Abstract
Objective
To determine the effect of mild fluid restriction on the hospital course of neonates with transient tachypnea of the newborn (TTN).
Study design
This is a pilot prospective randomized controlled trial of 64 late preterm and term neonates diagnosed with TTN at a single tertiary-care hospital in the United States. Patients were randomized to receive standard fluid management or mild fluid restriction. Primary outcome was duration of respiratory support. Secondary outcomes were duration of admission to the ICU, time to first enteral feed, and total and composite hospital charges. Results were analyzed by t-test, chi-square, Kaplan-Meier estimation and proportional hazards regression.
Results
Fluid restriction did not cause adverse events or unsafe dehydration. Fluid management strategy did not affect primary or secondary outcomes in the broad study population. Fluid restriction significantly reduced duration of respiratory support (p=0.008) and hospitalization costs (p=0.017) for neonates with severe TTN.
Conclusions
Mild fluid restriction appears safe in late preterm and term neonates with uncomplicated TTN. Fluid restriction may be of benefit in decreasing duration of respiratory support and hospitalization charges in term and late preterm neonates with uncomplicated severe TTN.
Transient tachypnea of the newborn (TTN) is a self-limited respiratory distress syndrome of term and late preterm neonates related to poor clearance of fetal lung fluid following delivery. Neonates with TTN have inefficient transition from in utero to ex utero pulmonary function due to delayed ion channel switching in the pulmonary epithelium.[1, 2] The absence of mechanical forces that normally aid pulmonary fluid clearance may also contribute to TTN in neonates who undergo Cesarean section delivery.[3] Signs of TTN include tachypnea, mild hypoxia, and respiratory distress. Distress from TTN typically resolves within the first 72-96 hours of life. Supportive care for TTN includes administration of low percentage supplemental oxygen and/or positive end expiratory pressure via continuous positive end-expiratory pressure (CPAP), high flow nasal CPAP (HFNCPAP), or conventional nasal cannula (NC).
Transient tachypnea of the newborn results in significant social and financial burden as affected neonates require admission to the intensive care unit (ICU). Separation from the parents and clinical illness result in delayed parent-child bonding and initiation of breast feeding. These costs, although individually minor, are increasingly important with the recent sharp rise in birthrate of late preterm neonates,[4, 5] and those delivered by Cesarean section,[4, 6] the groups most at-risk for TTN.[7] No effective treatment for TTN beyond supportive care has been identified.[8-10] A modest reduction in TTN symptom duration for a subset of patients with TTN could translate into thousands of hospital days and millions of dollars in savings.
We describe a randomized controlled trial of fluid management in neonates with TTN. We hypothesized that mild fluid restriction in the first days of life, mimicking physiologic low fluid intake by exclusively breastfed neonates, would speed resolution of TTN-related respiratory distress. We randomized patients to receive either standard-of-care daily total fluids or a more restrictive fluid management strategy. The primary study outcome was duration of respiratory support (CPAP, HFNCPAP, or NC). Additional secondary analysis focused on the cost of hospitalization of enrolled patients.
METHODS
This is a single-center study of inborn neonates at our urban tertiary care center that delivers over 6,000 babies annually. Neonates born between 34 0/7 and 41 6/7 weeks gestational age (GA) diagnosed with uncomplicated TTN in the first 12 hours of life were eligible for inclusion in this study. Uncomplicated TTN was defined as respiratory distress with chest x-ray (CXR) findings consistent with TTN (pulmonary fluid retention), in the absence of air leak syndrome (pneumothorax or pneumomediastinum). Respiratory distress was defined as flaring, grunting, and accessory muscle use with or without hypoxia. Respiratory support was initiated for those patients with hypoxia defined as oxygen saturation ≤ 95% and/or hypercapnia defined as PCO2 ≥ 50 mm Hg on room air. Mode of respiratory support (CPAP, HFNCPAP, or NC) was determined by the treating physician. Respiratory support was weaned and discontinued as respiratory distress resolved according to standard clinical practice.
