Abstract
The GJB2 gene is located on chromosome 13q12 and it encodes the connexin 26, a transmembrane protein involved in cell-cell attachment of almost all tissues. GJB2 mutations cause autosomal recessive (DFNB1) and sometimes dominant (DFNA3) non-syndromic sensorineural hearing loss. Moreover, it has been demonstrated that connexins are involved in regulation of growth and differentiation of epidermis and, in fact, GJB2 mutations have also been identified in syndromic disorders with hearing loss associated with various skin disease phenotypes. GJB2 mutations associated with skin disease are, in general, transmitted with a dominant inheritance pattern. Nonsyndromic deafness is caused prevalently by a loss-of-function, while literature evidences suggest for syndromic deafness a mechanism based on gain-of-function. The spectrum of skin manifestations associated with some mutations seems to have a very high phenotypic variability. Why some mutations can lead to widely varying cutaneous manifestations is poorly understood and in particular, the reason why the skin disease-deafness phenotypes differ from each other thus remains unclear. This review provides an overview of recent findings concerning pathogenesis of syndromic deafness imputable to GJB2 mutations with an emphasis on relevant clinical genotype-phenotype correlations. After describing connexin 26 fundamental characteristics, the most relevant and recent information about its known mutations involved in the syndromic forms causing hearing loss and skin problems are summarized. The possible effects of the mutations on channel expression and function are discussed.
Keywords: GJB2, connexin 26, skin, hearing loss.
1. INTRODUCTION
Sensorineural hearing loss is a very heterogeneous disorder showing different pattern of inheritance and involving a multitude of different genes. Several forms of hearing loss have been imputated to connexins mutations and prevalently to connexin 26 (Cx26) codified by the GJB2 gene (gap junction protein, beta 2).
Up to now 21 connexins have been established in humans [1], each coding for a transmembrane protein with the same protein topology. Connexins oligomerize with five other connexin molecules to form a connexon (Fig. 1A-B). Connexins are synthesized in the endoplasmic reticulum (ER) and oligomerize in the ER/Golgi or trans-Golgi network to form connexons [2]. Connexons are subsequently transported to the plasma membrane by vesicular carriers travelling along microtubules. Connexons in adjoining cells fuse through disulfide bonding to form gap junctions (GJ) (Fig. 1C) [3]. The combination of several connexins lead to diverse connexons and GJ channels with different properties according to the needs of each cell type [4]. Connexins are, in fact, expressed throughout the body and most cells express more than one type of connexin, thus connexons may either stem from a single species (homomeric) or different Cx (heteromeric) and depending on the compatibility of interacting connexons. This diversity is amplified at the level of intercellular channels, which can be formed by similar (homotypic channels) or different homomeric connexons (heterotypic channels), or two heteromeric Cx (heteromeric channels) [5]. Newly formed connexons tend to move laterally towards existing GJ channels, thus forming large gap junction plaques. Depending on the cell type and the connexin expressed, connexons can function as hemichannels, forming a direct transmembrane communication pathway, enabling the permeation of ions, but also of small metabolites such as ATP, cAMP, IP3 and glutamate. Connexons can thus act as secretory pathways and mediate a paracrine signalling. However, they generally must be tightly regulated to avoid loss of metabolites and of ionic gradients across the membrane. In fact, aberrant opening of hemichannels usually leads to cell death, and most connexons remain in closed conformation in the physiological state. GJ channels instead, will enable a direct communication pathway between the cytoplasms of adjacent cells mediating a direct exchange of ions but also of small molecules up to 1 kDa between the cytoplasms of adjacent cells [6].
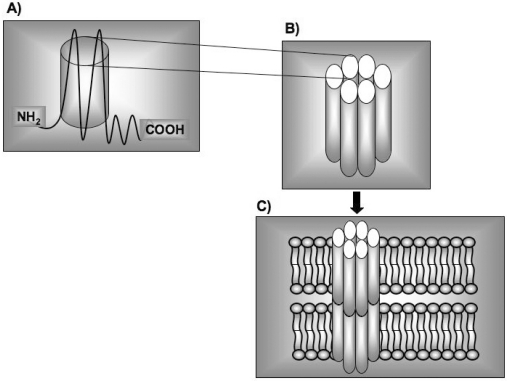
Fig. (1).
Schematic structure of connexins, hemichannels (connexons) and gap junctions. A) Connexins are integral membrane proteins involved in many biological functions. B) Connexons are constituted by six connexins wich are joined to form in their center an aqueous pore that extends connecting the cytoplasms of the two cells. C) The gap junction units are made up of twelve copies of the connexin molecules arranged into two hexamers (connexons), one associated with each cellular membrane and providing a pathway for the transfer of ions and other small molecules between the cells.
According to structural similarity, connexins are divided into two major groups: connexins alpha and beta [7, 1]. GJ appeared to be highly selective as many connexins are restricted in their capability to form functional channels with other members of this family especially when it concerns the connexins from alfa and beta groups [7].
Several connexins are involved in human pathologies [6]. It is actually reported that connexins 26, 29, 30, 30.3, 31, 32, and 43 codified respectively by GJB2, GJE1, GJB6, GJB4, GJB3, GJB1 and GJA1 genes are involved in human diseases affecting cochlea and some of them (Cx26, Cx30, Cx30.3, Cx31 and Cx43) have been involved too in syndromic form of hearing loss affecting coclea in combination with epidermis [8-10].
Mutations in the gene GJB2 (Cx26) are responsible for the major portion of genetic forms of sensorineural hearing loss (SNHL) (http://hereditaryhearingloss.org/). GJB2 gene (OMIM 121011) mutations account for up to 50% of prelingual recessive non-syndromic deafness, in Caucasian and European populations [11-13] and it is also involved in vestibular dysfunction [14]. Few specific mutations in the GJB2 gene also underlie autosomal non-syndromic deafness DFNA3 [15]. A digenic model of inheritance has been proposed in some cases; mutations affecting the GJB6 gene, which encodes connexin-30 (OMIM 604418), have been described in association with GJB2 mutations thereby determining a double heterozygous genotype [16, 17].
2. GJB2 (CX26) STRUCTURE
GJB2 gene is located on chromosome 13q12. The structure of the GJB2 gene, as well the structure of other gap junction genes, is simple (Fig. 2). An untranslated exon 1 is separated by an intron of 3179 bp length from exon 2, containing the uninterrupted coding region and the 3'-untranslated region (3'-UTR) (Fig. 2). GJB2 gene encodes Cx26 protein, that consists of four alfa-helical transmembrane domains (TM1–TM4), two extracellular loops (EC1 and EC2), a cytoplasmic aminoterminal domain (IC1), a cytoplasmic loop (IC2) between TM2 and TM3, and a carboxy-terminal (IC3) domain [18] (Fig. 3). The two latter domains are characteristic of each connexin, while the membrane spanning and the extra cellular domains are highly conserved among the entire protein family [7, 19]. Under physiological conditions the extra cellular portions of connexons interact with connexons of opposing cells in the intercellular space to complete functional active channels. The cytoplasmic loop and the N-terminus of the protein located at the cytoplasmic side of the channel pore are involved in voltage and ion gating and are thought to be essential for the regulation of channel selectivity [20].
