Abstract
Thymoma-associated multi-organ autoimmunity is a rare, autoimmune disease that causes colitis, liver dysfunction and cutaneous graft-versus-host (GVH)-like skin damage. This paraneoplastic autoimmune disorder may be due to inadequate T cell selection in the tumour environment of the thymus. Although sporadic case reports have revealed its clinical features, little is known about its pathological mechanism. By comparing the skin-infiltrating T cell subsets with those of GVH disease (GVHD) and other inflammatory skin diseases, we sought to elucidate the pathological mechanism of thymoma-associated multi-organ autoimmunity. Histopathological and immunohistochemical analysis of skin biopsies was performed for three patients with thymoma-associated multi-organ autoimmunity. Histopathological findings of thymoma-associated multi-organ autoimmunity were indistinguishable from those of patients with acute GVHD, although the aetiologies of these diseases are completely different. The frequency of regulatory T cells (Tregs) is reduced in cutaneous lesions and CD8+ cytotoxic T lymphocytes that massively infiltrate into the epidermis of patients with thymoma-associated multi-organ autoimmunity. Additionally, the ratio of T helper type 17 (Th17) cells to CD4+ cells in patients with thymoma-associated multi-organ autoimmunity and acute GVHD was higher than that in healthy controls, but similar to that in psoriasis vulgaris patients. Similarity of the skin-infiltrating T cell subsets with those of acute GVHD suggested that skin damage in patients with thymoma-associated multi-organ autoimmunity might be induced by self-reactive cytotoxic T lymphocytes under the diminished suppressive capacity of Tregs.
Keywords: autoimmunity, graft-versus-host disease (GVHD), regulatory T cells, thymus, Th17
Introduction
Thymoma-associated multi-organ autoimmunity, a rare autoimmune disorder, is known to cause colitis, liver dysfunction and cutaneous graft-versus-host (GVH)-like skin damage [1–9]. Although sporadic case reports have revealed its clinical features, little is known about its pathological mechanism.
As reported previously, thymoma patients occasionally develop paraneoplastic autoimmunity, such as myasthenia gravis (MG), pure red cell aplasia and acquired hypogammaglobulinaemia [10,11], probably because of abnormal T cell maturation within the tumour environment.
GVH disease (GVHD) is caused by the activation of donor T cells that recognize recipient antigens in normal tissues and show clonal expansion after haematopoietic cell transplantation [12]. In normal individuals, peripheral tolerance is maintained by regulatory T cells (Tregs), even if self-reactive T cells escape negative selection in the thymus. The development of CD4+CD25+ Tregs depends on the forkhead box P3 transcription factor (FoxP3), which is a specific marker for Tregs[13,14]. Recent studies have revealed that the number of Tregs is reduced in allografts, peripheral blood and the skin lesions of recipients of transplants with acute or chronic GVHD [15–18]. More recently, it was demonstrated that increased numbers of interleukin (IL)-17-producing CD4+[T helper type 17 (Th17)] cells in the peripheral blood correlate strongly with inflammatory processes and the clinical status of acute GVHD (aGVHD) and active, chronic GVHD [19].
Here, we demonstrate that the frequency of Tregs is reduced in the cutaneous lesions of patients with thymoma-associated multi-organ autoimmunity compared with healthy individuals or individuals with other inflammatory skin diseases. Similar to aGVHD, dominant CD8+ cytotoxic T lymphocyte (CTL) infiltration in the epidermis suggests that skin damage in patients with thymoma-associated multi-organ autoimmunity might be induced by self-reactive CTLs under the diminished, suppressive capacity of Tregs.
Case reports
The clinical data of our three thymoma patients are summarized in Table 1).
Table 1.
Summary of patient characteristics
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Sex | Female | Female | Female |
| WHO classification of thymoma | B1 | B1 | B2 |
| Age of thymoma detection (year) | 23 | 31 | 39 |
| Duration from thymoma detection to GVH-like disease onset (year) | 18 | 5 | 3 |
| Damaged organ due to GVH reaction | |||
| Skin | + | + | + |
| Liver | + | + | − |
| Intestine | + | − | − |
| Complications | MG | MG | MG, SLE |
| Relapse of thymoma | + | + | + |
| Course of skin lesion | Relapse | Relapse | Better |
| Prognosis | Died after 5 months | Died after 3 years | Exacerbating thymoma |
GVH, graft-versus-host; MG, myasthenia gravis; SLE, systemic lupus erythematosus.