Patients were recruited at our institution from August 1, 2008 through September 2, 2010. Neonates with a genetic abnormality or congenital anomaly of the lungs, heart, airway or other system likely to produce respiratory distress were excluded. Neonates undergoing an evaluation for sepsis (blood culture and/or antibiotic administration) were excluded from the study to avoid inadvertent inclusion of neonates with pneumonia. Neonates born with meconium noted at delivery were similarly excluded to avoid inadvertent inclusion of neonates with meconium aspiration syndrome. Patients meeting our institutional criteria for hyaline membrane disease (HMD) receiving surfactant treatment (CXR with reticular-granular markings and hypoxia requiring FiO2 ≥ 40% to maintain O2 saturation ≥ 92%) were not eligible for study enrollment or were removed from the study cohort at time of diagnosis of HMD. Patients were withdrawn from the study protocol if any respiratory diagnosis other than TTN was made. Informed consent was obtained from one or both parents prior to patient randomization. This study was approved by the Mount Sinai Program for the Protection of Human Subjects.
Neonates were alternately assigned to receive standard-of-care or restricted fluids based on order of diagnosis of TTN. Standard-of care total fluids were defined as 80 mL/kg/day on day of life (DOL) 1 for preterm neonates (born between 34 0/7 and 36 6/7 weeks GA) and 60 mL/kg/day on DOL 1 for term neonates (born between 37 0/7 and 41 6/7 weeks GA). Restricted fluids were defined as 60 mL/kg/day on DOL 1 for preterm neonates and 40 mL/kg/day on DOL 1 for term neonates. Total fluids were calculated as a combination of intravenous (IV) and any enteral fluid intake. All fluid management decisions following the initial starting volume for IV fluids were made by the treating medical team. Typically, total fluids were increased by 20 mL/kg/day daily for all patients until 150 mL/kg/day or ad lib feeds were achieved. Collection of fluid intake data ceased when patients achieved ad lib feeding, or at 72 hours of life. Collection of respiratory support data ceased when patients no longer received respiratory support. Patients, their families, and medical staff were not blinded to study group assignment.
Study safety was assessed daily for all enrolled patients. Predetermined indicators of dehydration including daily weight, daily urine output, and daily serum sodium, blood urea nitrogen (BUN), creatinine, glucose, and bilirubin level were monitored. Primary study outcome was total duration of respiratory support. Secondary clinical outcomes were time from birth to first enteral feed and total duration of admission to the NICU. Financial outcomes included total cost of hospitalization and component costs, including physician, direct, and indirect costs. Hospital charge data including breakdown by fee for type of service was obtained through hospital and physician faculty practice billing divisions at our institution. Charges were converted to costs using conversion information developed as part of the Healthcare Cost and Utilization Project (HCUP), a Federal-State-Industry partnership sponsored by the Agency for Healthcare Research and Quality. These cost-to-charge ratios specific for our Medical Center were based on all-payer inpatient cost information obtained from the hospital accounting reports collected by the Centers for Medicare and Medicaid Services (CMS). [11]
Sample size determination was made assuming a meaningful difference in duration of respiratory support of eight hours, standard deviation of eleven hours, a two-sided alpha of 0.05 and a power of 80%. Calculated sample size goal of 32 patients completing treatment in each group was met. All study outcome and financial data were evaluated on an intention to treat basis. Analysis was performed by t-test, chi-square analysis, Kaplan-Meier estimation, and Cox regression modeling as appropriate. Additional analyses were performed based on observed duration of respiratory support with mild TTN defined as not requiring respiratory support during the hospitalization, moderate TTN defined as requiring respiratory support < 48 hours, and severe TTN defined as requiring respiratory support ≥ 48 hours after birth (Table I).
Table 1.