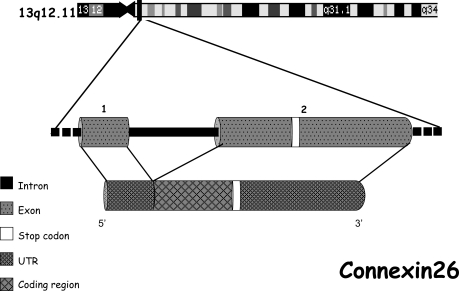
Fig. (2).
GJB2 molecular structure and localization. GJB2 gene is localized in 13q11 chromosome and it is constituted by two exons: only the exon 2 is a coding one.
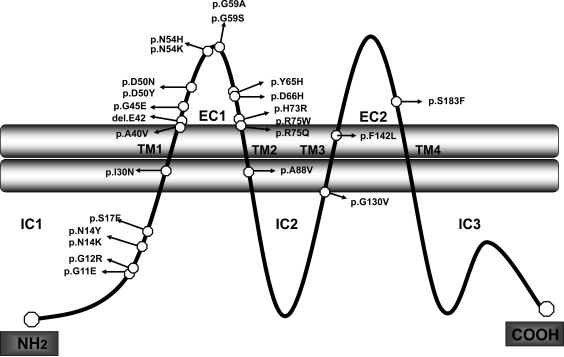
Fig. (3).
Connexin26 molecular structure and known syndromic mutations. Connexins are proteins constituted by four transmembrane domain: TM1-TM4, two extracellular loops: EC1 and EC2, and four intracellular domains: IC1 (NH2-terminal), IC2, and IC3 (COOH-terminal). Known mutations in Cx26 associated with syndromic forms of hearing loss are reported.
3. CONNEXIN EXPRESSION IN COCHLEA AND EPIDERMIS
Several connexin are expressed in the cochlea, but the most abundant expression was found for Cx26 and Cx30 proteins which co-localize and can form heteromeric channels. They are expressed in nonsensory epithelial cells among which hair cells are dispersed and connective tissue cells at more distal locations to the hair cells. Despite several studies their function in inner ear is poorly understood. In these cells it has been supposed that gap junctions are important for maintaining the endocochlear potential being involved in recycling endolymphatic K+ ions from the sensory hair cells back to the endolymph [21]. Data suggest that the role of gap junction communication in the cochlea may not be limited to K+ recycling and it has been shown alteration in biochemical coupling, thus proposing that metabolite transfer between cells is the determinant role of gap junction for K+ homeostasis of the inner ear [22, 23].
In the epidermis several connexins are expressed in a partially overlapping pattern. Noteworthy, non-differentiated basal keratinocytes express alfa connexins, while beta connexins prevail in differentiated keratinocytes. Cxs known to be expressed in human epidermis include Cx26, Cx30, Cx30.3, Cx31, Cx31.1, Cx37 and Cx43 [6, 24, 25]. In human skin, only the expression patterns of Cx43 and Cx26 have been studied in detail to date. Cx43 is expressed in interfollicular epidermis, particularly in the spinous and granular cell layers, in sebaceous glands and in hair follicles. Cx26 is present in hair follicles, and in eccrine sweat glands and ducts, but it is much less abundant in normal epidermis, being seen mainly in the skin of palms and soles [26, 27].
Cx26 plays an important role in the regulation of both keratinocyte proliferation and differentiation even though it is barely expressed in normal adult epidermis in humans and in rodents [7]. Cx26 expression is induced during wound healing, psoriasis, and skin hyperplasia stimulated by tumor promoters. In hyperplastic proliferating epidermis, Cx26 is co-expressed with Cx43 typical for basal and suprabasal keratinocytes. As Cx26 and Cx43 can not form permeable gap junctions, their co-expression may alter the gap junctional communication between keratinocytes and induce proliferation [28, 29].
4. SYNDROMIC FORMS IMPUTABLE TO GJB2 MUTATIONS
GJB2 mutations, as well as other connexins, have been reported as causative for several syndromic forms of hearing loss associated to skin problems. The GJB2 mutations are reported in Table 1.
Table 1.
Syndromic Forms Imputable to GJB2 Mutations
| Syndrome | Mutations | OMIM code |
|---|---|---|
| Keratitis-ichthyosis- deafness (KID) | G11E, G12R, N14K, N14Y, S17F, I30N, A40V, G45E, D50N, D50Y, A88V | 148210 |
| Ichthyosis, hystrix-like-deafness (HID) | D50N, D50Y | 602540 |
| Palmoplantar keratoderma- deafness (PPK) | Delta E42, N54H, G59A, G59R, H73R, R75Q, R75W, G130V, S183F | 148350 |
| Vohwinkel | G59S, Y65H, D66H, G130V | 124500 |
| Burt-Pumphrey | N54K, G59S | 149200 |
| Unususal mucocutaneous- deafness | F142L | - |
All these syndromic forms have an autosomal dominant pattern of transmission. As well as for the hearing loss, the clinical phenotypes associated have a large variability, the type and the severity of the skin disorders caused by Cx26 mutations are very heterogeneous and sometimes are present only features of incomplete syndrome (Table 2). The phenotypic variability is observed even among carriers of the same connexin mutation [30-33] (Table 2).
Table 2.
GJB2 Mutations Involved in SHL: Principal Phenotypic Characteristics and Proteic Domains Affected
| Mutations | Syndrome | Phenotypes Ear Skin | Protein domain | References | |
|---|---|---|---|---|---|
| G11E | KID | Profound SNHL | Hyperkeratosis, ocular problems, alopecia | IC1 | [20] |
| G12R | KID | Mild SNHL | Limited hyperkeratosis. Mild ocular problems | IC1 | [8,73] |
| N14K | KID/EKV | Severe SNHL | EKV-like: hypotrichosis, nail dystrophy, mucositis and skin lesions | IC1 | [74] |
| N14Y | KID | Profound SNHL | Hyperkeratosis palms and soles, impetiginous plaques on neck, axilla, perianal areas and occipital area, ocular problems | IC1 | [75] |
| S17F | KID | SNHL | Visual impairment, in one case lethal carcinoma of the tongue; sometimes trichothiodystrophy-like hair abnormalities | IC1 | [8,76,77] |
| I30N | KID | Profound SNHL | Skin necrosis | TM1 | [78] |
| A40V | KID | Profound SNHL | Mild palmoplantar keratoderma, follicular hyperkeratosis, occlusion triad | TM1/EC1 boundary | [79] |
| Delta E42 | PPK | Profound SNHL | Diffuse PPK | TM1/EC1 boundary | [9] |
| G45E | KID | Profound SNHL; inner ear abnormality: dysplasia of the cochlear and saccular neuroepithelium. | Severe skin lesion infections and septicaemia:fatal form. In japanese population is not fatal: it is responsible only for a recessive non syndromic form of SNHL |
TM1/EC1 boundary | [34,56,80,81] |
| D50N | KID/HID | Profound SNHL and sometimes conductive HL | Photophobia, keratitis, and erythrokeratoderma; sometimes in association with follicular occlusion triad | EC1 | [76,82-84] |
| D50Y | KID/HID | Profound SNHL | Photophobia, keratitis, and erythrokeratoderma | EC1 | [85] |
| N54H | PPK | Profound SNHL | PPK and knuckle pads | EC1 | [86] |
| N54K | BPS | Profound SNHL | PPK, prominent knuckle pads, and leukonychia | EC1 | [42] |
| G59A | PPK | H.F. SNHL | PPK | EC1 | [30] |
| G59R | PPK | SNHL: Mild at L.F.-severe at H.F. | Striate PPK | EC1 | [87] |
| G59S | Atypical BPS or Atypical Vohwinkel | Severe SNHL Profound SNHL |
Knuckle pads, PPK/ ichthyosis, massive mutilating keratoderma, proneness to skin cancer | EC1 | [88,89] |
| Y65H | Vohwinkel | Moderate SNHL | Mutilating PPK | EC1 | [90] |
| D66H | Vohwinkel | Moderate to severe SNHL | Mutilating PPK | EC1 | [91] |
| H73R | PPK | Severe progressive SNHL | Focal PPK similar to Vohwinkel with large intrafamilial variability | EC1 | [49] |
| R75W | PPK | Severe to profound SNHL | Variable: generally PPK and some times knuckle pads | EC1/TM2 boundary | [92,93] |
| R75Q | PPK | Severe to profound SNHL | PPK (not ever present) | EC1/TM2 boundary | [33,55] |
| A88V | KID | Severe to profound SNHL | Severe skin lesion infections and septicaemia:fatal form | TM2 | [94] |
| G130V | PPK or Vohwinkel | Severe SNHL Profound HL | Mild PPK PPK and skin constrictions |
IC2 | [31,57] |
| F142L | Unususal mucocutaneous- deafness | Severe to profound SNHL | Psoriasiform mucocutaneous involvement, inflammation of mucous membranes | TM3 | [95] |
| S183F | PPK | H. F. SNHL | Focal PPK and some times knuckle pads | EC2 | [49] |
EKV: erythrokeratodermia variabilis; H. F. : high frequency; L. F. : low frequency.
