Abstract
Objective
The objective of this study was to compare the language growth of children with connexin-related deafness (DFNB1) who received cochlear implants versus the language growth of implanted children with non-DFNB1 deafness.
Study Design
A prospective longitudinal observational study and analysis.
Setting
Two tertiary referral centers.
Patients
There were 37 children with severe to profound hearing loss who received cochlear implants before the age of 5 years.
Interventions
A standardized language measure, the section for expressive language of the Reynell Developmental Language Scale (RDLS) was used to assess expressive language skills at two times post-implantation (14 months and 57 months post-implantation). Molecular screening for DFNB1 gene variants.
Main outcome measures
Language quotient (LQ) scores (i.e. age equivalent score obtained on the RDLS divided by the child’s chronological age), results of genotyping.
Results
The mean language age at the second time interval (mean ± standard deviation [SD]: 51.8 ± 13 months) was greater than at the first testing session (mean ± SD: 19 ± 8 months, p<0.001, Wilcoxon signed rank test). When divided by genotype, DFNB1 children exhibited a higher LQ and less variability in scores than non-DFNB1 children at the second testing interval (Wilcoxon sign rank test, p=0.0034). A regression analysis (linear-fit by least squares) conducted on 26 children with pre-implantation audiometric data showed that DFNB1 status was the independent variable with greater predictive effect on LQ at the second testing interval, followed by age at implantation (R Square = 0.35, p=0.0479).
Conclusion
Deaf children who received cochlear implants before the age of 5 years and use oral-communication show substantial improvement in language abilities. In this study, DFNB1 children who use cochlear implants show greater gains in expressive language than non-DFNB1 children, independent of residual hearing, age at implantation and duration of implant use.
Introduction
Congenital deafness significantly impairs the acquisition of oral and spoken language. Even after accounting for intelligence, habilitation and instruction, children with severe and profound hearing loss fall behind their normal peers in the development of English language in all its domains (1,2). Although cochlear implants do not restore normal hearing, they provide important auditory cues to maximize the chances for a deaf child to develop speech. There is mounting evidence that cochlear implants have revolutionized the outcome of children who are born or become deaf before the age of 3 years. Not only do cochlear implants improve auditory perception, but also recent evidence shows that early cochlear implantation improves spoken language of severely to profound deaf children.(3–5)
When counseling parents about cochlear implantation, clinicians discuss speech development as one of the benefits of this procedure. It should also be recognized that the variability of performance in terms of speech perception and production after cochlear implantation is quite high, and our ability to predict individual outcomes is still limited. Moreover, even unimplanted deaf children show a limited amount of improvement in language abilities with instruction over time.(2) Nevertheless, implantation before the age of 2 years, greater pre-implant residual hearing, higher levels of speech perception, auditory-verbal rehabilitation, and improvements in speech coding strategies are variables that have shown a positive impact on the proportion of implanted deaf children who approach language skills of normal hearing children.(1–5)
Even in environments where children receive similar habilitation and instruction, there are differences in auditory performance with cochlear implants that are not attributable to age at implantation, residual hearing and mode of communication. The impact of the etiology of congenital deafness on cochlear implant performance has been investigated, but most studies focus on auditory perception outcomes rather than language development. Inheritance is perhaps the single most frequent etiology of congenital hearing loss, followed by viral labyrinthitis and other perinatally acquired causes. It is estimated that up to 60% of deaf children who are cochlear implant candidates have hereditary deafness, and that 30% to 60% of children with hereditary deafness have pathologic mutations in the connexin-26 and connexin-30 genes (DFNB1).(6) Connexin genes code for gap junction intracellular channels that are important for electrolyte transport, preservation of the endocochlear potential and signaling within the cochlea. DFNB1 mutations appear to cause hearing loss mainly due to hair cell dysfunction and degeneration, as temporal bone analysis have demonstrated preservation of spiral ganglion cells and no neural degeneration.(7) In contrast, the etiology in deaf children who do not carry mutations in the DFNB1 locus is highly heterogeneous. It is speculated that non-DFNB1 children have a greater complexity of structural and molecular defects as cause of their hearing loss than what is known to occur with DFNB1, and that subtle difference in cochlear implant performance between DFNB1 and non-DFNB1 children may be due to preservation of the peripheral and central neural substrate in DFNB1.(8)
Despite the fact that DFNB1 children do very well with cochlear implants, studies of matched cohorts comparing auditory perception outcomes between DFNB1 and non-DFNB1 children have not consistently shown differences; clinical variables such as age at implantation and duration of implant use seem stronger predictors of performance than genotype.(9–11) However, Bauer et al. (12) reported that DFNB1 children scored significantly higher on nonverbal cognitive measures and on a measure of reading comprehension. Green et al. (13) reported that cochlear implant recipients with DFNB1 performed within one standard deviation of hearing controls in reading tests better than other congenitally deaf cochlear implant recipients and non-cochlear implant recipients. Similarly, a previous report from our institution suggested that DFNB1 implanted children may have faster and greater benefits on tests of language expression and comprehension than children with non-syndromic sensorineural hearing loss who tested negative for DFNB1 mutations. (9) However, the significance of these findings is uncertain given that this study did not include longitudinal testing or strict control of independent variables known to affect cochlear implant performance.