Proposed classification system for patients with TTN. Neonates with TTN can be dichotomized into uncomplicated or complicated TTN based on the absence or presence of air leak. Further characterization can be made to distinguish the severity of disease based on duration of respiratory support.
| Classification | Patient Description |
|---|---|
| Uncomplicated | No air leak (pneumothorax or pneumomediastinum) |
| Complicated | Air leak present on CXR |
| Mild | No respiratory support (CPAP, HFNCPAP, NC) required |
| Moderate | Respiratory support required < 48 hours |
| Severe | Respiratory support required ≥ 48 hours |
RESULTS
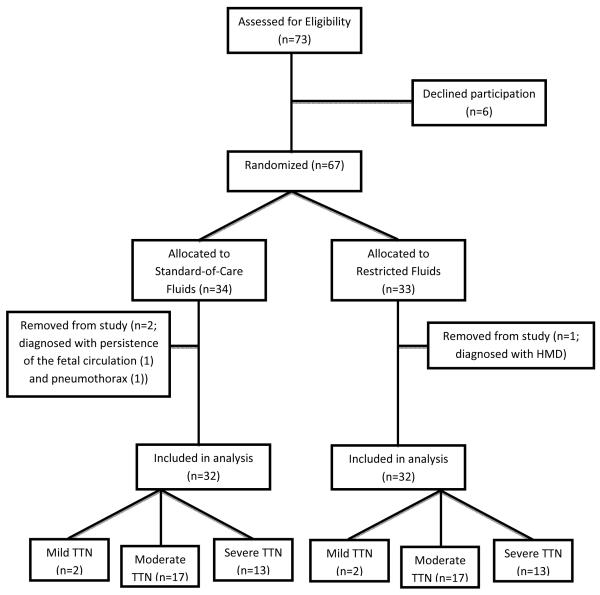
Seventy-three patients met enrollment criteria, and the parents of 67 eligible neonates agreed to the participate. Thirty-four were assigned to standard-of-care fluid management and 33 were assigned to the restricted fluid protocol. Two patients from the standard-of-care group and one patient from the restricted fluid group were withdrawn for a non-TTN respiratory diagnosis. Thirty-two patients in each group completed the study protocol and were included in data analysis (Figure 1; available at www.jpeds.com). Baseline characteristics of the two groups are demonstrated in Table II.
Figure 1.
Patient enrollment. All patients approached for study enrollment and eventual severity classification of disease are depicted.
Table 2.
Patient characteristics and study outcomes.
Baseline study subject characteristics for both the entire patient cohort (top panel) and those patients categorized as having severe TTN (bottom panel). Significant results are starred. Overall, the standard of care and intervention groups differed only in receipt of antenatal steroids, although no mother received antenatal steroids within two weeks or less of delivery. Fluid restricted patients with severe TTN were slightly more mature at birth than those receiving standard fluid management
| Standard Fluids | Restricted Fluids | p Value |
||
|---|---|---|---|---|
| Full patient cohort | White | 21 (65.6%) | 20 (62.5%) | 0.377 |
| Black or Hispanic | 8 (25%) | 9 (28.2%) | ||
| Asian | 2 (6.3%) | 3 (9.4%) | ||
| Other/Unreported | 1 (3.1%) | 0 (0%) | ||
| Male sex | 17 (53.1%) | 16 (50%) | 0.806 | |
| Birth weight (grams) | 2620 ± 546 | 2739 ± 544 | 0.387 | |
| Gestational age (weeks) | 35.8 ± 1.6 | 36.4 ± 1.5 | 0.160 | |
| Multiple gestation | 13 (40.6%) | 10 (31.3%) | 0.311 | |
| Received antenatal steroids | 7 (21.9%) | 1 (3.1%) | 0.023* | |
| C-section delivery | 24 (75%) | 18 (56.3%) | 0.118 | |
| In labor prior to C-section | 12 (37.5%) | 8 (25%) | 0.281 | |
| Maternal Pre-eclampsia | 5 (15.6%) | 6 (18.8%) | 0.745 | |
| Maternal GDM | 3 (9.4%) | 2 (6.3%) | 0.648 | |
| Maternal Asthma | 2 (6.3%) | 3 (9.4%) | 0.648 | |
| Apgar at 5 mintues = 9 | 27 (84.4%) | 29 (90.6%) | 0.308 | |
| Patients with Severe TTN (n=26) | White | 9 (69%) | 10 (77%) | 0.522 |
