Abstract
A 60-year-old female was admitted with epigastric pain lasting a month. Preoperative diagnosis was choledochal cyst with anomalous pancreaticobiliaryductal union (APBDU), C-P type. A papillary mass measuring 2.5 × 1.9 cm was found adjacent to the pancreaticocholedochal junction. Gallbladder (GB) cancer was also observed. Pyloric-preserving pancreaticoduodenectomy (PPPD) was performed. The patient received adjuvant chemotherapy/radiation therapy on the tumor bed. The gallbladder cancer showed serosal invasion, while the bile duct cancer extended into the pancreas. Although common bile duct (CBD) cancer lesion showed focally positive for p53 and the gallbladder cancer lesion showed negative for p53, the Ki-67 labeling index of the CBD cancer and GB cancer were about 10% and 30%, respectively. Nine months after curative resection, a stricture on the subhepatic colon developed due to adjuvant radiation therapy. Localized peritoneal seedings were incidentally found during a right hemicolectomy. The patient underwent chemotherapy and had no evidence of tumor recurrence for two years after PPPD.
Keywords: Choledochal cyst, Gallbladder neoplsms, Bile duct neoplasms, Synchronous multiple primary neoplasms
INTRODUCTION
In anomalous pancreaticobiliary ductal union (APBDU), the common channel is abnormally long and the connection between the choledochus and pancreatic duct is outside of the duodenal wall [1]. This congenital disorder is frequently associated with choledochal cysts, another congenital condition consisting of localized or diffused dilatation of the biliary tract [1]. Moreover, APBDU are prone to have benign as well as malignant complications including carcinoma of biliary tract [2].
Although several cases of single carcinoma in the biliary tract associated with APBDU have been reported, synchronous double cancer is very rare [3]. We describe here a case of a choledochal cyst combined with APBDU that was first detected in a patient in her sixties as a double primary cancer of the gallbladder and CBD.
CASE REPORT
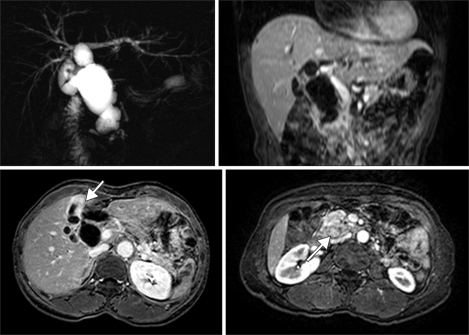
A 60-year-old female was admitted with a one month history of epigastric pain. Her medical records indicated she had taken medication for hypertension. Upon admittance, right upper quardrant pain were found and the other symptoms including fever and weight loss were absent. On abdominal physical examination, no palpable mass or tenderness was discovered. Laboratory results revealed a total bilirubin level of 1.2 mg/dL, alanine aminotransferase of 201 IU/L, aspartate aminotransferase of 318 IU/L, and alkaline phosphatase of 126 IU/L. The levels of tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9 were 6.69 ng/mL and 0.1 U/mL, respectively. Magnetic resonance imaging and magnetic resonance cholangiopancreatico-graphy identified a choledochal cyst with APBDU, choledocho-pancreatic (C-P) type. An irregular-shaped papillary mass measuring 2.5 × 1.9 cm was found adjacent to the pancreaticocholedochal junction with pancreatic invasion and the irregular wall thickening at the fundus and body of gallbladder was also seen. There was no lymph node enlargement or distant metastasis (Fig. 1).
Fig. 1.
Magnetic resonance cholangiopancreatography (MRCP) image including vertical and horizontal sections. MRCP shows fusiform cystic changes involving the entire common bile duct and hilar bile duct compatible with a choledochal cyst (type Ia) associated with the long common channel of the pancreaticobiliary junction (C-P type). Irregular wall thickening at the fundus and body of the gallbladder was seen. A 2.5 × 1.9 cm mass is also seen around the pancreaticobiliary junction.