The purpose of this study is to assess whether the DFNB1 genotype has an effect on expressive language development of children with non-syndromic deafness who received their cochlear implant before the age of 5 years. To establish meaningful comparisons between sub-groups of implanted children it is paramount to use age-appropriate tests of language development and longitudinal assessment of language skills. This study uses a test of language skills, the Reynell Developmental Language Scales (RDLS), which has been standardized in normal-hearing children between the ages of 1 and 7 years.(14) To minimize the effect of age at testing, we used the ratio between the language age and chronological age (Language Quotient) as a measure of language development. To control for duration of implant use, we included children with at least 1 year of experience with the implant, and speech skills were assessed longitudinally at least 2 years apart. To control for residual hearing, we analyzed a sub-group of children within this cohort with reliable hearing levels pre-implantation. In this manner, this study attempts to avoid those limitations that many of the outcome studies of pediatric cochlear implantation suffer derived from the effect of these confounding variables.
Materials and Methods
Subjects
Children who received cochlear implants between the years 2000 and 2007 underwent DFNB1 mutations as part of a hereditary deafness study protocol at the University of Miami Ear Institute. The inclusion criteria for this study were: 1) severe or profound sensorineural hearing loss; 2) diagnosis of hearing loss at birth or before 2 years of age; 3) surgery for cochlear implantation before the age of 5 years; 4) non-syndromic hearing loss, i.e. absence of clinical manifestation other than hearing loss; 5) serial evaluation of language skills with the RDLS a minimum of two times, at least two years apart; 7) minimum cochlear implant use of 11 months; 8) auditory-verbal therapy as the main instruction and mode of communication; and 9) complete screening for DFNB1. Thirty-seven subjects were selected: 28 children from the University of Miami Ear Institute and 9 children from our partner institution in Montevideo, Uruguay (British Hospital). These nine children received cochlear implants and underwent speech testing in Uruguay. Patients from Uruguay underwent Spanish equivalents of RDLS and open and closed set word testing. The subjects from Uruguay all spoke Spanish and had similar auditory-verbal therapy to their American counterparts. Auditory-verbal therapy with cochlear implants consisted in weekly visits for the first two years after implantation, and then supervised visits varied based on individual patient needs. The project has approval by the institutional review board of the University of Miami Miller School of Medicine and by the Ethics Committee of the British Hospital in Montevideo, Uruguay. Informed consent was obtained from the parents of each individual prior to any data collection.
Molecular Testing for DFNB1
The technique for molecular screening of DFNB1 has been previously described.(6) In summary, blood samples were obtained either in the clinic by the clinical research nurse or at time of cochlear implant surgery. Deoxyribonucleic acid was extracted from peripheral blood leukocytes. The open reading frame of GJB2 (Gap Junction Beta 2 gene, connexin 26 gene) was examined by direct sequencing. The coding exon of GJB2 (exon 2, 681 bp) was amplified by polymerase chain reaction (PCR) with the following primers F1 (5-GCT TAC CCA GAC TCA GAG AAG-3) and R1 (5-CTA CAG GGG TTT CAA ATG GTT GC-3) (product size 900 bp). The amplification conditions were 95°C for 5 min, then 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min, with a final extension for 5 min at 72°C. The obtained PCR product was subsequently sequenced with an ABI 377 sequencer with both forward and reverse internal primers, F1(5-CTG TCC TAG CTA TGT TCC-3) and R1(5-TGA GCA CGG GTT GCC TCA TC-3).