Below is reported a brief description for each syndrome:
A. Keratitis-Ichthyosis-Deafness (KID) Syndrome
KID syndrome is a rare congenital ectodermal disorder, characterized by the presence of skin lesions, mild to profound sensorineural hearing loss, and vascularizing keratitis that can result in progressive decline of visual acuity and may eventually lead to blindness. The skin lesions, described as erythrokeratoderma are not restricted to particular regions of the body and show marked ichthyosis with increased susceptibility to mucocutaneous infection sometimes fatal in the neonatal period [34]. Additional phenomena are dystrophic nails, dental abnormalities and scarring alopecia. Sometimes, it is reported increased carcinogenic potential [35-37].
B. Hystrix-Like Icthyosis Deafness Syndrome (HID)
HID deafness syndrome is similar to KID syndrome and displays all of the common features of KID. Symptoms are bilateral hearing loss and spiky hyperkeratotic masses which cover the whole body though the palms and soles are less badly affected. It can be differentiated from KID syndrome which also has symptoms of deafness and ichthyosis by the different distribution of hyperkeratosis. Actually, it is supported the idea that these two syndromes might represent a single form of syndromic deafness with a heterogeneous phenotype. Combined with the similarities between Vohwinkel syndrome (VS), Bart–Pumphrey syndrome (BPS) and palmoplantar keratoderma (PPK), the emerging view is that there are two broad types of skin disorder associated with syndromic deafness: the VS–BPS–PPK group and the KID–HID group [38].
C. Palmoplantar Keratoderma with Deafness Syndrome
Palmoplantar keratoderma is another syndromic complication of deafness (mild to profound SNHL). Hereditary palmoplantar keratodermas (PPK) comprise a clinically and genetically heterogeneous group of genodermatoses, which share impaired epidermal differentiation resulting in prominent palmoplantar hyperkeratosis. Classically, keratodermas have been separated according to their clinical appearance into diffuse, focal, and as a feature of ectodermal dysplasias and many other syndromes [39].
D. Vohwinkel Syndrome
Vohwinkel sindrome is another skin disease associated with SNHL. The skin problem is characterized by disturbed epidermal differentiation manifested by hyperkeratosis especially on the palms and soles (keratoderma), which, in the case of VS, often becomes mutilating with starfish-shaped proximal extensions and hyperkeratotic bands around the fingers, so-called pseudoainhum, sometimes leading to auto-amputation [40,41].
E. Bart–Pumphrey Syndrome
Bart–Pumphrey syndrome, is a rare autosomal dominant disorder characterized by congenital SNHL, palmoplantar hyperkeratosis, knuckle pads, and leukonychia (nail thickening and crumbling) [42-44].
5. FUNCTIONAL STUDIES
The properties of several specific mutations in connexins involved in ear-skin problems have been investigated in more detail in several studies principally at a cellular level, using either transfected mammalian cells or Xenopus oocyte. These studies give first of all several information about dominant inhibition on wild type connexins [9,45-50]. The more recent and convincing study on dominant inhibition has been realized by Zhang [51] transfecting HeLa cells stably expressing wild type with three Cx26 mutants associated with hearing loss and palmoplantar keratoderma (p.G59A, p.R75Q, and p.R75W). All mutants co-localized and coimmunoprecipitated with wild type Cx26, indicating that they interact physically, moreover, these mutants inhibited the transfer of calcein in cells stably expressing Cx26, demonstrating that they each have dominant negative effects on wild type Cx26. Functional studies gives also several information on connexin trafficking and presence and properties of hemichannels and gap junctions (Tables 3 and 4).
Table 3.
Altered Properties Identified by Functional Assays for Cx26 KID Mutations
| Mutation | Trafficking | Hemichannels | Functional GJ channels | Observations | References |
|---|---|---|---|---|---|
| G11E | Altered | Aberrant opening | Absent | Increase of calcium uptake, increase of cellular death by necrosis | [20] |
| G12R | OK | Increased function | Absent | Increased cell death | [59] |
| N14K | OK | Increased function | Present, but with loss of voltage gating | Increased cell death | [59] |
| N14Y | - | - | Absent | Reduced GJ intercellular communication | [75] |
| S17F | OK | Complete loss of functions | Absent | No increased cellular lethality | [59] |
| A40V | OK | Leaky hemichannels | Present | Increased cell death | [79,96,97] |
| G45E | OK | Excessive entry of Ca2+ | Present | Increased cell death | [79,96,97] |
| D50N | - | Increased function | - | Increase of calcium uptake, increase of cellular death by necrosis and apoptosis | [20,59] |
Table 4.