| Black or Hispanic | 3 (23%) | 3 (23%) | ||
| Asian | 1 (8%) | 0 (0%) | ||
| Other/Unreported | 0 (0%) | 0 (0%) | ||
| Male sex | 5 (38%) | 7 (54%) | 0.431 | |
| Birth weight (grams) | 2446 ± 439 | 2756 ± 549 | 0.126 | |
| Gestational age (weeks) | 35.1 ± 1.1 | 36.2 ± 1.5 | 0.037* | |
| Multiple gestation | 4 (31%) | 4 (31%) | 1 | |
| Received antenatal steroids | 4 (31%) | 0 (0%) | 0.030* | |
| C-section delivery | 8 (62%) | 6 (62%) | 0.431 | |
| In labor prior to C-section | 3 (23.1%) | 4 (30.8%) | 0.658 | |
| Maternal Pre-eclampsia | 3 (23%) | 1 (8%) | 0.277 | |
| Maternal GDM | 0 (0%) | 0 (0%) | 1 | |
| Maternal Asthma | 1 (8%) | 0 (0%) | 0.308 | |
| Apgar at 5 mintues = 9 | 11 (85%) | 13 (100%) | 0.141 | |
Patients were admitted to the study with initiation of fluid and respiratory support when indicated between 30 minutes and 8 hours of life. No patient enrolled in the study experienced an adverse event or unsafe fluid or glycemic balance due to the study protocol (Table III). Urine output after the first 12 hours of life was significantly lower for patients in the restricted fluids group (p=0.008), but never fell below the predetermined safety threshold of 1mL/kg/hour for any patient in any 12-hour period. Serum sodium, creatinine, and BUN were not different between the two groups at any point in time, and never deviated from the predetermined safety criteria ranges. No patient lost more than the predetermined threshold of 10% of birth weight. There was no difference in incidence of hypoglycemia or hyperbilirubinemia requiring phototherapy based on published guidelines[12, 13] between the two groups. No patient in the study received diuretics or vasopressors during the neonatal hospitalization.
Table 3.
Intention to treat analysis of safety parameters.
Study outcomes for both the entire patient cohort (top panel) and those patients categorized as having severe TTN (bottom panel). Results are displayed as mean ± standard deviation for normally distributed data and as median (interquartile range) for skewed data. Significant results are starred. Weight loss, urine output, and serum electrolytes are reported from the study endpoint DOL 3.
| Standard Fluids | Restricted Fluids | p Value | ||
|---|---|---|---|---|
| Full patient cohort | % of birth weight lost | −3.8 ± 2.3 | −3.0 ± 2.6 | 0.238 |
| UOP (mL/kg/hour) | 2.8 ± 0.6 | 2.2 ± 0.4 | 0.008* | |
| Serum sodium (mEq/L) | 137.4 ± 2.7 | 137.6 ± 3.6 | 0.836 | |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.508 | |
| Serum BUN (mg/dL) | 11.0 ± 3.1 | 11.0 ± 3.7 | 1 | |
| Hyperbilirubinemia requiring phototherapy | 23 patients | 21 patients | 0.59 | |
| Hypoglycemia (blood glucose < 40mg/dL) | 0 cases | 0 cases | 1 | |
| Total fluids received (mL/kg/day) 0-12 hr (n=64) | 65.5 ± 16.7 | 48.6 ± 16.6 | <0.001* | |
| Total fluids received (mL/kg/day) 0-24 hr (n=60) | 71.9 ± 19.8 | 52.8 ± 12.2 | <0.001* | |