To examine the possibility of the second mutant allele in heterozygous individuals, amplification of the first (non-coding) exon and the flanking donor splicing site was performed with PCR primers: GJB2-Exon1F(5-TCC GTA ACT TTC CCA GTC TCC GAG GGA AGA GG-3), GJB2-Exon1R(5-CCC AAG GAC GTG TGT TGG TCC AGC CCC-3). The amplification conditions were 95°C for 7 min, then 35 cycles of 92°C for 50 seconds, 53°C for 50 seconds, and 72°C for 1 min, with a final extension for 5 min at 72°C. The obtained PCR product was subsequently sequenced with both forward and reverse primers. We also screened heterozygous individuals for the large (342 kb) deletion in the GJB6 gene (connexin 30) corresponding to the DFNB1 locus with the method described by Wu et al.(15) Based on screening results, children were classified to have DFNB1 or deafness of other origins (non-DFNB1). DFNB1 patients had either biallelic deafness-causing mutations in the GJB2 and/or GJB6. Non-DFNB1 patients were those that had biallelic wild-type GJB2. The genetic analysis of all patients, including the children from Uruguay was done in Miami. In general, we did not know the cause of congenital hearing loss in non-DFNB1 children despite a full work-up including a detailed pre- and post-natal history, physical examination, and imaging evaluation of the inner ears.
Language Assessment
Language skills were assessed with the section for expressive language of the Reynell Developmental Language Scales (RDLS). This test has been used extensively in deaf children and exists in several languages. The children from Miami underwent testing with the English version and the children from Uruguay were tested with the Spanish version. The expressive section of the RDLS provides a score of expressive language, and the raw score can be transformed into a language age for individual and group comparisons. The language age is calculated based on a normative of 1,320 normal-hearing children between ages 1 and 7 years.(14) The expressive section of the RDLS has three components: (1) vocal language structure, (2) the use of vocabulary to name and describe word meanings, and (3) content, which assesses the use of language to create and express ideas. All children were tested in a quiet room and in the oral communication mode. Tests were conducted approximately one year after cochlear implantation surgery, and again two to five years after surgery.
Statistical Analysis
The dependent variables were the language age and language quotient (language quotient = language age/chronological age) which were calculated from the raw scores of the expressive version of the RDLS. The independent variables were age at implantation, duration of implant use, pre-implantation hearing level, and genotype (DFNB1 status). Wilcoxon signed rank test was used for the comparison of the language quotient (LQ) between DFNB1 and non-DFNB1 groups at the two time intervals (1 year and 2–5 years after implantation). Descriptive statistics, correlations, group comparison, and regression analyses (linear-fit by least squares) were conducted using the statistical package JMP version 9.0 for Mac (SAS Institute Inc., Cary, North Carolina).
Results
A total of 44 children were screened and 9 of them were excluded due to incomplete records (7 children) and/or ambiguous genotype (4 children). A total of 37 children comprise the population of this study. Table 1 shows demographic and clinical data. All of the 37 children were diagnosed with either severe or profound bilateral hearing loss on the basis of absent responses to 95 dB click stimuli on auditory brainstem response testing and/or pure-tone audiometry. Pre-implantation pure-tone threshold average (PTA) data were available for 26 children who were old-enough to undergo behavioral testing prior to surgery. The audiometric PTA represents the average threshold for 500, 1000 and 2000 Hertz in the best hearing ear. Table 2 shows audiometric data. In the DFNB1 group, 10 of 11 children had profound hearing loss (PTA greater than 95 dB), and one child had severe (PTA of 71 to 95 dB) hearing loss. In the non-DFNB1 group, 12 out of 15 children had profound hearing loss and 3 had severe hearing loss. There were no statistically significant differences between groups in terms of the proportion of children with severe hearing loss (Fisher’s exact test, p=0.617), and PTA values (Wilcoxon rank test, p=0.1744).
Table 1.
