleavage in the CS1 region have been found in both normal and OA cartilage [228], suggesting that calpains may be involved in normal aggrecan turnover.
9.3. Caspases
Chondrocyte death is a central feature of OA, and is thought to occur through a combination of autophagy and apoptosis [229, 230]. Expression of caspase 3 is increased in OA cartilage [230] and intra-articular injection of caspase inhibitors has been shown to reduce cartilage degradation in a rabbit surgical OA model [231].
10. Conclusions and future prospects
OA remains a disease with insufficient disease-modifying treatments. With an increasing number of people suffering from the disease, the identification of novel therapeutic targets is a priority. The central role of aggrecanases and collagenases in cartilage degradation has been verified in recent years by studies on transgenic mice. While these enzymes are also thought to play pivotal roles in human OA, there are likely to be some differences in the roles of individual enzymes between the two species. For example, murine MMP-1 differs considerably from the mammalian enzyme, so its role in OA cannot be studied in transgenic mice. Also, ADAMTS-4 plays little role in murine OA, but may contribute to human cartilage degradation. Further research is needed to fully delineate the role of individual proteinases in human OA.
Studies on mice with specific gene ablations have also identified a network of factors that regulate MMP13 and ADAMTS expression in chondrocytes (Figure 3). Mechanical damage is a primary risk factor for OA, and is now understood that one of the ways in such stimuli can act on chondrocytes is by stimulating proteinase expression via RUNX2 and Indian hedgehog. Conversely, protective mechanical stimuli can inhibit proteinase expression through CITED2 and FGF-2. Obesity may act as a risk factor not only through increasing mechanical strain but also through the pro-inflammatory properties of adipokines [232]. The mechanisms by which other OA risk factors such as gender and age increase proteinase expression remain unknown. Mechanisms regulating cartilage expression of enzymes such as HtrA1, matriptase and cathepsin K require further study. Similarly, while TIMP-3 is known to be able to protect cartilage by inhibiting MMPs and ADAMTSs, the role of endogenous serine and cysteine proteinase inhibitors in OA remains poorly studied. Given that many pathways can stimulate an increase in proteinase expression, development of inhibitors targeting the effector proteinases and using them in combination may block cartilage damage more effectively than therapies aimed at only one activating factor.
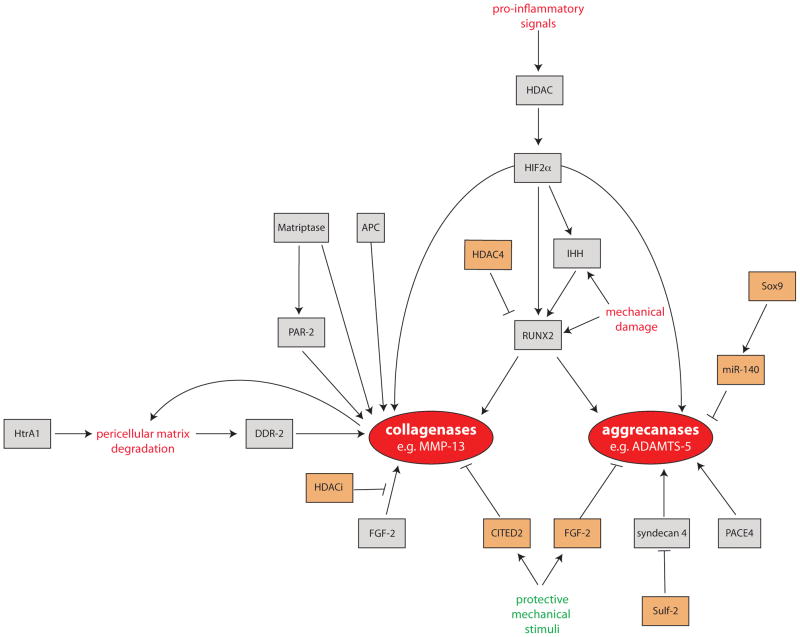
Figure 3. Factors regulating expression and activity of collagenases and aggrecanases in OA.
The expression and activity of collagenases (e. g. MMP-13 and MMP-1) and aggrecanases (e. g. ADAMTS-4 and ADAMTS-5) can be stimulated (orange boxes) or inhibited (grey boxes) by a number of inter-related mechanisms. Expression of the central transcription factor RUNX2 is increased by mechanical and pro-inflammatory stimuli, which act via HIF-2α, HDACs and Indian hedgehog. Expression of collagenolytic MMPs can also be increased in response to FGF-2 and DDR-2 signalling, and collagenase activity increased by matriptase activation of proMMP-1 and proMMP-3 zymogens and PAR-2 signalling. These catabolic stimuli can be counteracted by a variety of chondroprotective signals. For example, MMP13 expression can be reduced by the mechano-sensitive transcription factor CITED, and ADAMTS5 expression can be reduced by miR-140 and the mechano-responsive growth factor FGF-2.
OA is a disease involving the whole joint. To date, the role of proteinases in cartilage structural changes has been studied extensively, but the role of proteinases and proteinase inhibitors in synovial hypertrophy, osteophyte formation and subchondral bone remodelling is less well understood. Interestingly, Mmp13−/− mice develop osteophytes more rapidly than wild-type animals after surgical induction of OA [65]. The role of proteinases in joint components other than cartilage is important to understand if proteinase inhibitors are to be developed as OA therapeutics.
Highlights.
Osteoarthritis is a characterised by degradation of the cartilage extracellular matrix
Collegen is degraded by matrix metalloproteinases such as MMP-13
Aggrecan is degraded by related ADAMTS metalloproteinases
Less abundant cartilage components are degraded by a variety of proteinases
Factors such as inflammation and mechanical damage stimulate enzyme expression
Acknowledgments
Linda Troeberg is the recipient of an Arthritis Research UK Career Development Fellowship (grant number 19466). Hideaki Nagase is supported by Arthritis Research UK Core Grant to the Kennedy Institute of Rheumatology, and grant AR40994 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAMS or NIH.