Pylorus preserving pancreaticoduodenectomy (PPPD) was performed without additional hepatic resection. The proximal resection margin was at the level of the common hepatic duct bifurcation. Lymph node dissection was performed around the hepatoduodenal ligament, common hepatic artery, celiac axis, and paraaortic area.
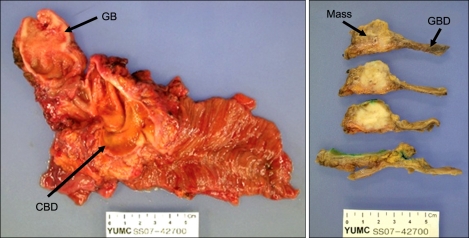
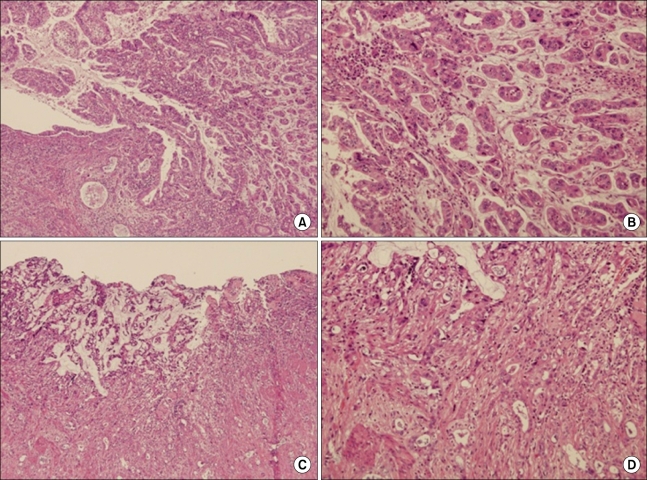
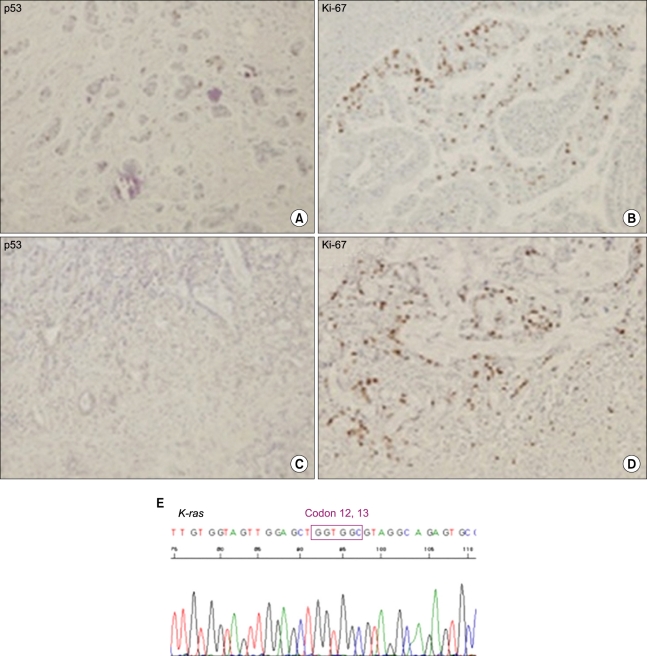
On pathologic evaluation, the CBD was markedly dilated, measuring 7 cm in inner circumference and 6.5 cm in length. Upon opening, we found an ill-defined yellowish tan solid mass abutting on the ampulla of Vater, measuring 2 × 1.5 × 1.5 cm. The gallbladder showed an ill-defined thickened wall area in the fundus, measuring 3 × 3 cm (Fig. 2). Microscopic examination revealed moderately-differentiated adenocarcinoma of the CBD with extension to the pancreatic head and moderately-differentiated adenocarcinoma of the gallbladder with extension to the serosa (Fig. 3). Only one regional node was involved by the tumor out of a total of 27 resected lymph nodes. There is no K-ras mutation on codon 12 and 13 in both gallbladder and CBD cancer lesions. In immunohistochemical staining with p53 and Ki-67, although CBD cancer lesion showed focally positive for p53 and the gallbladder (GB) cancer lesion showed negative for p53, the Ki-67 labeling index of the CBD cancer and GB cancer were about 10% and 30%, respectively (Fig. 4)
Fig. 2.