Demographic and clinical data of 37 implanted children grouped by genotype (DFNB1 and non-DFNB1).
| All children | DFNB1 | non-DFNB1 | |
|---|---|---|---|
| N | 37 | 14 | 23 |
| Age at implantation (months) | |||
| Mean±SD | 29±13 | 28±12.3 | 29.7±13.8 |
| Median | 33 | 30 | 33 |
| Minimum | 10 | 10 | 11 |
| Maximum | 60 | 48 | 60 |
| Gender | |||
| Male | 22 | 10 | 12 |
| Female | 15 | 4 | 11 |
| Device | |||
| Nucleus 24 | 23 | 11 | 12 |
| Nucleus Freedom | 10 | 1 | 8 |
| Clarion | 2 | 1 | 1 |
| Medel | 2 | 1 | 1 |
| Age at first RDLS test (months) | |||
| Mean± SD | 45±14.6 | 44.9±16 | 45±14 |
| Median | 47 | 42.5 | 48 |
| Minimum | 22 | 22 | 24 |
| Maximum | 72 | 72 | 72 |
| Age at second RDLS test (months) | |||
| Mean± SD | 85±19 | 84±24 | 85±16 |
| Median | 84 | 81.5 | 88 |
| Minimum | 36 | 36 | 54 |
| Maximum | 120 | 120 | 120 |
| Duration of implant use (months) | |||
| At first RDLS test | |||
| Mean± SD | 16.4±6 | 16.1±5 | 16.3±7 |
| Median | 14 | 16 | 14 |
| Minimum | 10 | 11 | 10 |
| Maximum | 44 | 24 | 44 |
| Duration of implant use (months) | |||
| At second RDLS test | |||
| Mean± SD | 56.8±15 | 55.9±16 | 57.4±15 |
| Median | 57 | 54 | 60 |
| Minimum | 24 | 24 | 33 |
| Maximum | 93 | 84 | 93 |
| Language Quotient | |||
| At first RDLS test
| |||
| Mean± SD | 0.438±0.14 | 0.46±0.13 | 0.425±0.15 |
| Median | 0.5 | 0.464 | 0.5 |
| Minimum | 0.167 | 0.25 | 0.167 |
| Maximum | 0.667 | 0.667 | 0.643 |
| At second RDLS test
| |||
| Mean± SD | 0.695±0.19 | 0.803±0.13 | 0.629±0.19 |
| Median | 0.681 | 0.830 | 0.6 |
| Minimum | 0.353 | 0.6 | 0.353 |
| Maximum | 1.133 | 1 | 1.133 |
SD: standard deviation. RDLS, Reynell Developmental Language Scales
Table 2.
Hearing level prior to cochlear implantation based on the three-frequency pure-tone threshold average (PTA) for the better hearing ear in 26 children. The table depicts the number of children with severe (71 to 95 dB) and profound (>95 dB) sensorineural hearing loss. PTA values greater than 115 were rounded off to 115 dB.
| Pre-implantation hearing | All children | DFNB1 | nonDFNB1 |
|---|---|---|---|
| N | 26 | 11 | 15 |
| Severe | 4 | 1 | 3 |
| Profound (>95dB) | 22 | 10 | 12 |
| Mean PTA±SD (dB) | 101±7.5 | 103±8 | 99.5±7 |
| Median | 99 | 100 | 98 |
| Minimum | 90 | 93 | 98 |
| Maximum | 115 | 115 | 113 |
SD: standard deviation
dB: decibels
All of the children had a period or hearing aid use prior to implantation that varied from 4 months to 13 months. All of the 37 children underwent cochlear implantation surgery with a multichannel device and relied exclusively on the cochlear implant for hearing. Only one child had bilateral cochlear implants; this child was in the DFNB1 group and received his second implant 2 years after the first implant. There were no significant differences between DFNB1 and non-DFNB1 groups in terms of age at implantation (2-sample Wilcoxon rank test, p=0.9875) and duration of implant use at neither the first (2-sample Wilcoxon rank test, p=0.9624) and last (2-sample Wilcoxon rank test, p=0.6486) testing intervals. Similarly, there were no significant differences between Spanish- and English-speaking children in any of the demographic and clinical variables. Table 3 shows the results of the genotyping.
Table 3.