| Total fluids received (mL/kg/day) 0-48 hr (n=45) | 87.6 ± 21.2 | 63.4 ± 13.7 | <0.001* | |
| Total fluids received (mL/kg/day) 0-72 hr (n=36) | 112.7 ± 21.2 | 92.2 ± 18.6 | 0.004* | |
| Duration of respiratory support (hours) | 45 (26; 85) | 42 (26; 69) | 0.209 | |
| Duration of stay in the NICU (calendar days) | 8 (5; 12) | 7 (4; 10) | 0.589 | |
| Time to first enteral feed (hours) | 31 (21; 51) | 35 (23; 44) | 0.667 | |
| Total Cost of Hospitalization (US dollars) | 7,034 (4,541; 12,369) | 7,073 (5,153; 10,104) | 0.718 | |
| Total Direct Costs (US dollars) | 3,062 (1,539; 4,998) | 2,731 (1,659; 4,109) | 0.832 | |
| Total Indirect Costs (US dollars) | 2,349 (1,243; 3,777) | 2,057 (1,437; 3,194) | 0.763 | |
| Physician Costs (US dollars) | 2,120 (1,365; 3,453) | 2,366 (1,533; 2,688) | 0.533 | |
| Patients with Severe TTN (n=26) | % of birth weight lost | −3.9 ± 2.4 | −2.3 ± 2.5 | 0.120 |
| UOP (mL/kg/hour) | 2.8 ± 0.7 | 2.2 ± 0.4 | 0.066 | |
| Serum sodium (mEq/L) | 136.3 ± 2.3 | 136.5 ± 4.1 | 0.908 | |
| Serum creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.637 | |
| Serum BUN (mg/dL) | 12.1 ± 2.9 | 12.3 ± 4.2 | 0.874 | |
| Hyperbilirubinemia requiring phototherapy | 11 patients | 11 patients | 1 | |
| Hypoglycemia (blood glucose < 40mg/dL) | 0 cases | 0 cases | 1 | |
| Total fluids received (mL/kg/day) 0-12 hr (n=64) | 66.1 ± 17.5 | 48.8 ± 10.8 | 0.002* | |
| Total fluids received (mL/kg/day) 0-24 hr (n=60) | 71.7 ± 16.0 | 50.4 ± 9.9 | 0.001* | |
| Total fluids received (mL/kg/day) 0-48 hr (n=45) | 81.0 ± 16.7 | 60.1 ± 11.4 | 0.002* | |
| Total fluids received (mL/kg/day) 0-72 hr (n=36) | 109.0 ± 16.7 | 89.1 ± 17.2 | 0.014* | |
| Duration of respiratory support (hours) | 113 (71; 141) | 75 (67; 86) | 0.048* | |
| Duration of stay in the NICU (calendar days) | 12 (9; 13) | 7 (7; 9) | 0.096 | |
| Time to first enteral feed (hours) | 51 (31; 76) | 50 (28; 66) | 0.42 | |
| Total Cost of Hospitalization (US dollars) | 13,555 (10,677; 15,547) | 7,747 (6,535; 9,641) | 0.017* | |
| Total Direct Costs (US dollars) | 5,294 (4,108; 5,955) | 2,810 (2,666; 3,786) | 0.033* | |
| Total Indirect Costs (US dollars) | 4,052 (3,125; 4,594) | 2,117 (2,040; 2,908) | 0.031* | |
| Physician Costs (US dollars) | 4,067 (4,489; 4,445) | 2,564 (2,061; 3,076) | 0.005* | |
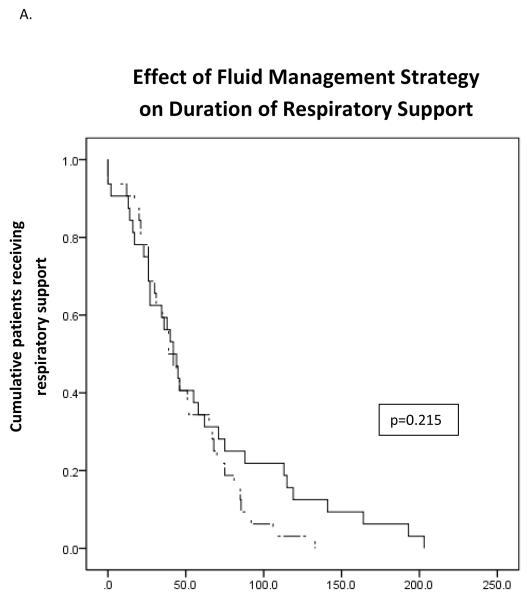
As was intended, patients in the standard-of-care group received significantly greater total fluids during the study period than did patients in the restricted fluid group (p<0.001) (Table III). No significant difference was found between the study groups in terms of the primary or secondary study outcomes on analysis of the entire patient cohort. Kaplan-Meier survival curves based on duration of respiratory support demonstrated no significant difference between the two groups (Figure 2, A). There was no relationship between age at inititation of study protocol and duration of respiratory support.