Specimens from patient before and after fixation. The common bile duct (CBD) is markedly dilated, measuring 7 cm in inner circumference and 6.5 cm in length. Opening along the CBD revealed an ill-defined mass measuring 2 × 1.5 cm. The gallbladder shows an ill-defined thickened wall area in the fundus measuring 3 × 3 cm. GB, gallbladder.
Fig. 3.
Hematoxylin & Eosin stain image of tumor. Tumor shows polypoid growth into the common bile duct (CBD) lumen with pancreatic invasion. (H&E; A, ×40; B, ×100). Adenocarcinoma of the gallbladder invaded fibromuscular connective tissue (H&E; C, ×40; D, ×100).
Fig. 4.
Immunostaining of tumor. p53 and Ki-67 immunostaining (×100; A, B, common bile duct cancer; C, D, gallbladder cancer) and K-ras mutation analysis (E).
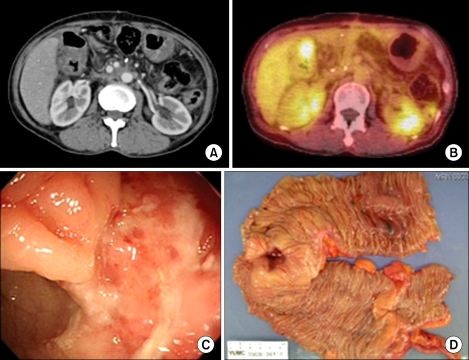
After an uneventful postoperative course, the patient received adjuvant chemotherapy with cisplatin, tegafur-uracil (UFT), and radiation therapy on the tumor bed of the gallbladder with 5,040 cGy. Nine months after curative resection, a stricture developed on the subhepatic colon due to adjuvant radiation therapy (Fig. 5). Localized peritoneal seedings were incidentally found during right hemicolectomy. The patient underwent chemotherapywith 5FU, Leucovorin and oxaliplatin 6 times. There is no evidence of tumor recurrence for 29 months after Rt. hemicolectomy with chemotherapy.
Fig. 5.
Images of computed tomography (CT), positron emission tomography (PET), colonoscopy and gross specimen for a complication caused by adjuvant radiation therapy. A stricture is shown on the subhepatic colon. (A, CT; B, PET; C, colonoscopy; D, gross specimen).
DISCUSSION
The mode of anomalous union is classified into two types: the pancreatico-choledochal (P-C) type, in which the main pancreatic duct enters the common bile duct, and the C-P type, where the CBD enters the main pancreatic duct [1]. The incidence of P-C type ductal junctions is higher in patients with GB cancer [4], while the incidence of C-P type junctions is higher in patients with carcinoma in congenital cystic dilatation [5]. In our experience, seven out of 10 patients with gallbladder cancer associated with APBDU have the P-C type [6].
APBDU is frequently associated with choledochal cysts and the incidence of malignancy in choledochal cysts ranges from 2.5 to 26% [3]. The number of bile duct cancer cases exceeds the number of cases of gallbladder cancer in the dilated bile duct with APBDU. Gallbladder cancer frequently occurs, but bile duct cancer also arises, in APBDU not associated with dilatation [7]. According to recent 10-year data from the Japanese Study Group on Pancreaticobiliary Maljunction, however, the rate of gallbladder cancer is higher than bile duct cancer with biliary dilatation [3]. The incidence of gallbladder cancer associated with APBDU is reported to range from 16.7 to 18.3% [4].
The gallbladder experiences conditions with significantly greater malignant potential in patients with APBDU, especially in cases not associated with choledochal cysts. Neoplastic development in the bile duct of patients with APBDU evolves through a multistep process associated with hyperproliferation and genetic alterations [3]. In our case, the K-ras mutation was not observed at any site of cancer. However, for epithelial cells in both cancerous areas with positive Ki-67, it might have caused another signal regulation apart from K-ras gene.