Mutation spectrum in the 14 DFNB1 children (28 alleles) with non-syndromic sensorineural hearing loss.
| Mutation | Number | Percentage | Type |
|---|---|---|---|
| c.35delG | 23 | 82% | Frameshift, truncating |
| del(GJB6-D13S1830) | 3 | 11% | Frameshift, large deletion |
| c.l67delT | 2 | 7% | Frameshift |
Language Skills
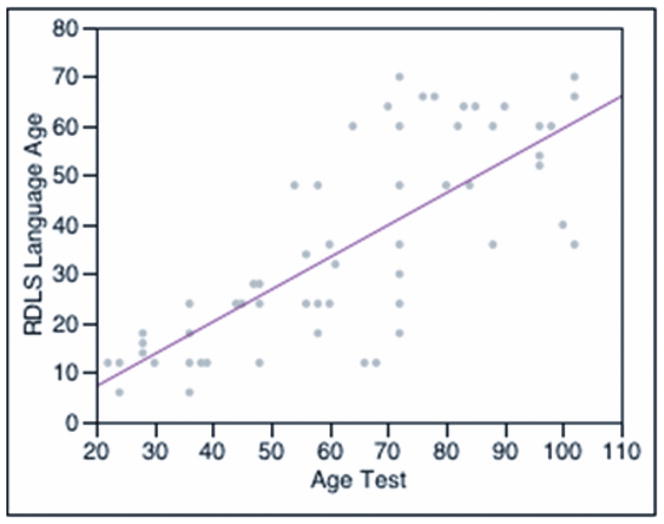
Figure 1 depicts the language age obtained at the two time intervals in all 37 children. Language age was calculated from the composite raw score of the expressive section of the RDLS. Language age improves with age and implant experience and approaches the language growth of normal-hearing children. The slope of this cohort’s regression line is 0.65, and the expected slope for normal-hearing children is a value of 1. All of the children had better language age at the second time interval (mean ± standard deviation [SD]: 51.8 ± 13 months) than they had at the first testing session (mean ± SD: 19 ± 8 months, p<0.001, Wilcoxon signed rank test).
Figure 1.
Language age (calculated from the raw scores of the expressive section of the Reynell Developmental Language Scales [RDLS]) by age at testing in months. The line indicates the linear fit by least-squares regression analysis (Language Age prediction expression = −5.85 + 0.65 × Age Test. R Square = 0.60, p<0.001).
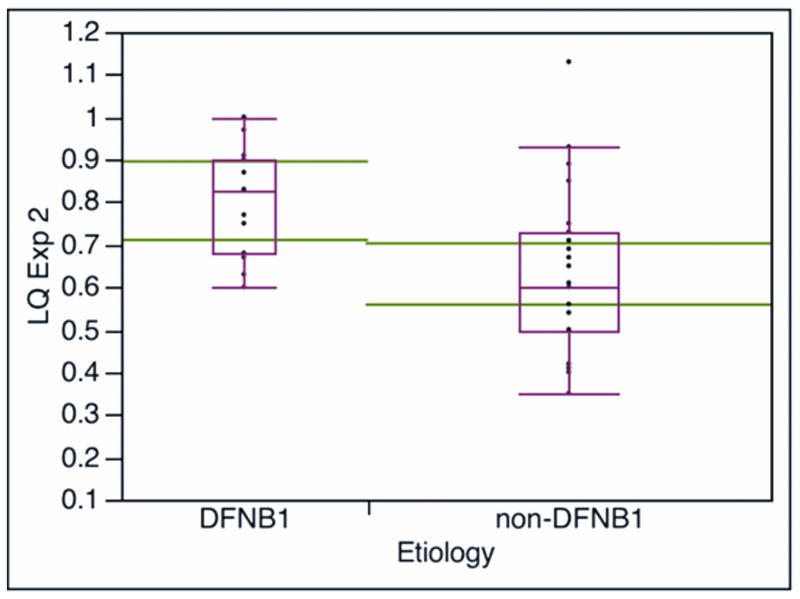
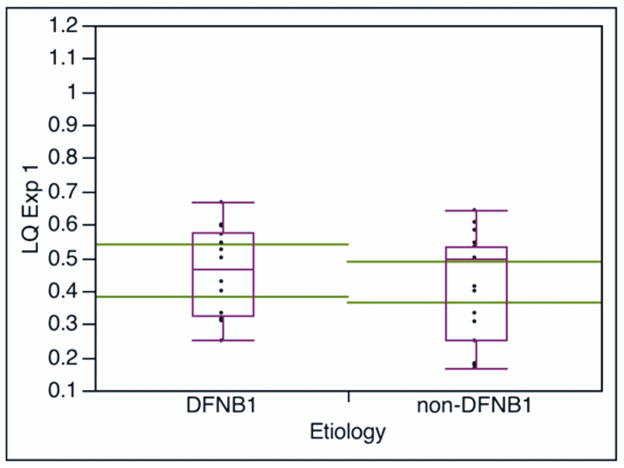
Language quotient was calculated from the ratio language age/chronological age, and the language quotient (LQ) was used to compare the language growth between the DFNB1 and non-DFNB1 groups. Both groups showed statistically significant (p<0.001) growth in LQ at the second testing interval: the DFNB1 group had a 0.34 LQ growth (difference of first and second LQ) and the non-DFNB1 children had a LQ growth of 0.20. Figure 2 shows values of LQ for both groups at the two testing intervals. There was no difference in terms of age at testing or duration of implant use between the groups at the two testing times (table 1). There was no difference in LQ values between the groups at the first testing time. However, at the second testing interval, the DFNB1 group had a higher LQ value (median, mean ± SD: 0.83, 0.803± 0.13) than the LQ value of the non-DFNB1 group (median, mean ± SD: 0.6, 0.629 ± 0.19); this difference in LQ values between groups was statistically significant (P<0.0034, Wilcoxon rank test). There were children who scored at normal LQ values (LQ value of 1 or higher) in both groups, but the non-DFNB1 group showed greater variability in scores; two non-DFNB1 children had low LQ scores of 0.4 or below, which can be seen in deaf unimplanted children.(2)
Figure 2.