Abbreviations
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- APC
activated protein C
- CITED2
cAMP-responsive element-binding protein/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2
- ECM
extracellular matrix
- ERK
extracellularly-regulated kinase
- FAPα
fibroblast activation protein α
- FGF-2
fibroblast growth factor 2
- Gla
γ-carboxyglutamate
- HDAC
histone deacetylase
- HIF-2α
hypoxia-inducible factor 2α
- IGD
interglobular domain
- IGF
insulin-like growth factor
- IGFBP
IGF binding protein
- MMP
matrix metalloproteinase
- OA
osteoarthritis
- PACE4
paired basic amino acid cleaving enzyme 4
- PAR
protease-activated receptor
- PC
proprotein convertase
- RUNX2
runt-related transcription factor 2
- SIRT1
Sirtuin 1
- TIMP
tissue inhibitor of metalloproteinases
- tPA
tissue-type plasminogen activator
- uPA
urokinase-type plasminogen activator
- WISP-1
Wnt-induced signalling protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nature Reviews Drug discovery. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11:203. doi: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 5.Thomas L. Reversible collapse of rabbit ears after intravenous papain, and prevention of recovery by cortisone. J Exp Med. 1956;104:245–252. doi: 10.1084/jem.104.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas L, McCluskey RT, Potter JL, Weissmann G. Comparison of the effects of papain and vitamin A on cartilage. I. The effects in rabbits. J Exp Med. 1960;111:705–718. doi: 10.1084/jem.111.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucy JA, Dingle JT, Fell HB. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali SY, Evans L, Stainthorpe E, Lack CH. Characterization of cathepsins in cartilage. Biochem J. 105(1967)(102):549–157. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woessner JFJ. Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973;248:1634–1642. [PubMed] [Google Scholar]
- 10.Sapolsky AI, Altman RD, Woessner JF, Howell DS. The action of cathepsin D in human articular cartilage on proteoglycans. J Clin Invest. 1973;52:624–633. doi: 10.1172/JCI107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingle JT, Barrett AJ, Weston PD. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971;123:1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapolsky AI, Howell DS. Further characterization of a neutral metalloprotease isolated from human articular cartilage. Arthritis Rheum. 1982;25:981–988. doi: 10.1002/art.1780250811. [DOI] [PubMed] [Google Scholar]
- 13.Galloway WA, Murphy G, Sandy JD, Gavrilovic J, Cawston TE, Reynolds JJ. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983;209:741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunja-Smith Z, Nagase H, Woessner JFJ. Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989;258:115–119. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosang AJ, Neame PJ, Hardingham TE, Murphy G, Hamilton JA. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991;266:15579–15582. [PubMed] [Google Scholar]
- 16.Fosang AJ, Neame PJ, Last K, Hardingham TE, Murphy G, Hamilton JA. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992;267:19470–19474. [PubMed] [Google Scholar]
- 17.Fosang AJ, Last K, Knäuper V, Neame PJ, Murphy G, Hardingham TE, Tschesche H, Hamilton JA. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993;295:273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fosang AJ, Last K, Knäuper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;381:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 19.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- 21.Lark MW, Gordy JT, Weidner JR, Ayala J, Kimura JH, Williams HR, Mumford RA, Flannery CR, Carlson SS, Iwata M, Sandy JD. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the “aggrecanase” site (Glu373-Ala374) is a primary event in proteolysis of the interglobular domain. J Biol Chem. 1995;270:2550–2556. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CE, Little CB, Büttner FH, Bartnik E, Caterson B. Differential expression of aggrecanase and matrix metalloproteinase activity in chondrocytes isolated from bovine and porcine articular cartilage. J Biol Chem. 1998;273:30576–30582. doi: 10.1074/jbc.273.46.30576. [DOI] [PubMed] [Google Scholar]
- 23.Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 24.Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC, Hollis GF, Newton RC, Magolda RL, Trzaskos JM, Arner EC. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- 25.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–562. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 26.Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 27.Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, Wynn R, Duke JL, George HJ, Hillman MC, Murphy K, Wiswall BH, Copeland RA, Decicco CP, Bruckner R, Nagase H, Itoh Y, Newton RC, Magolda RL, Trzaskos JM, Burn TC. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- 28.Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. European Cells and Materials. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- 29.Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- 30.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thøgersen IB, Hughes C, Caterson B, Nagase H. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 31.Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000;478:241–245. doi: 10.1016/s0014-5793(00)01854-8. [DOI] [PubMed] [Google Scholar]
- 32.Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- 33.Collins-Racie LA, Flannery CR, Zeng W, Corcoran C, Annis-Freeman B, Agostino MJ, Arai M, DiBlasio-Smith E, Dorner AJ, Georgiadis KE, Jin M, Tan XY, Morris EA, LaVallie ER. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004;23:219–230. doi: 10.1016/j.matbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Yamaji N, Nishimura K, Abe K, Ohara O, Nagase T, Nomura N. Metalloprotease having aggrecanase activity. 6,716,613. US Patent. 2004