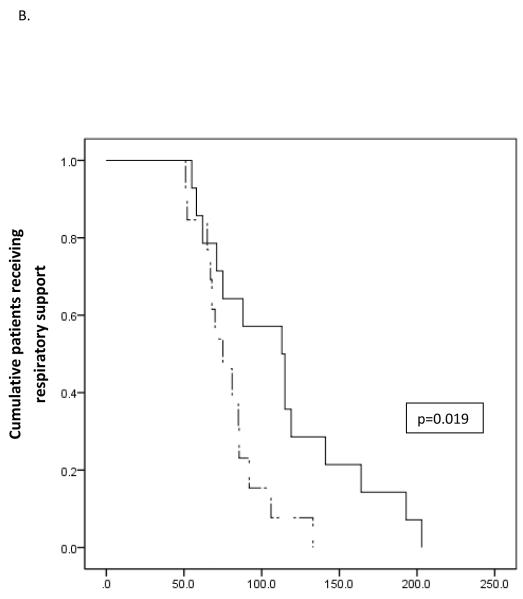
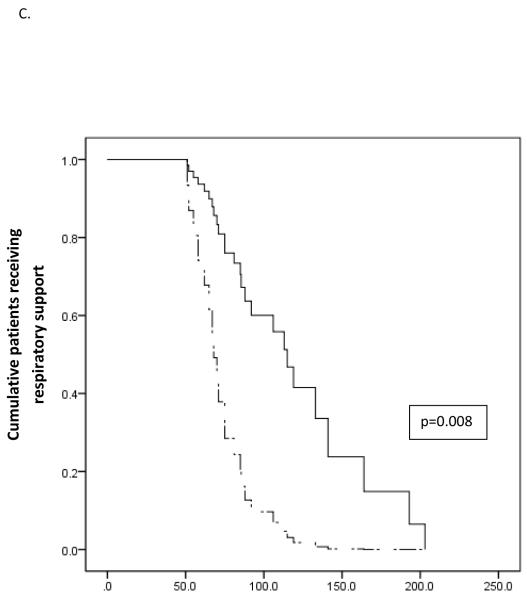
Figure 2.
Survival analysis of the effect of fluid management on duration of respiratory support. Solid lines represent standard fluid management while dash lines represents restrictive fluid management. A) In the overall study cohort there was no statistically significant effect of fluid management strategy on duration of respiratory support. In patients with severe TTN an unadjusted model (B) and a model adjusted for gestational age and receipt of antenatal steroids (C) demonstrate significant decreased duration of respiratory support for neonates receiving restricted total fluids.
Post-hoc classification of patients as experiencing mild, moderate, or severe TTN proved enlightening. Kaplan-Meier curves for the subpopulation of 26 neonates with severe TTN demonstrated significantly shorter duration of respiratory support for neonates receiving restricted fluids (p=0.019) (Figure 2, B). This effect persisted in Cox regression analysis adjusting for gestational age and receipt of antenatal steroids, the only baseline characteristics significantly different between the two groups (p=0.008) (Figure 2, C). Secondary outcomes were not affected by the study intervention in either the full patient cohort or in any disease category cohort.
Mean total hospital costs for patients receiving standard-of-care fluid management were $7,034, compared $7,073 for those receiving restricted fluid management (p>0.05) (Table III). Subgroup analysis of patients with severe TTN demonstrated significant between-group difference in hospital and physician costs. Patients with severe TTN receiving standard fluid management had mean total hospital costs of $13,555, and those receiving restricted fluids had mean total hospital charges of $7,747 (p=0.017). This significant savings persisted at the level of physician costs, hospital direct and hospital indirect costs.