Comparison of language quotient LQ (LQ = language age/chronological age) by DFNB1 status at the two testing intervals. LQ Exp1 and LQ Exp2: LQ calculated with the expressive section of the Reynell Developmental Language Scales at the first (Figure 2A, median duration of implant use is 14 months) and second (Figure 2B, median duration of implant use is 57 months) testing interval, respectively. The large horizontal lines across the boxes represent the population means 95% confidence intervals. The ends of the boxes are the 25th and 75th quartiles. The line across the middle of the boxes identifies the median sample values. The whiskers at the ends of the boxes extend to the outermost data points.
A regression analysis (linear-fit by least squares) conducted on the 26 children with pre-implantation audiometric data showed that DFNB1 status was the independent variable with greater predictive effect on LQ at the second testing interval, followed by age at implantation. The only independent variable in this analysis with a p value less than 0.05 was DFNB1 status. The R Square of this regression analysis was 0.35 (p=0.0479) and the parameters estimates, p values for the two-tailed t-test and standard beta coefficients are shown in table 4.
Table 4.
Linear-fit by least squares regression analysis conducted on the 26 children with pre-implantation audiometric data in order to identify the predictive effect of independent variables on language quotient at the second testing interval (the median duration of implant use at this testing interval is 57 months). The only independent variable in this analysis with a p value less than 0.05 (*) was DFNB1 status. The R Square of this regression analysis was 0.35 (p=0.0479).
| Term | Estimate | Std Error | t Ratio | P | St Beta |
|---|---|---|---|---|---|
| Intercept | 1.17014 | 0.594 | 1.97 | 0.062 | 0 |
| DFNB1 status | 0.08696 | 0.037 | 2.35 | 0.028* | 0.442 |
| Age at CI surgery | −0.0059 | 0.003 | −1.74 | 0.096 | −0.327 |
| Pre-implant PTA | −0.0035 | 0.005 | −0.69 | 0.497 | −0.132 |
| Duration of CI use | 0.00160 | 0.002 | 0.65 | 0.522 | 0.118 |
CI: cochlear implant
Std Error: standard error
St Beta: standard Beta coefficient
PTA: pure-tone threshold average for 500, 1000, and 2000 Hertz in the better hearing ear
Discussion
This study shows that deaf children who receive a cochlear implant before the age of 5 years and use oral communication as the main mode of instruction can achieve gains in expressive language that are substantial. The language growth of implanted children approaches the language growth of normal-hearing children with continued use of the implant and instruction. This finding has been reported previously (2–5) and supports the premise that cochlear implantation in the adequate educational setting can empower deaf children to achieve age-appropriate language abilities.