- 35.Zeng W, Corcoran C, Collins-Racie LA, Lavallie ER, Morris EA, Flannery CR. Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS-5, -9, -16 and -18. Biochim Biophys Acta. 2006;1760:517–524. doi: 10.1016/j.bbagen.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615–626. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandy JD. A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis Cartilage. 2006;14:95–100. doi: 10.1016/j.joca.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, Fosang AJ. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117:1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma H-L, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 40.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 41.Little CB, Mittaz L, Belluoccio D, Rogerson FM, Campbell IK, Meeker CT, Bateman JF, Pritchard MA, Fosang AJ. ADAMTS-1-knockout mice do not exhibit abnormalities in aggrecan turnover in vitro or in vivo. Arthritis Rheum. 2005;52:1461–1472. doi: 10.1002/art.21022. [DOI] [PubMed] [Google Scholar]
- 42.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, Yang Z, Majumdar MK, Morris EA. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 43.Song R-H, Tortorella MD, Malfait A-M, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 44.Powell AJ, Little CB, Hughes CE. Low molecular weight isoforms of the aggrecanases are responsible for the cytokine-induced proteolysis of aggrecan in a porcine chondrocyte culture system. Arthritis Rheum. 2007;56:3010–3019. doi: 10.1002/art.22818. [DOI] [PubMed] [Google Scholar]
- 45.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, Yatabe T, Komiya K, Enomoto H, Fujikawa K, Okada Y. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol Int. 2007;57:703–711. doi: 10.1111/j.1440-1827.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- 46.Yao W, Wasserman ZR, Chao M, Reddy G, Shi E, Liu RQ, Covington MB, Arner EC, Pratta MA, Tortorella M, Magolda RL, Newton R, Qian M, Ribadeneira MD, Christ D, Wexler RR, Decicco CP. Design and synthesis of a series of (2R)-N(4)-hydroxy-2-(3-hydroxybenzyl)-N(1)- [(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]butanediamide derivatives as potent, selective, and orally bioavailable aggrecanase inhibitors. J Med Chem. 2001;44:3347–3350. doi: 10.1021/jm015533c. [DOI] [PubMed] [Google Scholar]
- 47.Tortorella MD, Tomasselli AG, Mathis KJ, Schnute ME, Woodard SS, Munie G, Williams JM, Caspers N, Wittwer AJ, Malfait A-M, Shieh H-S. Structural and inhibition analysis reveals the mechanism of selectivity of a series of aggrecanase inhibitors. J Biol Chem. 2009;284:24185–24191. doi: 10.1074/jbc.M109.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chockalingam PS, Sun W, Rivera-Bermudez MA, Zeng W, Dufield DR, Larsson S, Lohmander LS, Flannery CR, Glasson SS, Georgiadis KE, Morris EA. Elevated aggrecanase activity in a rat model of joint injury is attenuated by an aggrecanase specific inhibitor. Osteoarthritis Cartilage. 2010:315–323. doi: 10.1016/j.joca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 49.De Savi C, Pape A, Cumming JG, Ting A, Smith PD, Burrows JN, Mills M, Davies C, Lamont S, Milne D, Cook C, Moore P, Sawyer Y, Gerhardt S. The design and synthesis of novel N-hydroxyformamide inhibitors of ADAM-TS4 for the treatment of osteoarthritis. Bioorg Med Chem Lett. 2011;21(5):1376–81. doi: 10.1016/j.bmcl.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, Lohmander LS. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100:93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48:3130–3139. doi: 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]
- 53.Fell HB, Barratt MEJ, Welland H, Green R, PAR The capacity of pig articular cartilage in organ culture to regenerate after breakdown induced by complement-sufficient antiserum to pig erythrocytes. Calc Tissue Res. 1976;20:3–21. doi: 10.1007/BF02546393. [DOI] [PubMed] [Google Scholar]
- 54.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu R-Q, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 55.Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10:R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim NH, Kashiwagi M, Visse R, Jones J, Enghild JJ, Brew K, Nagase H. Reactive-site mutants of N-TIMP-3 that selectively inhibit ADAMTS-4 and ADAMTS-5: biological and structural implications. Biochem J. 2010;431:113–122. doi: 10.1042/BJ20100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehrlich MG, Mankin HJ, Jones H, Wright R, Crispen C, Vigliani G. Collagenase and collagenase inhibitors in osteoarthritic and normal cartilage. J Clin Invest. 1977;59:226–233. doi: 10.1172/JCI108632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 61.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 62.Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3:e3740. doi: 10.1371/journal.pone.0003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chia S, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 64.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Little C, Barai A, Burkhardt D, Smith S, Fosang A, Werb Z, Shah M, Thompson E. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balbín M, Fueyo A, Knäuper V, López JM, Alvarez J, Sánchez LM, Quesada V, Bordallo J, Murphy G, López-Otín C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- 67.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 68.Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, Banotai CA, Mueller WT, McConnell P, Yan C, Baragi V, Lesch C, Roark WH, Wilson M, Datta K, Guzman R, Han HK, Dyer RD. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem. 2007;282:27781–27791. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- 69.Piecha D, Weik J, Kheil H, Becher G, Timmermann A, Jaworski A, Burger M, Hofmann MW. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflamm Res. 2010;59:379–389. doi: 10.1007/s00011-009-0112-9. [DOI] [PubMed] [Google Scholar]
- 70.Settle S, Vickery L, Nemirovskiy O, Vidmar T, Bendele A, Messing D, Ruminski P, Schnute M, Sunyer T. Cartilage degradation biomarkers predict efficacy of a novel, highly selective matrix metalloproteinase 13 inhibitor in a dog model of osteoarthritis. Arthritis Rheum. 2010;62:3006–3015. doi: 10.1002/art.27596. [DOI] [PubMed] [Google Scholar]