Altered Properties Identified by Functional Assays for Cx26 PPK and Vohwinkel Mutations
| Mutation | Trafficking | Hemichannels | Functional GJ channels | Observations | References |
|---|---|---|---|---|---|
| Delta E42 | OK | - | Absent | - | [9,98] |
| G59A | OK | Heteromeric: inhibition of calcein transfer | Present | - | [52,99] |
| Y65H | Altered | - | Absent | - | [90] |
| D66H | Altered | - | Absent | - | [45,49,100] |
| H73R | Altered | - | - | - | [49] |
| R75W | OK | Heteromeric: inhibition of calcein transfer | Present | - | [51,99] |
| R75Q | OK | Heteromeric: inhibition of calcein transfer | Present | - | [51,99] |
6. DISCUSSION
It has been demonstrated that connexins have a great importance in many human biological processes. Connexins are expressed in several tissues, but in many cases their effects are restricted to particular organs and mutations in a connexin can affect one organ expressing this protein, but these mutations do not affect other tissues expressing the same protein. The precise mechanism for this selectivity for organ involvement is actually unknown and it can only partially be explained by redundancy in connexin expression or by a specific regulation at the transcriptional level as an alternative splicing [52]. Several data have highlighted, in particular, the importance of some connexins in the cochlea and in the skin. The essential role of connexins in the human cochlea and skin was, in fact, evident from association between mutational changes, deafness [12, 53] and/or skin problems. A chief role in deafness and skin pathologies is carried out by the GJB2 gene. In fact, mutations in the GJB2 gene coding for Cx26 can cause a variety of deafness and hereditary hyperproliferative skin disorders in humans. The majority of these mutations, causative of a non–syndromic form of hearing loss, are inherited in an autosomal recessive manner, but several mutations have been also associated with dominantly inherited forms both non-syndromic and syndromic.
In syndromes associated to GJB2 mutations (Table 1) are affected specifically the ear and the epidermis. The lack of associated skin disorders in cases of nonsyndromic SNHL shows that the function and development of the epidermis is not affected by the simple loss of Cx26 function as in the case of homozygous c.35delG patients. In this case a likely explanation is that one or more of the several other connexins expressed in epidermis can compensate for homozygous loss of Cx26. However, while other connexins may compensate for loss of Cx26 from epidermis (but not in the inner ear), they cannot overcome the effects of the dominant mutations. Thus, the Cx26 mutations that can cause syndromic deafness associated with skin disease must show some type of alteration of function, but the mechanisms whereby Cx26 mutation leads to pathological changes remain to be elucidated.
Phenotype associated to identified mutations is largely variable, broad clinical patterns emerge for the wide range of clinical manifestations and rates of progression (Table 2). Sometimes the clinical pattern is very serious, even fatal (p.G45E and p.A88V mutations) (Table 2). A high phenotypic variability has been observed in close aminoacids (p.G11E, p.G12R), sometimes giving also origin to different syndromes (p.A40V: KID and Delta E42: PPK) (Table 2). It has been observed a variable expressivity also for different mutations in the same amino acid: p.N14K- p.N14Y, p.G59A- p.G59R- p.G59S, p.R75W- p.R75Q. [32, 54]. The amino acid N14 is near two of the residues mutated in Keratitis-like ichthyosis deafness (KID) syndrome (p.G12R and p.S17F), yet the phenotype associated with p.N14K strongly differs from the KID phenotype, having a phenotype more similar to Clouston syndrome (caused by mutations in GJB6). However, a different mutation at the same location, p.N14Y, was reported to cause a disorder similar to KID. p.G59A and p.G59R have been indicated as causative of PPK, while p.G59S has been indicated as causative in an atypical form of BPS as well as in an atypical form of Vohwinkel (Table 2). One hypothesis to explain the phenotypic differences in these cases may be that distinct substitutions cause different conformational changes to the protein, each with unique consequences for its behaviour. Interestingly, for the p.R75Q mutation, sometimes a palmoplantar keratoderma phenotype does not consistently occur with the hearing loss giving origin to a non-syndromic form of hearing loss. This high phenotypic variability observed, even among carriers of the same connexin mutation [32, 55] (Table 2) it has been observed also for p.G45E and p.G130V mutations. The p.G45E mutation, reported as causative of a syndromic fatal form of KID (Table 2), is however, the third most common GJB2 mutation (16% of disease alleles) in Japanese patients with autosomal recessive non-syndromic HL [56]. This finding suggests different modes of action of the same GJB2 mutation depending on the genetic background. Instead, p.G130V is reported as causative of two different syndromic forms: PPK and Vohwinkel (Tables 1 and 2) [31, 57].
To explain the differences observed between the phenotypes it have been assessed several functional studies. Principal results are reported in Table 3 for KID analyzed mutations and in Table 4 for PPK and Vohwinkel analyzed mutations. Several studies in transfected cell systems, such as Xenopus oocytes or HeLa cells, have shown an aberrant hemichannel function for many mutations (Tables 3 and 4) [58, 59]. This aberrant hemichannel function may contribute to a loss of cell viability and tissue integrity, leading to rapid cell death, probably because of loss of ionic gradient and metabolites. By studying p.G12R and p.D50N mutations it has been determined a cellular death that could be rescued by introduction of Ca2+ to the extracellular media during incubation. Moreover, it has been observed [20] by studying the effects of mutations p.G11E and p.D50N that alteration of calcium ion fluxes can result in cell death by necrosis [60]. Free cytoplasmic calcium content is increased in samples with mutated connexins, indicating that a major function of Cx26 is the regulation of calcium flux, whose deregulation leads to predominantly necrotic death.
Recently, functional consequences of p.N14Y and p.N14K have been assessed, using fluorescently labelled proteins and parachute assay, and compared with that of the classical KID mutation p.D50N [61]. These analyses show that p.N14Y and p.N14K and p.D50N have different consequences for protein localization and gap junction permeability (Table 3). However, the differences between the phenotypes observed cannot be readily explained from effects on protein trafficking or gap junction permeability.
Besides aberrant hemichannel function, another putative mechanism of disease has also been proposed suggesting that the effect on functions can depends by the specific domain altered. Studies on a mutation associated with Vohwinkel syndrome (p.D66H) [62] and the GJB2 p.R75W missense mutation [63, 64], associated with sensorineural deafness and PPK, both located in EC1, have both a dominant negative effect on Cx26 gap junction assembly. It is thus suggested that alterations in the EC1 domain could impair proper docking of connexons. Moreover, mutations of some specific residues in the IC1 domains of connexins are consistently found in skin disorders (Table 2). The Glycine at position 11 or 12 of beta-group connexins seems to be of particular importance. The replacement of this glycine by a polar residue has been associated with skin disease in several connexins (Cx26 p.G12R in relation to KID, Cx31 p.G12R, p.G12D Cx30.3, p.G12D in EKV, and Cx30 p.G11R in Clouston syndrome) [59, 65-68]. Thus, it has been hypothesized that this conserved glycine maintains the flexibility of the NT and enables the gating of the channel by this domain.
By analyzing all identified mutations (Table 2), it could be observed that KID mutations essentially cluster in the first 50 aminoacid (IC1-TM1-EC1 domains) (Table 2), with the exception of p.A88V (TM2 domain). Mutations in Cx26 causing PPK–deafness syndrome mostly cluster in the EC1 domain of Cx26 (with the exception of p.G130V located in the IC2 domain), but also mutation involved in other phenotypes BPS, Vohwinkel and Clouston syndromes are located in the same domains; so, further studies are necesary to determine if there is a clear correlation domain-involved and phenotype-associated.