Svirsky et al. (2) compared expressive language scores obtained with the RDLS and showed greater language gains during the first 2.5 years of implant use in implanted children than those predicted for unimplanted deaf children. These authors also showed that the cumulative gains were the same as those expected for normal hearing children, concluding that the implant keeps this delay from increasing further. In other words, cochlear implants can narrow or at least prevent the widening of the language gap that inexorably occurs in deaf unimplanted children. Moreover, these investigators also showed a large degree of individual variability, with some implanted children falling under 2 standard deviations of the predicted growth and some children scoring gains similar to normal-hearing peers, and this variability could not be explained by age of implantation or other clinical variables in their cohort.
The main finding of this study is the difference in LQ observed at the last testing interval between DFNB1 and non-DFNB1 children. Despite the fact that these groups were relatively homogeneous and no differences in age at implantation and duration of implant could be demonstrated, DFNB1 children attained a higher LQ with continued use of the implant after approximately 4 years. This inter-group difference could not be demonstrated at approximately 1 year of implant use, which suggests that, at least in terms of expressive language skills, DFNB1 children do not reach a plateau in language growth during this time period.
Our groups were matched in terms of age at implantation, device, duration of cochlear implant use, educational exposure (oral-communication), and age at test. At least in the 26 children with data on pre-implantation PTA, we could not demonstrate a significant difference in residual hearing between the groups. All of the children received cochlear implantation with multichannel devices and used the most updated coding strategies. Furthermore, this study included only children without obvious co-morbidities or manifestations of syndromic deafness. Consequently, DFNB1 status should be the most plausible explanation for the observed difference in language growth. We speculate that children in the DFNB1 group have better preservation of the peripheral and central neural substrate than non-DFNB1 children. The etiology of the hearing loss in non-DFNB1 children is probably due to multiple causes, which makes this group heterogeneous in terms of the anatomy and pathophysiology of the impairment. This is perhaps illustrated by the greater variability in LQ scores: although some children in the non-DFNB1 group were star performers achieving LQ scores on 1 or higher, there were a few non-DFNB1 children who scored similar to what is expected of unimplanted deaf children; moreover, two non-DFNB1 children showed a regression of their LQ. This level of variability, including children whose language regressed, was not observed in DFNB1 children.
Despite controlling for most clinical variables, one important limitation of this study is that we could not control for other important variables that are known to affect speech outcomes. For instance, we were unable to gather data on all of the children to study the effect of residual hearing on the results. One of the most important prognostic factors for speech-intelligibility appears to be the degree of residual hearing before implantation. Yoshinaga-Itano et al. (1998) showed that even a small amount of residual hearing such as in severe hearing loss vastly improves the outcome when compared with profound hearing loss.(16) We were unable to record residual hearing before implantation in 11 of 37 of our patients; this occurred in part because some children were diagnosed with “severe to profound” hearing loss on the basis of absent responses to 95 click stimuli on ABR and were implanted at infancy before reliable behavioral responses could be obtained. Another important variable with important influence on language development appears to be the age of identification and intervention of the hearing loss. Using the Minnesota Child Development Inventory, a standardized parent-reported measure of child development that includes two scales for expressive language and comprehension, significantly better language skills were demonstrated in children whose hearing loss was identified before 6 months of age than in children whose hearing impairment was identified at later age.(17) Miyamoto et al. (5) and Svirsky et al. (3) showed a significant benefit in RDLS scores and language development when children were implanted before 2 years of age when compared with children implanted after this age. This study did not investigate the effect of early (before 2 years of age) versus late implantation but the average age at implantation for all of the children in this cohort was 29 months, and there were no inter-group statistical differences in age at implantation. Similarly, we did not control for other important prognostic variables such as children’s intelligence, motivation, attention, pre-implantation language level, and personality, as well as socio-educational status of the parents.(18) Nevertheless, our results offer an insight into the effect of etiology of hearing loss on speech development in implanted children with non-syndromic deafness. Given that DFNB1 is the most common identifiable etiology of non-syndromic prelingual deafness both in sporadic and familial cases, exploring the etiology of deafness is useful not only to provide accurate factual information about the disorder, but also to provide parents and children with important information regarding available therapies, support and prognosis. Most DFNB1 children with biallelic frameshift mutations have a congenital hearing loss that is severe to profound, stable, symmetrical, without associated manifestations, and respond well to habilitation.(6) This study in this ethnically diverse cohort provides data that can be applied to assist in the clinical evaluation and counseling of families of children with DFNB1. Additional studies of genotype/phenotype correlations are needed to develop norms and recommendations for diagnosis and therapy that can be extrapolated across diverse populations.