- 71.Böhm BB, Aigner T, Gehrsitz A, Blobel CP, Kalden JR, Burkhardt H. Up-regulation of MDC15 (metargidin) messenger RNA in human osteoarthritic cartilage. Arthritis Rheum. 1999;42:1946–1950. doi: 10.1002/1529-0131(199909)42:9<1946::AID-ANR21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 72.Kerna I, Kisand K, Laitinen P, Tamm AE, Kumm J, Lintrop M, Tamm AO. Association of ADAM12-S protein with radiographic features of knee osteoarthritis and bone and cartilage markers. Rheumatol Int. 2011 doi: 10.1007/s00296-010-1717-6. In press. [DOI] [PubMed] [Google Scholar]
- 73.Zack MD, Malfait AM, Skepner AP, Yates MP, Griggs DW, Hall T, Hills RL, Alston JT, Nemirovskiy OV, Radabaugh MR, Leone JW, Arner EC, Tortorella MD. ADAM-8 isolated from human osteoarthritic chondrocytes cleaves fibronectin at Ala271. Arthritis Rheum. 2009;60:2704–2713. doi: 10.1002/art.24753. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez-Lopez J, Pombo-Suarez M, Loughlin J, Tsezou A, Blanco FJ, Meulenbelt I, Slagboom PE, Valdes AM, Spector TD, Gomez-Reino JJ, Gonzalez A. Association of a nsSNP in ADAMTS14 to some osteoarthritis phenotypes. Osteoarthritis Cartilage. 2009;17:321–327. doi: 10.1016/j.joca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Little CB, Fosang AJ. Is cartilage matrix breakdown an appropriate therapeutic target in osteoarthritis - insights from studies of aggrecan and collagen proteolysis? Curr Drug Targets. 2010;11:561–575. doi: 10.2174/138945010791011956. [DOI] [PubMed] [Google Scholar]
- 76.Swingler TE, Waters JG, Davidson RK, Pennington CJ, Puente XS, Darrah C, Cooper A, Donell ST, Guile GR, Wang W, Clark IM. Degradome expression profiling in human articular cartilage. Arthritis Res Ther. 2009;11:R96. doi: 10.1186/ar2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- 78.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Meurs J, van Lent P, Stoop R, Holthuysen A, Singer I, Bayne E, Mudgett J, Poole R, Billinghurst C, van der Kraan P, Buma P, van den Berg W. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum. 1999;42:2074–2084. doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 80.Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48:3452–3463. doi: 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- 81.Heilpern AJ, Wertheim W, He J, Perides G, Bronson RT, Hu LT. Matrix metalloproteinase 9 plays a key role in lyme arthritis but not in dissemination of Borrelia burgdorferi. Infect Immun. 2009;77:2643–2649. doi: 10.1128/IAI.00214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8:367–376. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 83.De Croos JN, Jang B, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Membrane type-1 matrix metalloproteinase is induced following cyclic compression of in vitro grown bovine chondrocytes. Osteoarthritis Cartilage. 2007;15:1301–1310. doi: 10.1016/j.joca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Miller M, Manning H, Jain A, Troeberg L, Dudhia J, Essex D, Sandison A, Seiki M, Nanchahal J, Nagase H, Itoh Y. Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis. Arthritis Rheum. 2009;60:686–697. doi: 10.1002/art.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 86.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 87.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 88.Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiménez MJ, Balbín M, López JM, Alvarez J, Komori T, López-Otín C. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol Cell Biol. 1999;19:4431–4442. doi: 10.1128/mcb.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, Saito T, Ozaki T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage. 2010:1–42. doi: 10.1016/j.joca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, Olsen BR. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev. 2001;106:97–106. doi: 10.1016/s0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 93.Drissi H, Zuscik M, Rosier R, O’Keefe R. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Mol Aspects Med. 2005;26:169–179. doi: 10.1016/j.mam.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, Nakamura K, Tokunaga K, Chung U, Kawaguchi H. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nature Medicine. 2010;16:678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 95.Husa M, Liu-Bryan R, Terkeltaub R. Shifting HIFs in osteoarthritis. Nature Medicine. 2010;16:641–644. doi: 10.1038/nm0610-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 98.Thirunavukkarasu K, Pei Y, Moore TL, Wang H, Yu XP, Geiser AG, Chandrasekhar S. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun. 2006;345:197–204. doi: 10.1016/j.bbrc.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 99.Thirunavukkarasu K, Pei Y, Wei T. Characterization of the human ADAMTS-5 (aggrecanase-2) gene promoter. Mol Biol Rep. 2007;34:225–231. doi: 10.1007/s11033-006-9037-3. [DOI] [PubMed] [Google Scholar]
- 100.Wong M, Siegrist M, Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–693. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 101.Saito T, Kawaguchi H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthritis Cartilage. 2010 doi: 10.1016/j.joca.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 102.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, Hsu C, Ali SA, Alman BA. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 103.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 104.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 105.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 106.Sawaji Y, Hynes J, Vincent T, Saklatvala J. Fibroblast growth factor 2 inhibits induction of aggrecanase activity in human articular cartilage. Arthritis Rheum. 2008;58:3498–3509. doi: 10.1002/art.24025. [DOI] [PubMed] [Google Scholar]
- 107.Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W. Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage. 2003;11:738–746. doi: 10.1016/s1063-4584(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 108.Yang S, Kim J, Ryu J-H, Oh H, Chun C-H, Kim BJ, Min BH, Chun J-S. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nature Medicine. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 109.Tamiya H, Ikeda T, Jeong JH, Saito T, Yano F, Jung YK, Ohba S, Kawaguchi H, Chung UI, Choi JY. Analysis of the Runx2 promoter in osseous and non-osseous cells and identification of HIF2A as a potent transcription activator. Genes Dev. 2008;416:53–60. doi: 10.1016/j.gene.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Takamoto M, Tsuji K, Yamashita T, Sasaki H, Yano T, Taketani Y, Komori T, Nifuji A, Noda M. Hedgehog signaling enhances core-binding factor a1 and receptor activator of nuclear factor-kappaB ligand (RANKL) gene expression in chondrocytes. J Endocrinol. 2003;177:413–421. doi: 10.1677/joe.0.1770413. [DOI] [PubMed] [Google Scholar]