Autosomal dominant mutations of the Cx26 gene may manifest effects on skin, perhaps due to their dominant-negative effect on wild-type Cx26 and other connexins as determined by several studies [9, 45, 62, 63, 69, 70]. Recent studies have, in fact, shown that mutant forms of Cx26 associated with skin disorders have an impact on the function of Cx43 and Cx30 [28, 45, 71, 72]. These results suggest that the precise phenotypes (deafness versus skin disease) resulting from the different dominant Cx26 mutants may depend upon both the nature of the physical interactions between different mutant and different wild-type connexins and upon their relative levels of expression in particular tissues. It is possible that the cell death associated with dominant connexin mutants results from causes other than direct disruption of tissue gap junction networks. Mutant connexins may exhibit entirely novel interactions with other key cellular proteins, reflected by their mislocation within the cell. This could lead to premature cell death independently of gap junction networks.
Mutations in connexin-associated protein could also explain the phenotypic variability observed among carriers of the same connexin mutation. It is likely that several syndromes here described are in fact multigenic disorders. Direct and in direct protein partners of connexins are still largely unknown. Further studies in this direction may explain the symptomatic variations of connexins diseases.
REFERENCES
- 1.Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell. Commun. Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 2.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–166. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hormuzdi SG, Filippov MA, Mitropoulou G, Monyer H, Buzzone R. Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks. Biochim. Biophys. Acta. 2004;1662:113–137. doi: 10.1016/j.bbamem.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 5.White TW, Buzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J. Bioenerg. Biomembr. 1996;28:339–350. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- 6.Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur. J. Clin. Invest. 2011;41:103–116. doi: 10.1111/j.1365-2362.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 7.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 8.Richard G. Connexin disorders of the skin. Adv. Dermatol. 2001;17:243–277. [PubMed] [Google Scholar]
- 9.Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G. trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J. Cell Sci. 2001;114:2105–2113. doi: 10.1242/jcs.114.11.2105. [DOI] [PubMed] [Google Scholar]
- 10.López-Bigas N, Arbonés ML, Estivill X, Simonneau L. Expression profiles of the connexin genes, Gjb1 and Gjb3, in the developing mouse cochlea. Gene Expr. Patterns. 2002;2:113–117. doi: 10.1016/s0925-4773(02)00299-x. [DOI] [PubMed] [Google Scholar]
- 11.Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell D, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dodé C, Marlin S, Boulila-ElGaïed A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Levilliers J, Garabédian EN, Mueller RF, McKinlay Gardner RJ, Petit C. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum. Mol. Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- 12.Kelsell DP, Dunlop J, Stevens H-P, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 13.Zelante L, Gasparini P, Estivill X, Melchionda S, D'Agruma L, Govea N, Milá M, Monica MD, Lutfi J, Shohat M, Mansfield E, Del grosso K, Rappaport E, Surrey S, Fortina P. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 1997;6:1605–1609. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- 14.Dodson KM, Blanton SH, Welch KO, Norris VW, Nuzzo RL, Wegelin JA, Marin RS, Nance WE, Panda A, Arnos KS. Vestibular dysfunction in DFNB1 deafness. Am. J. Med. Genet. A. 2011;155A:993–1000. doi: 10.1002/ajmg.a.33828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denoyelle F, Weil D, Levilliers J, Petit C. DFNA3. Adv. Otorhinolaryngol. 2000;56:78–83. doi: 10.1159/000059086. [DOI] [PubMed] [Google Scholar]
- 16.Lerer I, Sagi M, Ben-Neriah Z, Wang T, Levi H, Abeliovich D. A deletion mutation in GJB6 cooperating with a GJB2 mutation in trans in non-syndromic deafness: A novel founder mutation in Ashkenazi Jews. Hum. Mutat. 2001;18:460. doi: 10.1002/humu.1222. [DOI] [PubMed] [Google Scholar]
- 17.del Castillo FJ, Rodríguez-Ballesteros M, Alvarez A, Hutchin T, Leopardi E, de Oliveira CA, Azaiez H, Brownstein Z, Avenarius MR, Marlin S, Panda A, Shahin H, Siemering KR, Weil D, Wuyts W, Aguirre LA, Martín Y, Moreno-Pelayo MA, Villamar M, Avraham KB, Dahl HH, Kanaan M, Nance WE, Petit C, Smith RJ, Van Camp G, Sartorato EL, Murgia A, Moreno F, del Castillo I. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J. Med. Genet. 2005;42:588–594. doi: 10.1136/jmg.2004.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans WH, Martin PE. Gap junctions: structure and function. Mol. Membr. Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 19.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 20.Terrinoni A, Codispoti A, Serra V, Bruno E, Didona B, Paradisi M, Nisticò S, Campione E, Napoletano B, Diluvio L, Melino G. Connexin 26 (GJB2) mutations as a cause of the KID syndrome with hearing loss. Biochem. Biophys. Res. Commun. 2010;23:25–30. doi: 10.1016/j.bbrc.2010.03.098. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi T, Rimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res. Rev. 2000;32:163–166. doi: 10.1016/s0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 22.Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell. Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez VH, Bortolozzi M, Pertegato V, Beltramello M, Giarin M, Zoccolo M, Pantano S, Mammano F. Unitary permeability of gap junction channels to second messengers measured by FRET microscopy. Nat. Methods. 2007;4:353–358. doi: 10.1038/nmeth1031. [DOI] [PubMed] [Google Scholar]
- 24.Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaître G, Hand C, Hayflick SJ, Zonata J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat. Genet. 2000;26:142–144. doi: 10.1038/79851. [DOI] [PubMed] [Google Scholar]