Conclusions
Deaf children who received cochlear implants before the age of 5 years and use oral-communication show substantial improvement in expressive language abilities.
In this study, DFNB1 children who use cochlear implants show greater gains in expressive language than non-DFNB1 children, independent of residual hearing, age at implantation and duration of implant use.
Footnotes
This paper will be presented at the American Otological Society meeting in Chicago, IL, May 2011
References
- 1.Moog J, Geers A. EPIC: A program to accelerate academic progress in profoundly hearing-impaired children. Volta Review. 1985;87:259–77. [Google Scholar]
- 2.Svirsky M, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychological Review. 2000;11(2):153–8. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurotol. 2004;9(4):224–33. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- 4.Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto RT, Hay-McCutcheon MJ, Kirk KI, Houston DM, Bergeson-Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Oto-Laryngol. 2008;128:373–7. doi: 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angeli SI. Phenotype/genotype correlations in a DFNB1 cohort with ethnical diversity. Laryngoscope. 2008;118:2014–23. doi: 10.1097/MLG.0b013e31817fb7ad. [DOI] [PubMed] [Google Scholar]
- 7.Jun AI, McGuirt WT, Hinojosa R, Green GE, Fischel-Ghodsian N, Smith RJ. Temporal bone histopathology in connexin 26-related hearing loss. Laryngoscope. 2000;110(2 Pt 1):269–75. doi: 10.1097/00005537-200002010-00016. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki A, Fukushima K, Kataoka Y, Fukuda S, Nishizaki K. Using assessment of higher brain functions of children with GJB2-associated deafness and cochlear implants as a procedure to evaluate language development. Int J Pediatr Otorhinolaryngol. 2006;70(8):1343–9. doi: 10.1016/j.ijporl.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Connell SS, Angeli SI, Suarez H, et al. Performance after cochlear implantation in DFNB1 patients. Otolaryngol Head Neck Surg. 2007;137(4):596–602. doi: 10.1016/j.otohns.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Cullen RD, Buchman CA, Brown CJ, et al. Cochlear implantation for children with GJB2-related deafness. Laryngoscope. 2004;114(8):1415–9. doi: 10.1097/00005537-200408000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Sinnathuray AR, Toner JG, Geddis A, et al. Auditory perception and speech discrimination after cochlear implantation in patients with connexin 26 (GJB2) gene-related deafness. Otol Neurotol. 2004;25(6):930–4. doi: 10.1097/00129492-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Bauer PW, Geers AE, Brenner C, Moog JS, Smith RJ. The effect of GJB2 allele variants on performance after cochlear implantation. Laryngoscope. 2003;113(12):2135–40. doi: 10.1097/00005537-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Green GE, Scott DA, McDonald JM, et al. Performance of cochlear implant recipients with GJB2-related deafness. Am J Med Genet. 2002;109(3):167–70. doi: 10.1002/ajmg.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynell JK, Huntley M. Reynell Developmental Language Scales: 2nd revision. Windsor, UK: NFER-Nelson; 1985. [Google Scholar]
- 15.Wu BL, Kenna M, Lip V, et al. Use of a multiplex PCR/sequencing strategy to detect both connexin 30 (GJB6) 342 kb deletion and connexin 26 (GJB2) mutations in cases of childhood deafness. Am J Me Genet. 2003;121(2):102– 8. doi: 10.1002/ajmg.a.20210. [DOI] [PubMed] [Google Scholar]
- 16.Yoshinaga-Itano C. Early speech development in children who are deaf or hard of hearing: Interrelationships with the language and hearing. Volta Review. 1998;100(5):181–212. [Google Scholar]
- 17.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102(5):1161–71. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 18.Pisoni DB, Cleary M, Geers AE, Tobey EA. Individual differences in effectiveness of cochlear implants in children who are prelingually deaf: New process measures of performance. Volta Review. 1999;101(3):111–65. [PMC free article] [PubMed] [Google Scholar]