- 111.Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60:1406–1415. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakajima M, Shi D, Dai J, Tsezou A, Zheng M, Norman PE, Takahashi A, Ikegawa S, Jiang Q. Replication studies in various ethnic populations do not support the association of the HIF-2α SNP rs17039192 with knee osteoarthritis. Nature Med. 2011;17:26–27. doi: 10.1038/nm0111-26. [DOI] [PubMed] [Google Scholar]
- 113.Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- 114.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young DA, Lakey RL, Pennington CJ, Jones D, Kevorkian L, Edwards DR, Cawston TE, Clark IM. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther. 2005;7:R503–512. doi: 10.1186/ar1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X, Song Y, Jacobi JL, Tuan RS. Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes. Growth Factors. 2009;27:40–49. doi: 10.1080/08977190802625179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Higashiyama R, Miyaki S, Yamashita S, Yoshitaka T, Lindman G, Ito Y, Sasho T, Takahashi K, Lotz M, Asahara H. Correlation between MMP-13 and HDAC7 expression in human knee osteoarthritis. Modern Rheumatol. 2010;20:11–17. doi: 10.1007/s10165-009-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin HS, Hu CY, Chan HY, Liew YY, Huang HP, Lepescheux L, Bastianelli E, Baron R, Rawadi G, Clément-Lacroix P. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol. 2007;150:862–872. doi: 10.1038/sj.bjp.0707165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hong S, Derfoul A, Pereira-Mouries L, Hall DJ. A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J. 2009;23:3539–3552. doi: 10.1096/fj.09-133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fath DM, Kong X, Liang D, Lin Z, Chou A, Jiang Y, Fang J, Caro J, Sang N. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. J Biol Chem. 2006;281:13612–13619. doi: 10.1074/jbc.M600456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279:41966–41974. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- 122.Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 123.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 124.Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, Kurosaka M, Kuroda R. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 125.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 127.Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, Clark I, Dalmay T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006;580:4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 128.Lee RC, Feinbaum RL, Ambros V. The C. elegans hetero- chronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 129.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, Takada S, Lotz MK, Ueno-Kudo H, Asahara H. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tardif G, Hum D, Pelletier JP, Duval N, Martel-Pelletier J. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2009;10:148. doi: 10.1186/1471-2474-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, Needham MR, Read SJ, Newham P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of MMP-13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vanwanseele B, Eckstein F, Knecht H, Stüssi E, Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46:2073–2078. doi: 10.1002/art.10462. [DOI] [PubMed] [Google Scholar]
- 134.Buckwalter JA, Martin JA, Brown TD. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology. 2006;43:603–609. [PubMed] [Google Scholar]
- 135.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JH, Fitzgerald JB, DiMicco MA, Cheng DM, Flannery CR, Sandy JD, Plaas AH, Grodzinsky AJ. Co-culture of mechanically injured cartilage with joint capsule tissue alters chondrocyte expression patterns and increases ADAMTS5 production. Arch Biochem Biophys. 2009;489:118–126. doi: 10.1016/j.abb.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–47280. doi: 10.1074/jbc.M304652200. [DOI] [PubMed] [Google Scholar]
- 138.Leong DJ, Li YH, Gu XI, Sun L, Zhou Z, Nasser P, Laudier DM, Iqbal J, Majeska RJ, Schaffler MB, Goldring MB, Cardoso L, Zaidi M, Sun HB. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25:182–191. doi: 10.1096/fj.10-164277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leong DJ, Gu XI, Li Y, Lee JY, Laudier DM, Majeska RJ, Schaffler MB, Cardoso L, Sun HB. Matrix metalloproteinase-3 in articular cartilage is upregulated by joint immobilization and suppressed by passive joint motion. Matrix Biol. 2010;29:420–426. doi: 10.1016/j.matbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 141.Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Genes Dev. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uría JA, Balbín M, López JM, Alvarez J, Vizoso F, Takigawa M, López-Otín C. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol. 1998;153:91–101. doi: 10.1016/S0002-9440(10)65549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tardif G, Pelletier JP, Dupuis M, Geng C, Cloutier JM, Martel-Pelletier J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999;42:1147–1158. doi: 10.1002/1529-0131(199906)42:6<1147::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 144.Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, Werb Z, Rosen SD. HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: effects on VEGF, FGF-1, and SDF-1. BMC Biochem. 2006;7:2. doi: 10.1186/1471-2091-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Otsuki S, Taniguchi N, Grogan SP, D’Lima D, Kinoshita M, Lotz M. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10:R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Otsuki S, Hanson SR, Miyaki S, Grogan SP, Kinoshita M, Asahara H, Wong C-H, Lotz MK. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0913897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, Mackie SA, McDonagh T, Crawford TK, Tomkinson KN, LaVallie ER, Morris EA. Autocatalytic cleavage of ADAMTS-4 (Aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- 151.Yu WH, Yu S, Meng Q, Brew K, Woessner JF. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- 152.Barre PE, Redini F, Boumediene K, Vielpeau C, Pujol JP. Semiquantitative reverse transcription-polymerase chain reaction analysis of syndecan-1 and -4 messages in cartilage and cultured chondrocytes from osteoarthritic joints. Osteoarthritis Cartilage. 2000;8:34–43. doi: 10.1053/joca.1999.0286. [DOI] [PubMed] [Google Scholar]