DISCUSSION
This prospective evaluation of fluid management in late pre-term and term neonates with TTN has three major findings: 1) Mild fluid restriction appears safe in term and late preterm neonates with uncomplicated TTN; 2) fluid restriction significantly decreases duration of respiratory support for neonates with severe uncomplicated TTN; and 3) for neonates with severe uncomplicated TTN, fluid restriction leads to significant cost savings.
At diagnosis with uncomplicated TTN, patients can be categorized into mild or moderate-to-severe TTN. The distinction between moderate and severe TTN cannot be made until 48 hours of life. As our fluid restriction protocol appears safe in all patients with TTN, however, we suggest initiating fluid restriction for all patients with moderate or severe uncomplicated TTN to benefit those who ultimately demonstrate severe disease.
As fluid restriction in late preterm and term neonates with respiratory distress is a novel therapy, there was significant concern amongst both treating physicians in our NICU and committee members of the institutional review board that patients in our intervention cohort would face significant dehydration, weight loss, and/or hypoglycemia. With safety concerns in mind, the decision was made not to blind caregivers to patient group assignment. The fidelity of group assignment to total fluids received demonstrated in our study reinforces our belief that mild fluid restriction is safe in late preterm and term neonates, as significant corrections to fluid or glycemic balance were not required to maintain weight, urine output, and measured electrolytes within the normal range.
A significant strength of our study is the lack of antibiotic use in our patient cohort. Although we recognize that it is standard practice in many NICU settings in the United States to initiate antibiotic coverage for presumptive pneumonia in all neonates presenting with respiratory distress, we feel that this practice is not necessary. It has been a longstanding practice in our unit not to initiate an evaluation for sepsis in neonates without either historical risk factors for neonatal sepsis (intrapartum maternal fever, inadequately prophylaxed maternal group B streptococcus, etc) or clinical depression at birth. No neonate enrolled in our study received antibiotic therapy, and none manifested neonatal pneumonia or bacteremia. We are therefore confident that our patient population did not have congenital pneumonia. Due to the exclusion of neonates born with meconium noted at delivery, we can make similar inferences about meconium aspiration syndrome.
We found a significant $5,808 total cost savings per patient with severe TTN that received restricted fluid management. In 2007 there were 4,316,233 live births in the United States.[4] Published results[14] as well as unpublished data from our group estimate the current incidence of TTN at 1-2% of live births. In our study, 40% of patients met criteria for severe TTN. If the savings and patient demographics seen in our study are replicated nationally and the more conservative estimate of the true incidence of TTN is used, mild fluid restriction could result in an annual savings of over $100 million in the United States alone. Savings abroad could be even more significant where TTN is more common due to higher incidence of Cesarean section delivery.[15]
This study has several limitations. As a small, single center study, our results may not be fully generalizable to the TTN patient population in all hospital types on a national level. Our study was underpowered to detect the primary endpoint. As no study on this topic has been published, we made empiric estimation of effect size and standard deviation that proved incorrect, resulting in a power to detect the primary outcome of only 20%. Additionally, our patient mix is likely biased towards more severe cases of TTN, as we only enrolled patients on the neonatology service and patients with TTN not requiring respiratory support may be cared for by private practice-based pediatricians at our institution. Some patients enrolled in the study may have had mild HMD not meeting criteria for exogenous surfactant administration. There was no blinding to study group assignment. The randomization strategy used in this study was alternate assignment by order of diagnosis (most closely approximating order of birth). Although we believe that order of birth provided random patient assignment, as supported by the similarities between the two groups at enrollment, it is possible that patient assignment could have been biased for patients born in close temporal proximity. Finally, decisions to increase total fluids and initiate or adjust respiratory support were left to the treating medical team based on their standard practice and extensive experience managing TTN. We used these “real world” criteria in our study as we felt a protocol with strict criteria would not produce results generalizable to clinical care. There are no published guidelines for respiratory support management for patients with TTN, and support is generally initiated and later weaned based on clinical performance of the individual neonate. Of note, most decisions regarding weaning and cessation of respiratory support in our unit are made by bedside nurses who were largely unaware of patient group assignment. Future larger multi-center study is needed to address these deficiencies.