Case 1
We have reported previously the case of a 40-year-old Japanese woman presenting with psoriasiform erythroderma caused by thymoma-associated multi-organ autoimmunity [7], whereas others have reported similar cases [1–6,8,9]. This patient was diagnosed with MG associated with type-B1 thymoma, as defined by the World Health Organization (WHO) classification, at 23 years of age, and extended thymectomy followed by radiation therapy (RT) was performed [20]. After several years, tacrolimus was introduced for recurrent MG. At 37 years of age, ablative surgery and RT were performed for multiple disseminated tumours in the left pleural cavity. Two years later, multiple recurrent tumours appeared in the peritoneal cavity, which were resistant to chemotherapy. Subtotal resection of the peritoneal tumours with bilateral oophorectomy was performed. For mass reduction and relief of MG, steroid pulse therapy replaced treatment with tacrolimus. A few days after steroid pulse therapy was started, generalized erythema appeared. Generalized, psoriasiform erythematous patches were fused on the trunk, developing into generalized erythroderma (Fig. 1). Although high doses of oral steroids and cyclosporin were continued, the patient developed liver dysfunction and diarrhoea. A skin biopsy specimen was taken from an erythematous skin lesion of the left dorsal foot. Erythroderma gradually improved over 2 months after the initiation of high-dose oral steroid therapy, but reappeared with discontinuation of steroid therapy. Five months after the first appearance of erythroderma, the patient died of sepsis.
Fig. 1.
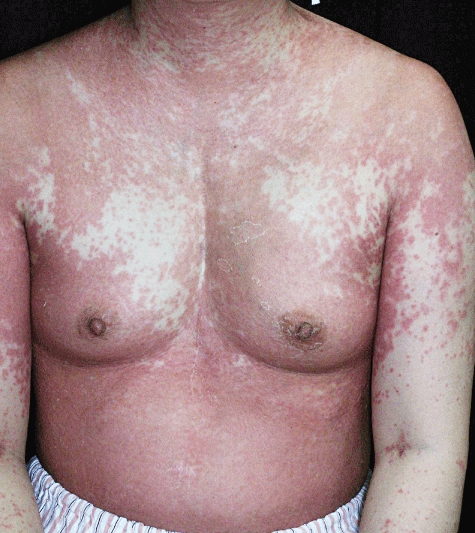
Clinical appearance of thymoma-associated multi-organ autoimmunity on the trunk of case 1. Scaly erythemas were fused on the chest.
Case 2
A 36-year-old Japanese woman presented with psoriasiform erythroderma after thymoma relapse (Fig. 2). Extended thymectomy was performed for type-B1 thymoma associated with MG when the patient was 31 years of age. Despite treatment with predonisolone (10 mg/day), tacrolimus and ambenonium, the thymoma reappeared. Generalized erythema was improved by higher-dose prednisolone (20 mg/day) and topical steroid treatments; however, skin lesions recurred after withdrawal of prednisolone administration. Generalized erythemas were fused and developed into erythroderma after systemic steroid withdrawal, accompanied by alopecia, pneumonia and liver dysfunction. A skin biopsy was performed on an erythematous area of the abdomen. Three years after the first appearance of erythroderma, the patient died of pneumonia.
Fig. 2.