- 25.Macari F, Landau M, Cousin P, Mevorah B, Brenner S, Panizzon R, Schorderet DF, Hohl D, Huber M. Mutation in the gene for connexin 30.3 in a family with erythrokeratodermia variabilis. Am. J. Hum. Genet. 2000;67:1296–1301. doi: 10.1016/s0002-9297(07)62957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucke T, Choudhry R, Thom R, Selmer IS, Burden AD, Hodgins MB. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J. Invest. Dermatol. 1999;112:354–361. doi: 10.1046/j.1523-1747.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 27.Salomon D, Masgrau E, Vischer S, Ullrich S, Dupont E, Sappino P, Saurat JH, Meda P. Topography of mammalian connexins in human skin. J. Invest. Dermatol. 1994;103:240–247. doi: 10.1111/1523-1747.ep12393218. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Ramirez A, Budunova I. Overexpression of connexin26 in the basal keratinocytes reduces sensitivity to tumor promoter TPA. Exp. Dermatol. 2010;19:633–640. doi: 10.1111/j.1600-0625.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 29.Kretz M, Euwens C, Hombach S, Eckardt D, Teubner B, Traub O, Willecke K, Ott TJ. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. Cell. Sci. 2003;116:3443–3452. doi: 10.1242/jcs.00638. [DOI] [PubMed] [Google Scholar]
- 30.Heathcote K, Syrris P, Carter ND, Patton MA. A connexin 26 mutation causes a syndrome of sensorineural hearing loss and palmoplantar hyperkeratosis (MIM 148350) J. Med. Genet. 2000;37:50–51. doi: 10.1136/jmg.37.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iossa S, Chinetti V, Auletta G, Laria C, De Luca M, Rienzo M, Giannini P, Delfino M, Ciccodicola A, Marciano E, Franzé A. New evidence for the correlation of the p.G130V mutation in the GJB2 gene and syndromic hearing loss with palmoplantar keratoderma. Am. J. Med. Genet. A. 2009;149A:685–688. doi: 10.1002/ajmg.a.32462. [DOI] [PubMed] [Google Scholar]
- 32.Kelsell DP, Di WL, Houseman MJ. Connexin mutations in skin disease and hearing loss. Am. J. Hum Genet. 2001;68:559–568. doi: 10.1086/318803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uyguner O, Tukel T, Baykal C, Eris H, Emiroglu M, Hafiz G, Ghanbari A, Baserei N, Yuksel-Apak M, Wollnik B. The novel R75Q mutation in the GJB2 gene causes autosomal dominant hearing loss and palmoplantar keratoderma in a Turkish family. Clin. Genet. 2002;62:306–309. doi: 10.1034/j.1399-0004.2002.620409.x. [DOI] [PubMed] [Google Scholar]
- 34.Jonard L, Feldmann D, Parsy C, Freitag S, Sinico M, Koval C, Grati M, Couderc R, Denoyelle F, Bodemer C, Marlin S, Hadj-Rabia S. A familial case of Keratitis-Ichthyosis-Deafness (KID) syndrome with the GJB2 mutation G45E. Eur. J. Med. Genet. 2008;51:35–43. doi: 10.1016/j.ejmg.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Grob JJ, Breton A, Bonafe JL, Sauvan-Ferdani M, Bonerandi JJ. Keratitis, ichthyosis, and deafness (KID) syndrome.Vertical transmission and death from multiple squamous cell carcinomas. . Arch. Dermatol. 1987;123:777–782. [PubMed] [Google Scholar]
- 36.Conrado LA, Marques SA, La storia JC, Cucé LC, Marques ME, Dillon NL. Keratitis-ichthyosis-deafness (KID) syndrome with squamous cell carcinoma. Int. J. Dermatol. 2007;46:403–406. doi: 10.1111/j.1365-4632.2007.02977.x. [DOI] [PubMed] [Google Scholar]
- 37.Janecke AR, Hennies HC, Günther B, Gansl G, Smolle J, Messmer EM, Utermann G, Rittinger O. GJB2 mutations in keratitis-ichthyosis-deafness syndrome including its fatal form. Am. J. Med. Genet. A. 2005;133A:128–131. doi: 10.1002/ajmg.a.30515. [DOI] [PubMed] [Google Scholar]
- 38.van Geel M, van Steensel MA, Küster W, Hennies HC, Happle R, Steijlen PM, König A. HID and KID syndromes are associated with the same connexin 26 mutation. Br. J. Dermatol. 2002;146:938–942. doi: 10.1046/j.1365-2133.2002.04893.x. [DOI] [PubMed] [Google Scholar]
- 39.Kelsell DP, Stevens HP. The palmoplantar keratodermas: much more than palms and soles. Mol. Med. Today. 1999;5:107–113. doi: 10.1016/s1357-4310(98)01428-2. [DOI] [PubMed] [Google Scholar]
- 40.Peris K, Salvati EF, Torlone G, Chimenti S. Keratoderma hereditarium mutilans (Vohwinkel's syndrome) associated with congenital deaf-mutism. Br. J. Dermatol. 1995;132:617–620. doi: 10.1111/j.1365-2133.1995.tb08721.x. [DOI] [PubMed] [Google Scholar]
- 41.Solis RR, Diven DG, Trina Z. Vohwinkel's syndrome in three generations. J. Am. Acad. Dermatol. 2001;44:376–378. doi: 10.1067/mjd.2001.106348. [DOI] [PubMed] [Google Scholar]
- 42.Richard G, Brown N, Ishida-Yamamoto A, Krol A. Expanding the phenotypic spectrum of Cx26 disorders: Bart-Pumphrey syndrome is caused by a novel missense mutation in GJB2. J. Invest. Dermatol. 2004;123:856–863. doi: 10.1111/j.0022-202X.2004.23470.x. [DOI] [PubMed] [Google Scholar]
- 43.Bart RS, Pumphrey RE. Knuckle pads, leukonychia and deafness.A dominantly inherited syndrome. N. Engl. J.Med. 1967;276:202–207. doi: 10.1056/NEJM196701262760403. [DOI] [PubMed] [Google Scholar]
- 44.Ramer JC, Vasily DB, Ladda RL. Familial leuconychia, knuckle pads, hearing loss, and palmoplantar hyperkeratosis: an additional family with Bart-Pumphrey syndrome. J. Med. Genet. 1994;31:68–71. doi: 10.1136/jmg.31.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marziano NK, Casalotti SO, Portelli AE, Becker DL, Forge A. Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum. Mol. Genet. 2003;12:805–812. doi: 10.1093/hmg/ddg076. [DOI] [PubMed] [Google Scholar]
- 46.Thomas T, Telford D, Laird DW. Functional domain mapping and selective trans-dominant effects exhibited by Cx26 disease-causing mutations. J. Biol. Chem. 2004;279:19157–19168. doi: 10.1074/jbc.M314117200. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Deng Y, Bao X, Reuss L, Altenberg GA. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J. 2005;19:1516–1518. doi: 10.1096/fj.04-3491fje. [DOI] [PubMed] [Google Scholar]
- 48.Piazza V, Beltramello M, Mentiti M, Colao E, Malatesta P, Argento R, Chiarella G, Gallo LV, Catalano M, Perrotti N, Mammano F, Cassandro E. Functional analysis of R75Q mutation in the gene coding for Connexin 26 identified in a family with nonsyndromic hearing loss. Clin. Genet. 2005;68:161–166. doi: 10.1111/j.1399-0004.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 49.de Zwart-Storm EA, Hamm H, Stoevesandt J, Steijlen PM, Martin PE, van Geel M, van Steensel MA. A novel missense mutation in GJB2 disturbs gap junction protein transport and causes focal palmoplantar keratoderma with deafness. J. Med. Genet. 2008;45:161–166. doi: 10.1136/jmg.2007.052332. [DOI] [PubMed] [Google Scholar]