This manuscript describes the first randomized controlled trial of fluid management in TTN. We demonstrate the safety of mild fluid restriction in late preterm and term neonates with TTN, and propose an evidence-based management strategy to speed symptom resolution in neonates with severe TTN. Additionally, we propose a new classification system for patients with TTN to better identify those who will benefit from fluid restriction. Our management strategy provides significant clinical and financial benefits to neonates with uncomplicated severe TTN. This is the first description of an effective management strategy that speeds the resolution of TTN in any patient cohort.
ACKNOWLEDGMENTS
We would like to thank the families of patients in our NICU for their willingness to allow their neonates’ participation in this investigation. Additional thanks go to the nursing and medical staff who aided with subject identification and embraced the study protocol. Thanks also to Debra Lunburg, Nelson Roberts, and Doran Ricks for their assistance in obtaining financial information for our study patients. Special thanks to Robert Green, MD, for statistical support.
A.S. is supported by NIH 5KL2RR029885, and L.T. is supported by NIH R21ES08723. .
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- [1].Helve O, Janer C, Pitkanen O, Andersson S. Expression of the epithelial sodium channel in airway epithelium of newborn infants depends on gestational age. Pediatrics. 2007;120:1311–6. doi: 10.1542/peds.2007-0100. [DOI] [PubMed] [Google Scholar]
- [2].Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006;30:34–43. doi: 10.1053/j.semperi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [3].Jain L, Dudell GG. Respiratory transition in infants delivered by cesarean section. Semin Perinatol. 2006;30:296–304. doi: 10.1053/j.semperi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- [4].Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- [5].Martin JA, Kirmeyer S, Osterman M, Shepherd RA. Born a bit too early: recent trends in late preterm births. NCHS Data Brief. 2009;1:8. [PubMed] [Google Scholar]
- [6].Ramachandrappa A, Jain L. Elective cesarean section: its impact on neonatal respiratory outcome. Clin Perinatol. 2008;35:373–93. vii. doi: 10.1016/j.clp.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419–25. doi: 10.1001/jama.2010.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kao B, de Ramirez SA Stewart, Belfort MB, Hansen A. Inhaled epinephrine for the treatment of transient tachypnea of the newborn. J Perinatol. 2008;28:205–10. doi: 10.1038/sj.jp.7211917. [DOI] [PubMed] [Google Scholar]
- [9].Lewis V, Whitelaw A. Furosemide for transient tachypnea of the newborn. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD003064. CD003064. [DOI] [PubMed] [Google Scholar]
- [10].Yurdakok M. Transient tachypnea of the newborn: what is new? J Matern Fetal Neonatal Med. 2010;23(Suppl 3):24–6. doi: 10.3109/14767058.2010.507971. [DOI] [PubMed] [Google Scholar]
- [11].HCUP Cost-to-Charge Ratio Files (CCR). Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: pp. 206–2009. [Google Scholar]
- [12].Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- [13].Wong RJ, DeSandre GH, Sibley E, Stevenson DK. Neonatal Jaundice and Liver Disease. In: Fanaroff AA, Martin RJ, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicine : diseases of the fetus and infant. 8th ed Mosby Elsevier; Philadelphia, Pa.: 2006. pp. 1419–66. [Google Scholar]
- [14].Dani C, Reali MF, Bertini G, Wiechmann L, Spagnolo A, Tangucci M, et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian Group of Neonatal Pneumology. Eur Respir J. 1999;14:155–9. doi: 10.1034/j.1399-3003.1999.14a26.x. [DOI] [PubMed] [Google Scholar]
- [15].Derbent A, Tatli MM, Duran M, Tonbul A, Kafali H, Akyol M, et al. Transient tachypnea of the newborn: effects of labor and delivery type in term and preterm pregnancies. Arch Gynecol Obstet. 2010 doi: 10.1007/s00404-010-1473-6. [DOI] [PubMed] [Google Scholar]