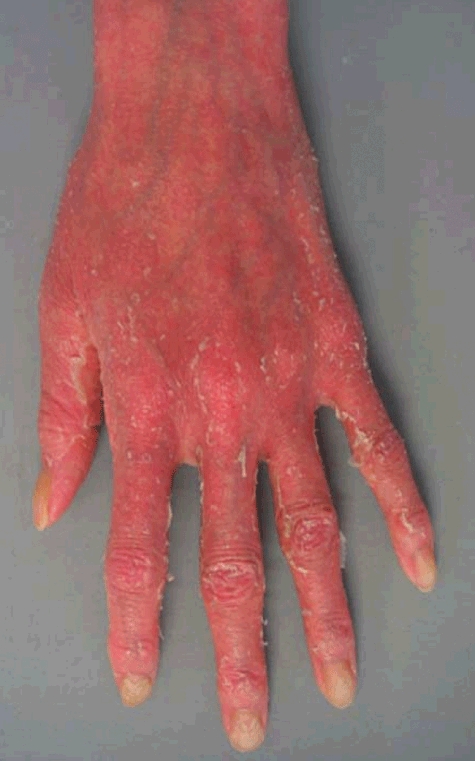
Clinical appearance of thymoma-associated multi-organ autoimmunity on the hand of case 2. Erythroderma lesions were seen on the entire body.
Case 3
A 42-year-old Japanese woman presented with scaling erythematous patches during the treatment of systemic lupus erythematosus with prednisolone (10 mg/day) since age 38 years. Thymoma was revealed by chest computed tomography, and needle biopsy showed type-B2 thymoma-infiltrating pleura. Subsequently, she developed eyelid ptosis and was diagnosed with MG. Chemotherapy was slightly effective in reducing the tumour size and subtotal resection was performed for thymoma removal, followed by chemotherapy and RT. Generalized psoriasiform erythroderma and oral erosions appeared during the RT course. A skin biopsy was taken from the involved area on the left upper arm (Fig. 3). Skin lesions disappeared with prednisolone (30 mg/day); however, oral aphthae recurred after withdrawal of systemic steroids. The patient developed pleural dissemination and thymoma metastasis to the lymph nodes and was started on a weekly docetaxel regimen. However, the adverse effect of glossitis was too severe to continue treatment.
Fig. 3.
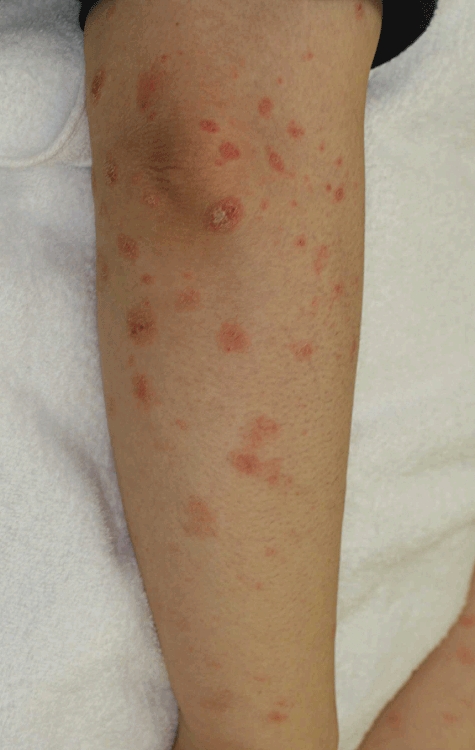
Clinical appearance of thymoma-associated multi-organ autoimmunity on the left upper arm of case 3. Multiple erythemas approximately 10 mm in diameter were seen on the entire body. Aphthas in the oral cavity were also observed.
Materials and methods
Samples and immunohistochemical analysis
Skin biopsy tissues were fixed with 10% formaldehyde, and paraffin-embedded sections were stained with haematoxylin and eosin and analysed by immunohistochemistry. Immunohistochemical staining was performed on skin sections from the three thymoma-associated multi-organ autoimmunity patients described in the case reports, three acute GVHD (aGVHD) patients, three lichen planus (LP) patients, three psoriasis vulgaris patients and three healthy controls. aGVHD, LP and psoriasis vulgaris were diagnosed on clinical appearance and histopathology. All aGVHD patients were treated with immunosuppressive therapy. All psoriasis vulgaris patients were treated only with topical steroids. No LP patients were treated. Because our thymoma-associated multi-organ autoimmunity patients and aGVHD patients were treated with immunosuppressive therapy, the effect of this medication on their immune condition cannot be excluded in this study. Three-micrometer-thick sections were stained with the following monoclonal antibodies (mAbs): anti-CD4 antibody (CD4 mAb, clone 1F6, dilution 1:25; Novocastra, Newcastle, UK); anti-CD8 mAb (CD8 mAb, clone C8/144B, dilution 1:100; DakoCytomation, Minneapolis, MN, USA); anti-CD1a mAb (CD1a mAb, clone 010, dilution 1:50; DakoCytomation); anti-FoxP3 mAb (FoxP3 mAb, clone 236A/E7, dilution 1:100; Abcam, Cambridge, UK); and anti-IL-17 antibody (polyclonal IL-17 antibody, dilution 1:150; R&D Systems, Minneapolis, MN, USA). Immunohistochemistry was performed as described previously [21,22]. For FoxP3 staining, Dako LSAB+/AP was used, whereas for other immunohistochemical staining, the Dako ChemMate Envision Kit/horseradish peroxidase (HRP) was used.