- 153.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nature Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 154.Longpré J-M, Mcculloch DR, Koo B-H, Alexander JP, Apte SS, Leduc R. Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2009;41:1116–1126. doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 155.Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC, Pei D. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J Biol Chem. 2004;279:15434–15440. doi: 10.1074/jbc.M312797200. [DOI] [PubMed] [Google Scholar]
- 156.Salminen-Mankonen H, Säämänen AM, Jalkanen M, Vuorio E, Pirilä L. Syndecan-1 expression is upregulated in degenerating articular cartilage in a transgenic mouse model for osteoarthritis. Scand J Rheumatol. 2005;34:469–474. doi: 10.1080/03009740500304338. [DOI] [PubMed] [Google Scholar]
- 157.Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- 158.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 159.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 160.Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 161.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 162.Sunk IG, Bobacz K, Hofstaetter JG, Amoyo L, Soleiman A, Smolen J, Xu L, Li Y. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum. 2007;56:3685–3692. doi: 10.1002/art.22970. [DOI] [PubMed] [Google Scholar]
- 163.Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, GMBLY Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–2673. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- 164.Xu L, Servais J, Polur I, Kim D, Lee PL, Chung K, Li Y. Attenuation of osteoarthritis progression by reduction of the discoidin domain receptor 2 in mice. Arthritis Rheum. 2010 doi: 10.1002/art.27582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tsuchiya A, Yano M, Tocharus J, Kojima H, Fukumoto M, Kawaichi M, Oka C. Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone. 2005;37:323–336. doi: 10.1016/j.bone.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 166.Polur I, Lee PL, Servais J, Xu L, Li Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol. 2010;25:599–608. doi: 10.14670/hh-25.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiang Y, Masuko-Hongo K, Sekine T, Nakamura H, Yudoh K, Nishioka K, Kato T. Expression of proteinase-activated receptors (PAR)-2 in articular chondrocytes is modulated by IL-1beta, TNF-alpha and TGF-beta. Osteoarthritis Cartilage. 2006;14:1163–1173. doi: 10.1016/j.joca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 168.Boileau C, Amiable N, Martel-Pelletier J, Fahmi H, Duval N, Pelletier JP. Activation of proteinase-activated receptor 2 in human osteoarthritic cartilage upregulates catabolic and proinflammatory pathways capable of inducing cartilage degradation: a basic science study. Arthritis Res Ther. 2007;9:R121. doi: 10.1186/ar2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Amiable N, Tat SK, Lajeunesse D, Duval N, Pelletier JP, Martel-Pelletier J, Boileau C. Proteinase-activated receptor (PAR)-2 activation impacts bone resorptive properties of human osteoarthritic subchondral bone osteoblasts. Bone. 2009;44:1143–1150. doi: 10.1016/j.bone.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferrell WR, Kelso EB, Lockhart JC, Plevin R, McInnes IB. Protease-activated receptor 2: a novel pathogenic pathway in a murine model of osteoarthritis. Ann Rheum Dis. 2010;69:2051–2054. doi: 10.1136/ard.2010.130336. [DOI] [PubMed] [Google Scholar]
- 171.Amiable N, Martel-Pelletier J, Lussier B, Tat SK, Pelletier JP, Boileau C. Proteinase-activated receptor-2 gene disruption limits the effect of osteoarthritis on cartilage in mice: a novel target in joint degradation. J Rheumatol. 2011;38:911–920. doi: 10.3899/jrheum.100710. [DOI] [PubMed] [Google Scholar]
- 172.Milner JM, Patel A, Davidson RK, Swingler TE, Desilets A, Young DA, Kelso EB, Donell ST, Cawston TE, Clark IM, Ferrell WR, Plevin R, Lockhart JC, Leduc R, Rowan AD. Matriptase is a novel initiator of cartilage matrix degradation in osteoarthritis. Arthritis Rheum. 2010;62:1955–1966. doi: 10.1002/art.27476. [DOI] [PubMed] [Google Scholar]
- 173.Corr M. Wnt-beta-catenin signaling in the pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol. 2008;4:550–556. doi: 10.1038/ncprheum0904. [DOI] [PubMed] [Google Scholar]
- 174.Papathanasiou I, Malizos KN, Tsezou A. Low-density lipoprotein receptor-related protein 5 (LRP5) expression in human osteoarthritic chondrocytes. J Orthop Res. 2010;28:348–353. doi: 10.1002/jor.20993. [DOI] [PubMed] [Google Scholar]
- 175.Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 176.Urano T, Narusawa K, Shiraki M, Usui T, Sasaki N, Hosoi T, Ouchi Y, Nakamura T, Inoue S. Association of a single nucleotide polymorphism in the WISP1 gene with spinal osteoarthritis in postmenopausal Japanese women. J Bone Miner Metab. 2007;25:253–258. doi: 10.1007/s00774-007-0757-9. [DOI] [PubMed] [Google Scholar]
- 177.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4) FEBS Lett. 2001;494:192–195. doi: 10.1016/s0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- 179.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 180.Gendron C, Kashiwagi M, Hughes C, Caterson B, Nagase H. TIMP-3 inhibits aggrecanase-mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. FEBS Lett. 2003;555:431–436. doi: 10.1016/s0014-5793(03)01295-x. [DOI] [PubMed] [Google Scholar]
- 181.Black R, Castner B, Slack J, Tocker J, Eisenman J, Jacobsen E, Delaney J, Winters D, Hecht R, Bendele A. Injected TIMP-3 protects cartilage in a rat meniscal tear model. Osteoarthritis Cartilage. 2006;14:S23–24. [Google Scholar]