- 50.Mani RS, Ganapathy A, Jalvi R, Srikumari Srisailapathy CR, Malhotra V, Chadha S, Agarwal A, Ramesh A, Rangasayee RR, Anand A. Functional consequences of novel connexin 26 mutations associated with hereditary hearing loss. Eur. J. Hum. Genet. 2009;17:502–509. doi: 10.1038/ejhg.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Scherer SS, Yum SW. Dominant Cx26 mutants associated with hearing loss have dominant-negative effects on wild type Cx26. Mol. Cell. Neurosci. 2011;47:71–78. doi: 10.1016/j.mcn.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essenfelder GM, Larderet G, Waksman G, Lamartine J. Gene structure and promoter analysis of the human GJB6 gene encoding connexin 30. Gene. 2005;350:33–40. doi: 10.1016/j.gene.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 53.Cohn ES, Kelley PM. Clinical phenotype and mutations in connexin 26 (DFNB1/GJB2), the most common cause of childhood hearing loss. Am. J. Med. Genet. 1999;89:130–136. [PubMed] [Google Scholar]
- 54.Feldmann D, Denoyelle F, Blons H, Lyonnet S, Loundon N, Rouillon I, Hadj-Rabia S, Petit C, Couderc R, Garabédian EN, Marlin S. The GJB2 mutation R75Q can cause nonsyndromic hearing loss DFNA3 or hereditary palmoplantar keratoderma with deafness. Am. J. Med. Genet. A. 2005;137:225–227. doi: 10.1002/ajmg.a.30765. [DOI] [PubMed] [Google Scholar]
- 55.Birkenhäger R, Lüblinghoff N, Prera E, Schild C, Aschendorff A, Arndt S. Autosomal dominant prelingual hearing loss with palmoplantar keratoderma syndrome: Variability in clinical expression from mutations of R75W and R75Q in the GJB2 gene. Am. J. Med. Genet. A. 2010;152A:1798–1802. doi: 10.1002/ajmg.a.33464. [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa S, Kawano A, Hayashi C, Nishiyama N, Kawaguchi S, Furuse H, Ikeda K, Suzuki M, Nakagawa M. The clinical features of patients with the homozygous 235delC and the compound-heterozygous Y136X/G45E of the GJB2 mutations (Connexin 26) in cochlear implant recipients. Auris Nasus Larynx. 2011;38:444–449. doi: 10.1016/j.anl.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Snoeckx RL, Hassan DM, Kamal NM, Van Den Bogaert K, Van Camp G. Mutation analysis of the GJB2 (connexin 26) gene in Egypt. Hum. Mutat. 2005;26:60–61. doi: 10.1002/humu.9350. [DOI] [PubMed] [Google Scholar]
- 58.Munhoz Essenfelder G, Buzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 59.Lee JR, De Rosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus Oocytes. J. Invest. Dermatol. 2009;129:870–878. doi: 10.1038/jid.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zong WX. Thompson CB Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 61.de Zwart-Storm EA, Rosa RF, Martin PE, Foelster-Holst R, Frank J, Bau AE, Zen PR, Graziadio C, Paskulin GA, Kamps MA, van Geel M, van Steensel MA. Molecular analysis of connexin26 asparagine14 mutations associated with syndromic skin phenotypes. Exp. Dermatol. 2011;20:408–412. doi: 10.1111/j.1600-0625.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 62.Bakirtzis G, Jamieson S, Aasen T, Bryson S, Forrow S, Tetley L, Finbow M, Greenhalgh D, Hodgins M. The effects of a mutant connexin 26 on epidermal differentiation. Cell. Commun. Adhes. 2003;10:359–364. doi: 10.1080/cac.10.4-6.359.364. [DOI] [PubMed] [Google Scholar]
- 63.Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum. Genet. 1998;103:393–399. doi: 10.1007/s004390050839. [DOI] [PubMed] [Google Scholar]
- 64.Oshima A, Doi T, Mitsuoka K, Maeda S, Fujiyoshi Y. Roles of Met-34, Cys-64, Arg-75 in the assembly of human connexin 26. J. Biol. Chem. 2003;278:1807–1816. doi: 10.1074/jbc.M207713200. [DOI] [PubMed] [Google Scholar]
- 65.Richard G. Connexin disorders of the skin. Clin. Dermatol. 2005;23:23–32. doi: 10.1016/j.clindermatol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Essenfelder MG, Buzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 67.van Steensel MA, Oranje AP, van der Schroeff J.G. Wagner, A. van Geel M. The missense mutation G12D in Connexin30. can cause both erythrokeratodermia variabilis of Mendes da Costa and progressive symmetric erythrokeratodermia of Gottron. Am. J. Med. Genet. A. 2009;149A:657–661. doi: 10.1002/ajmg.a.32744. [DOI] [PubMed] [Google Scholar]
- 68.Tattersall D, Scott CA, Gray C, Zicha D, Kelsell DP. EKV mutant connexin 31 associated cell death is mediated by ER stress. Hum. Mol. Genet. 2009;18:4734–4745. doi: 10.1093/hmg/ddp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin PE, Coleman SL, Casalotti SO, Forge A, Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum. Mol. Genet. 1999;8:2369–2376. doi: 10.1093/hmg/8.13.2369. [DOI] [PubMed] [Google Scholar]
- 70.Forge A, Marziano NK, Casalotti SO, Becker DL, Jagger D. The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell. Commun. Adhes. 2003;10:341–346. doi: 10.1080/cac.10.4-6.341.346. [DOI] [PubMed] [Google Scholar]
- 71.Schütz M, Auth T, Gehrt A, Bosen F, Körber I, Strenzke N, Moser T, Willecke K. The connexin26 S17F mouse mutant represents a model for the human hereditary keratitis-ichthyosis-deafness syndrome. Hum. Mol. Genet. 2011;20:28–39. doi: 10.1093/hmg/ddq429. [DOI] [PubMed] [Google Scholar]
- 72.Serrano Castro PJ, Naranjo Fernandez C, Quiroga Subirana P, Payan Ortiz M. Vohwinkel Syndrome secondary to missense mutation D66H in GJB2 gene (connexin 26) can include epileptic manifestations. Seizure. 2010;19:129–131. doi: 10.1016/j.seizure.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Neoh CY, Chen H, Ng SK, Lane EB, Common JE. A rare connexin 26 mutation in a patient with a forme fruste of keratitis-ichthyosis-deafness (KID) syndrome. Int. J. Dermatol. 2009;48:1078–1081. doi: 10.1111/j.1365-4632.2009.04136.x. [DOI] [PubMed] [Google Scholar]
- 74.Van Steensel MA, Van Geel M, Steijlen PM. Further delineation of the hypotrichosis-deafness syndrome. Eur. J. Dermatol. 2005;15:437–438. [PubMed] [Google Scholar]
- 75.Arita K, Akiyama M, Aizawa T, Umetsu Y, Segawa I, Goto M, Sawamura D, Depura M, Kawano K, Shimizu H. A novel N14Y mutation in Connexin26 in keratitis-ichthyosis-deafness syndrome: analyses of altered gap junctional communication and molecular structure of N terminus of mutated Connexin26. Am. J. Pathol. 2006;169:416–423. doi: 10.2353/ajpath.2006.051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazereeuw-Hautier J, Bitoun E, Chevrant-Breton J, Man SY, Bodemer C, Prins C, Antille C, Saurat JH, Atherton D, Harper JI, Kelsell DP, Hovnanian A. Keratitis-ichthyosis-deafness syndrome: disease expression and spectrum of connexin 26 (GJB2) mutations in 14 patients. Br. J. Dermatol. 2007;156:1015–1019. doi: 10.1111/j.1365-2133.2007.07806.x. [DOI] [PubMed] [Google Scholar]