Early resection of the cyst wall and bile duct with reconstruction of the biliary ducts is been recommended. To prevent biliary tract cancers, APBDU with cystic dilatations should be excised during childhood. It is usually a surgical disease of infancy or childhood, but 20 to 30% of patients are first diagnosed in adulthood due to incidental findings during medical check-ups or late onset of symptoms [2]. In the present case, the patient had a choledochal cyst and APBDU without symptoms until her 60s. Although she was symptom-free, the congenital anomalies of this patient eventually developed into biliary tract cancers of the gallbladder and CBD, both compatible with stage IIIB according to the 7th American Joint Committee on Cancer tumor-node-metastasis classification system. Radical cholecystectomy, removal of the GB with en-bloc subsegmental resection of the adjacent hepatic parenchyma of segments IVb and V plus a regional lymphadenectomy, is suggested for T2 or more advanced GB cancer [8]. Surgeons who prefer aggressive operation often perform PPPD plus hepatectomy for GB cancer, and the hospital mortalities of these cases were higher (10 to 15%) than those (2 to 5%) of PPPD or major hepatectomy [8]. Although the role of radiation therapy in patients with biliary malignancies remains a debate, favorable survivals have been reported in postoperative adjuvant radiation therapy for GB cancer with lymph node invasion [9]. Because simple cholecystectomy with external radiotherapy can be recommended as an alternative treatments, only cholecystectomy with adjuvant chemoradiation therapy performed for GB cancer in current report [10].
In conclusion, choledochal cysts and APBDU are closely associated biliary tract anomalies. The malignant potential of these disease entities usually requires early surgical intervention and life-long follow-up.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.The Japanese Study Group on Pancreaticobiliary Maljunction (JSPBM) The Committee of JSPBM for Diagnostic Criteria. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219–221. [Google Scholar]
- 2.Yamaguchi M. Congenital choledochal cyst. Analysis of 1,433 patients in the Japanese literature. Am J Surg. 1980;140:653–657. doi: 10.1016/0002-9610(80)90051-3. [DOI] [PubMed] [Google Scholar]
- 3.Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;394:159–169. doi: 10.1007/s00423-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 4.Sandoh N, Shirai Y, Hatakeyama K. Incidence of anomalous union of the pancreaticobiliary ductal system in biliary cancer. Hepatogastroenterology. 1997;44:1580–1583. [PubMed] [Google Scholar]
- 5.Jan YY, Chen HM, Chen MF. Malignancy in choledochal cysts. Hepatogastroenterology. 2002;49:100–103. [PubMed] [Google Scholar]
- 6.Kang CM, Kim KS, Choi JS, Lee WJ, Kim BR. Gallbladder carcinoma associated with anomalous pancreaticobiliary duct junction. Can J Gastroenterol. 2007;21:383–387. doi: 10.1155/2007/383949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, et al. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345–351. doi: 10.1007/s00534-002-0741-7. [DOI] [PubMed] [Google Scholar]
- 8.Pitt HA. Gallbladder cancer: what is an aggressive approach? Ann Surg. 2005;241:395–396. doi: 10.1097/01.sla.0000154119.55201.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho SY, Kim SH, Park SJ, Han SS, Kim YK, Lee KW, et al. Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol. 2010;102:87–93. doi: 10.1002/jso.21544. [DOI] [PubMed] [Google Scholar]
- 10.Mondragón-Sánchez R, González-Geroniz M, Oñate-Ocaña LF, Garduño-López AL, Mondragón-Sánchez A, Bernal-Maldonado R, et al. A retrospective analysis of patients with gallbladder cancer treated with radical resection versus cholecystectomy plus external radiotherapy. Hepatogastroenterology. 2003;50:1806–1810. [PubMed] [Google Scholar]