- 182.Sahebjam S, Khokha R, Mort JS. Increased collagen and aggrecan degradation with age in the joints of Timp3−/− mice. Arthritis Rheum. 2007;56:905–909. doi: 10.1002/art.22427. [DOI] [PubMed] [Google Scholar]
- 183.Mahmoodi M, Sahebjam S, Smookler D, Khokha R, Mort JS. Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am J Pathol. 2005;166:1733–1740. doi: 10.1016/S0002-9440(10)62483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Milner JM, Rowan AD, Cawston TE, Young DA. Metalloproteinase and inhibitor expression profiling of resorbing cartilage reveals pro-collagenase activation as a critical step for collagenolysis. Arthritis Res Ther. 2006;8:R142. doi: 10.1186/ar2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morris KJ, Cs-Szabo G, Cole AA. Characterization of TIMP-3 in human articular talar cartilage. Connect Tissue Res. 2010;51:478–490. doi: 10.3109/03008201003686958. [DOI] [PubMed] [Google Scholar]
- 186.Troeberg L, Fushimi K, Khokha R, Emonard H, Ghosh P, Nagase H. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. FASEB J. 2008;22:3515–3524. doi: 10.1096/fj.08-112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee HJ, Lee GH, Nah S, Lee KH, Yang H, Kim YM, Chun W, Hong S, Kim S. Association of TIMP-4 gene polymorphism with the risk of osteoarthritis in the Korean population. Rheumatol Int. 2008;28:845–850. doi: 10.1007/s00296-008-0545-4. [DOI] [PubMed] [Google Scholar]
- 188.Martel-Pelletier J, Faure MP, McCollum R, Mineau F, Cloutier JM, Pelletier JP. Plasmin, plasminogen activators and inhibitor in human osteoarthritic cartilage. J Rheumatol. 1991;18:1863–1871. [PubMed] [Google Scholar]
- 189.Milner JM, Elliott SF, Cawston TE. Activation of procollagenases is a key control point in cartilage collagen degradation: interaction of serine and metalloproteinase pathways. Arthritis Rheum. 2001;44:2084–2096. doi: 10.1002/1529-0131(200109)44:9<2084::AID-ART359>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 190.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin CY, Dickson RB, Kitamura H, Miyazaki K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2006;97:1327–1334. doi: 10.1111/j.1349-7006.2006.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 193.Jackson MT, Smith MM, Smith SM, Jackson CJ, Xue M, Little CB. Activation of cartilage matrix metalloproteinases by activated protein C. Arthritis Rheum. 2009;60:780–791. doi: 10.1002/art.24303. [DOI] [PubMed] [Google Scholar]
- 194.Garvican ER, Vaughan-Thomas A, Redmond C, Gabriel N, Clegg PD. MMP-mediated collagen breakdown induced by activated protein C in equine cartilage is reduced by corticosteroids. J Orthop Res. 2010;28:370–378. doi: 10.1002/jor.21001. [DOI] [PubMed] [Google Scholar]
- 195.Milner JM, Rowan AD, Elliott SF, Cawston TE. Inhibition of furin-like enzymes blocks interleukin-1alpha/oncostatin M-stimulated cartilage degradation. Arthritis Rheum. 2003;48:1057–1066. doi: 10.1002/art.10873. [DOI] [PubMed] [Google Scholar]
- 196.Malfait AM, Arner EC, Song RH, Alston JT, Markosyan S, Staten N, Yang Z, Griggs DW, Tortorella MD. Proprotein convertase activation of aggrecanases in cartilage in situ. Arch Biochem Biophys. 2008;478:43–51. doi: 10.1016/j.abb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 197.Lipinska B, Sharma S, Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Strauch KL, Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988;85:1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- 200.Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, Junker U, Jones SA, Clausen T, Ehrmann M. The Role of Human HtrA1 in Arthritic Disease. J Biol Chem. 2006;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 201.Chamberland A, Wang E, Jones AR, Collins-Racie LA, Lavallie ER, Huang Y, Liu L, Morris EA, Flannery CR, Yang Z. Identification of a novel HtrA1-susceptible cleavage site in human aggrecan: evidence for the involvement of HtrA1 in aggrecan proteolysis in vivo. J Biol Chem. 2009;284:27352–27359. doi: 10.1074/jbc.M109.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kielty CM, Lees M, Shuttleworth CA, Woolley D. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem Biophys Res Commun. 1993;191:1230–1236. doi: 10.1006/bbrc.1993.1349. [DOI] [PubMed] [Google Scholar]
- 203.Milner JM, Kevorkian L, Young DA, Jones D, Wait R, Donell ST, Barksby E, Patterson AM, Middleton J, Cravatt BF, Clark IM, Rowan AD, Cawston TE. Fibroblast activation protein alpha is expressed by chondrocytes following a pro-inflammatory stimulus and is elevated in osteoarthritis. Arthritis Res Ther. 2006;8:R23. doi: 10.1186/ar1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Busby WHJ, Yocum SA, Rowland M, Kellner D, Lazerwith S, Sverdrup F, Yates M, Radabaugh M, Clemmons DR. Complement 1s is the serine protease that cleaves IGFBP-5 in human osteoarthritic joint fluid. Osteoarthritis Cartilage. 2009;17:547–555. doi: 10.1016/j.joca.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Clemmons DR, Busby WH, Garmong A, Schultz DR, Howell DS, Altman RD, Karr R. Inhibition of insulin-like growth factor binding protein 5 proteolysis in articular cartilage and joint fluid results in enhanced concentrations of insulin-like growth factor 1 and is associated with improved osteoarthritis. Arthritis Rheum. 2002;46:694–703. doi: 10.1002/art.10222. [DOI] [PubMed] [Google Scholar]
- 206.Brömme D, Okamoto K, Wang BB, Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J Biol Chem. 1996;271:2126–2132. doi: 10.1074/jbc.271.4.2126. [DOI] [PubMed] [Google Scholar]
- 207.Konttinen YT, Mandelin J, Li TF, Salo J, Lassus J, Liljeström M, Hukkanen M, Takagi M, Virtanen I, Santavirta S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–960. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- 208.Takahashi D, Iwasaki N, Kon S, Matsui Y, Majima T, Minami A, Uede T. Down-regulation of cathepsin K in synovium leads to progression of osteoarthritis in rabbits. Arthritis Rheum. 2009;60:2372–2380. doi: 10.1002/art.24718. [DOI] [PubMed] [Google Scholar]
- 209.Morko J, Kiviranta R, Joronen K, Säämänen AM, Vuorio E, Salminen-Mankonen H. Spontaneous development of synovitis and cartilage degeneration in transgenic mice overexpressing cathepsin K. Arthritis Rheum. 2005;52:3713–3717. doi: 10.1002/art.21423. [DOI] [PubMed] [Google Scholar]