- 77.De Raeve L, Bonduelle M, Deconinck H, Roseeuw D, Stene JJ. Trichothiodystrophy-like hair abnormalities in a child with keratitis ichthyosis deafness syndrome. Pediatr. Dermatol. 2008;25:466–469. doi: 10.1111/j.1525-1470.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- 78.Arndt S, Aschendorff A, Schild C, Beck R, Maier W, Laszig R, Birkenhäger R. A novel dominant and a de novo mutation in the GJB2 gene (connexin-26) cause keratitis-ichthyosis-deafness syndrome: implication for cochlear implantation. Otol. Neurotol. 2010;31:210–215. doi: 10.1097/MAO.0b013e3181cc09cd. [DOI] [PubMed] [Google Scholar]
- 79.Montgomery JR, White TW, Martin BL, Turner ML, Holland SM. A novel connexin 26 gene mutation associated with features of the keratitis-ichthyosis-deafness syndrome and the follicular occlusion triad. J. Am. Acad. Dermatol. 2004;51:377–382. doi: 10.1016/j.jaad.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 80.Fuse Y, Doi K, Hasegawa T, Sugii A, Hibino H, Kubo T. Three novel connexin26 gene mutations in autosomal recessive non-syndromic deafness. Neuroreport. 1999;10:1853–1857. doi: 10.1097/00001756-199906230-00010. [DOI] [PubMed] [Google Scholar]
- 81.Griffith AJ, Yang Y, Pryor SP, Park HJ, Jabs EW, Nadol JB, Jr, Russell LJ, Wasserman DI, Richard G, Adams JC, Merchant SN. Cochleosaccular dysplasia associated with a connexin 26 mutation in keratitis-ichthyosis-deafness syndrome. Laryngoscope. 2006;116:1404–1408. doi: 10.1097/01.mlg.0000224549.75161.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Geel M, van Steensel MA, Küster W, Hennies HC, Happle R, Steijlen PM, König A. HID and KID syndromes are associated with the same connexin 26 mutation. Br. J. Dermatol. 2002;146:938–942. doi: 10.1046/j.1365-2133.2002.04893.x. [DOI] [PubMed] [Google Scholar]
- 83.Kelly B, Lozano A, Altenberg G, Makishima T. Connexin 26 mutation in keratitis-ichthyosis-deafness (KID) syndrome in mother and daughter with combined conductive and sensorineural hearing loss. Int. J. Dermatol. 2008;47:443–447. doi: 10.1111/j.1365-4632.2008.03603.x. [DOI] [PubMed] [Google Scholar]
- 84.Maintz L, Betz RC, Allam JP, Wenzel J, Jaksche A, Friedrichs N, Bieber T, Novak N. Keratitis-ichthyosis-deafness syndrome in association with follicular occlusion triad. Eur. J. Dermatol. 2005;15:347–352. [PubMed] [Google Scholar]
- 85.Yotsumoto S, Hashiguchi T, Chen X, Ohtake N, Tomitaka A, Akamatsu H, Matsunaga K, Shiraishi S, Miura H, Adachi J, Kanzaki T. Novel mutations in GJB2 encoding connexin-26 in Japanese patients with keratitis-ichthyosis-deafness syndrome. Br. J. Dermatol. 2003;148:649–653. doi: 10.1046/j.1365-2133.2003.05245.x. [DOI] [PubMed] [Google Scholar]
- 86.Akiyama M, Sakai K, Arita K, Nomura Y, Ito K, Kodama K, McMillan JR, Kobayashi K, Sawamura D, Shimizu H. A novel GJB2 mutation p.Asn54His in a patient with palmoplantar keratoderma, sensorineural hearing loss and knuckle pads. J. Invest. Dermatol. 2007; 127:1540–1543. doi: 10.1038/sj.jid.5700711. [DOI] [PubMed] [Google Scholar]
- 87.Leonard NJ, Krol AL, Bleoo S, Somerville MJ. Sensorineural hearing loss, striate palmoplantar hyperkeratosis, and knuckle pads in a patient with a novel connexin 26 (GJB2) mutation. J. Med. Genet. 2005;42:e2. doi: 10.1136/jmg.2003.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alexandrino F, Sartorato EL, Marques-de-Faria AP, Steiner CE. G59S mutation in the GJB2 (connexin 26) gene in a patient with Bart-Pumphrey syndrome. Am. J. Med. Genet. A. 2005;136:282–284. doi: 10.1002/ajmg.a.30822. [DOI] [PubMed] [Google Scholar]
- 89.Bondeson ML, Nyström AM, Gunnarsson U, Vahlquist A. Connexin 26 (GJB2) mutations in two Swedish patients with atypical Vohwinkel (mutilating keratoderma plus deafness) and KID syndrome both extensively treated with acitretin. Acta Derm. Venereol. 2006;86:503–508. doi: 10.2340/00015555-0164. [DOI] [PubMed] [Google Scholar]
- 90.de Zwart-Storm EA, van Geel M, Veysey E, Burge S, Cooper S, Steijlen PM, Martin PE, van Steensel MA. novel missense mutation in GJB2, p.Tyr65His, causes severe Vohwinkel syndrome. Br. J. Dermatol. 2011;164:197–199. doi: 10.1111/j.1365-2133.2010.10058.x. [DOI] [PubMed] [Google Scholar]
- 91.Maestrini E, Korge BP, Ocaña-Sierra J, Calzolari E, Cambiaghi S, Scudder PM, Hovnanian A, Monaco AP, Munro CS. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum. Mol. Genet. 1999;8:1237–1243. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- 92.Yuan Y, Huang D, Yu F, Zhu X, Kang D, Yuan H, Han D, Dai P. A de novo GJB2 (connexin 26) mutation, R75W, in a Chinese pedigree with hearing loss and palmoplantar keratoderma. Am. J. Med. Genet. A. 2009;149A:689–692. doi: 10.1002/ajmg.a.32461. [DOI] [PubMed] [Google Scholar]
- 93.Lee JY, In SI, Kim HJ, Jeong SY, Choung YH, Kim YC. Hereditary palmoplantar keratoderma and deafness resulting from genetic mutation of Connexin 26. J. Korean Med. Sci. 2010;25:1539–1542. doi: 10.3346/jkms.2010.25.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koppelhus U, Tranebjaerg L, Esberg G, Ramsing M, Lodahl M, Rendtorff ND, Olesen HV, Sommerlund M. A novel mutation in the connexin 26 gene (GJB2) in a child with clinical and histological features of keratitis-ichthyosis-deafness (KID) syndrome. Clin. Exp. Dermatol. 2011;36:142–148. doi: 10.1111/j.1365-2230.2010.03936.x. [DOI] [PubMed] [Google Scholar]
- 95.Brown CW, Levy ML, Flaitz CM, Reid BS, Manolidis S, Hebert AA, Bender MM, Heilstedt HA, Plunkett KS, Fang P, Roa BB, Chung P, Tang HY, Richard G, Alford RL. A novel GJB2 (connexin 26) mutation, F142L, in a patient with unusual mucocutaneous findings and deafness. J. Invest. Dermatol. 2003;121:1221–1223. doi: 10.1046/j.1523-1747.2003.12550_4.x. [DOI] [PubMed] [Google Scholar]
- 96.Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. Cell. Physiol. 2007;293:C337–345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 97.Sánchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 2010;136:47–62. doi: 10.1085/jgp.201010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas T, Aasen T, Hodgins M, Laird DW. Transport and function of cx26 mutants involved in skin and deafness disorders. Cell Commun. Adhes. 2003;10:353–358. doi: 10.1080/cac.10.4-6.353.358. [DOI] [PubMed] [Google Scholar]
- 99.Yum SW, Zhang J, Scherer SS. Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30. Neurobiol. Dis. 2010;38:226–236. doi: 10.1016/j.nbd.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas T, Telford D, Laird DW. Functional domain mapping and selective trans-dominant effects exhibited by Cx26 disease-causing mutations. J. Biol. Chem. 2004;279:19157–19168. doi: 10.1074/jbc.M314117200. [DOI] [PubMed] [Google Scholar]