- 210.Hou WS, Li W, Keyszer G, Weber E, Levy R, Klein MJ, Gravallese EM, Goldring SR, Brömme D. Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis Rheum. 2002;46:663–674. doi: 10.1002/art.10114. [DOI] [PubMed] [Google Scholar]
- 211.Fujita Y, Nakata K, Yasui N, Matsui Y, Kataoka E, Hiroshima K, Shiba RI, Ochi T. Novel mutations of the cathepsin K gene in patients with pycnodysostosis and their characterization. J Clin Endocrinol Metab. 2000;85:425–431. doi: 10.1210/jcem.85.1.6247. [DOI] [PubMed] [Google Scholar]
- 212.Boskey AL, Gelb BD, Pourmand E, Kudrashov V, Doty SB, Spevak L, Schaffler MB. Ablation of cathepsin K activity in the young mouse causes hypermineralization of long bone and growth plates. Calcif Tissue Int. 2009;84:229–239. doi: 10.1007/s00223-008-9214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kiviranta R, Morko J, Uusitalo H, Aro HT, Vuorio E, Rantakokko J. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J Bone Miner Res. 2001;16:1444–1452. doi: 10.1359/jbmr.2001.16.8.1444. [DOI] [PubMed] [Google Scholar]
- 214.Dejica VM, Mort JS, Laverty S, Percival MD, Antoniou J, Zukor DJ, Poole AR. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol. 2008;173:161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Connor JR, LePage C, Swift BA, Yamashita D, Bendele AM, Maul D, Kumar S. Protective effects of a cathepsin K inhibitor, SB-553484, in the canine partial medial meniscectomy model of osteoarthritis. Osteoarthritis Cartilage. 2009;17:1236–1243. doi: 10.1016/j.joca.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 216.Hou WS, Li Z, Büttner FH, Bartnik E, Brömme D. Cleavage site specificity of cathepsin K toward cartilage proteoglycans and protease complex formation. Biol Chem. 2003;384:891–897. doi: 10.1515/BC.2003.100. [DOI] [PubMed] [Google Scholar]
- 217.Li Z, Hou WS, Brömme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000;39:529–536. doi: 10.1021/bi992251u. [DOI] [PubMed] [Google Scholar]
- 218.Cherny MM, Lecaille F, Kienitz M, Nallaseth FS, Li Z, James MN, Bromme D. Structure-activity analysis of cathepsin K/chondroitin 4-sulfate interactions. J Biol Chem. 2010 doi: 10.1074/jbc.M110.126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bayliss MT, Ali SY. Studies on cathepsin B in human articular cartilage. Biochem J. 1978;171:149–154. doi: 10.1042/bj1710149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hembry RM, Knight CG, Dingle JT, Barrett AJ. Evidence that extracellular cathepsin D is not responsible for the resorption of cartilage matrix in culture. Biochim Biophys Acta. 1982;714:307–312. doi: 10.1016/0304-4165(82)90338-5. [DOI] [PubMed] [Google Scholar]
- 221.Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. TNFα-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011 doi: 10.1002/art.30279. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maehara H, Suzuki K, Sasaki T, Oshita H, Wada E, Inoue T, Shimizu K. G1-G2 aggrecan product that can be generated by M-calpain on truncation at Ala709-Ala710 is present abundantly in human articular cartilage. J Biochem. 2007;141:469–477. doi: 10.1093/jb/mvm052. [DOI] [PubMed] [Google Scholar]
- 223.Yamamoto S, Shimizu K, Shimizu K, Suzuki K, Nakagawa Y, Yamamuro T. Calcium-dependent cysteine proteinase (calpain) in human arthritic synovial joints. Arthritis Rheum. 1992;35:1309–1317. doi: 10.1002/art.1780351111. [DOI] [PubMed] [Google Scholar]
- 224.Fukui I, Tanaka K, Murachi T. Extracellular appearance of calpain and calpastatin in the synovial fluid of the knee joint. Biochem Biophys Res Commun. 1989;162:559–566. doi: 10.1016/0006-291x(89)92347-4. [DOI] [PubMed] [Google Scholar]
- 225.Suzuki K, Shimizu K, Hamamoto T, Nakagawa Y, Hamakubo T, Yamamuro T. Biochemical demonstration of calpains and calpastatin in osteoarthritic synovial fluid. Arthritis Rheum. 1990;33:728–732. doi: 10.1002/art.1780330516. [DOI] [PubMed] [Google Scholar]
- 226.Maddock KR, Huff-Lonergan E, Rowe LJ, Lonergan SM. Effect of pH and ionic strength on mu- and m-calpain inhibition by calpastatin. J Anim Sci. 2005;83:1370–1376. doi: 10.2527/2005.8361370x. [DOI] [PubMed] [Google Scholar]
- 227.Oshita H, Sandy JD, Suzuki K, Akaike A, Bai Y, Sasaki T, Shimizu K. Mature bovine articular cartilage contains abundant aggrecan that is C-terminally truncated at Ala719-Ala720, a site which is readily cleaved by m-calpain. Biochem J. 2004;382:253–259. doi: 10.1042/BJ20040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Struglics A, Hansson M. Calpain is involved in C-terminal truncation of human aggrecan. Biochem J. 2010;430:531–538. doi: 10.1042/BJ20100591. [DOI] [PubMed] [Google Scholar]
- 229.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15:631–638. doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 230.Sharif M, Whitehouse A, Sharman P, Perry M, Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50:507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 231.D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 232.Hu PF, Bao JP, Wu LD. The emerging role of adipokines in osteoarthritis: a narrative review. Mol Biol Rep. 2011;38:873–878. doi: 10.1007/s11033-010-0179-y. [DOI] [PubMed] [Google Scholar]
- 233.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 234.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.van Goor H, Melenhorst W, Turner A, Holgate S. Adamalysins in biology and disease. J Pathol. 2009;219:277–286. doi: 10.1002/path.2594. [DOI] [PubMed] [Google Scholar]
- 236.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, Furie B, Furie BC, Cines DB, Huang M. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656–659. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- 237.Mather T, Oganessyan V, Hof P, Huber R, Foundling S, Esmon C, Bode W. The 2.8 A crystal structure of Gla-domainless activated protein C. EMBO J. 1996;15:6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 238.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sivaraman J, Lalumière M, Ménard R, Cygler M. Crystal structure of wild-type human procathepsin K. Protein Sci. 1999;8:283–290. doi: 10.1110/ps.8.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biol. 2007;8:218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]