Quantification of the frequency of immunostained cells in the upper dermis was performed in single-stained serial sections. The number of FoxP3+ Tregs and IL-17+ Th17 cells was quantified (mean number/high power field calculated in three non-adjacent, high-power fields) and related to the number of CD4+ T lymphocytes (FoxP3+/CD4+ ratio and IL-17+/CD4+ ratio, respectively). The number of FoxP3+ Tregs was also related to the number of CD8+ T lymphocytes (i.e. FoxP3+/CD8+ ratio).
Results
Figure 4 shows the results from histopathological and immunohistochemical analyses of skin biopsies from three thymoma-associated multi-organ autoimmunity patients described in the case reports. As shown by haematoxylin and eosin staining, focal liquefaction degeneration of the basal epidermal layer, presence of superficial perivascular lymphocytes, infiltration with exocytosis and the presence of dyskeratotic keratinocytes (satellite cell necrosis) were found in all three cases. CD1a+ Langerhans cells disappeared completely from the epidermis, and CD8+ CTLs infiltrated massively into the epidermis (Fig. 4). These findings are consistent with the histopathological features of aGVHD (data not shown), as reported previously [23,24].
Fig. 4.
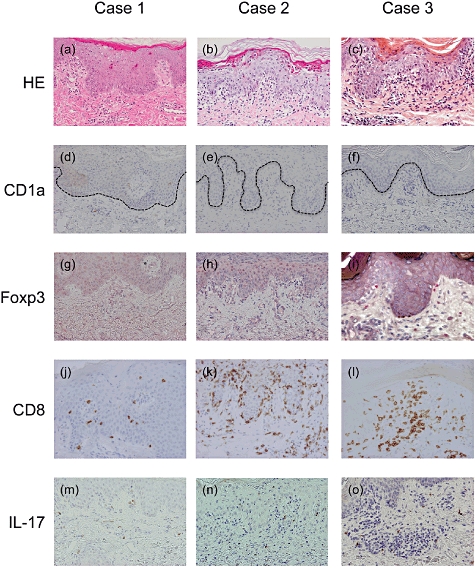
Haematoxylin and eosin staining revealed graft-versus-host (GVH)-like reactions in cases 1–3 (a–c). Immunohistochemical staining revealed the following: CD1a+ Langerhans cells disappearing from the epidermis (d–f); few forkhead box P3 (Fox P3)+ regulatory T cells (Tregs) expressed (g–i); CD8+ cytotoxic T lymphocytes infiltrating the epidermis (j–l); and presence of interkeukin (IL)-17+ cells in the dermis (m–o). Dotted lines represent the basal epidermal layer (original magnifications: ×100).
Because thymoma-associated multi-organ autoimmunity has many clinical and histopathological similarities with post-haematopoietic cell transplantation aGVHD, we further examined infiltrating T cell subsets in the skin for the presence of FoxP3+ Tregs. As reported previously, Tregs are found sparsely in the skin lesions of patients with aGVHD [18]. In this study, compared to healthy controls, the percentage of skin-infiltrating FoxP3+ Tregs per number of CD4 T cells decreased in patients with thymoma-associated multi-organ autoimmunity, whereas Tregs increased in LP and psoriasis vulgaris patients (Fig. 5a). The percentage of skin-infiltrating FoxP3+ Tregs per number of CD8+ T cells was also profoundly decreased in thymoma-associated multi-organ autoimmunity and aGVHD compared with psoriasis vulgaris (Fig. 5b).
Fig. 5.
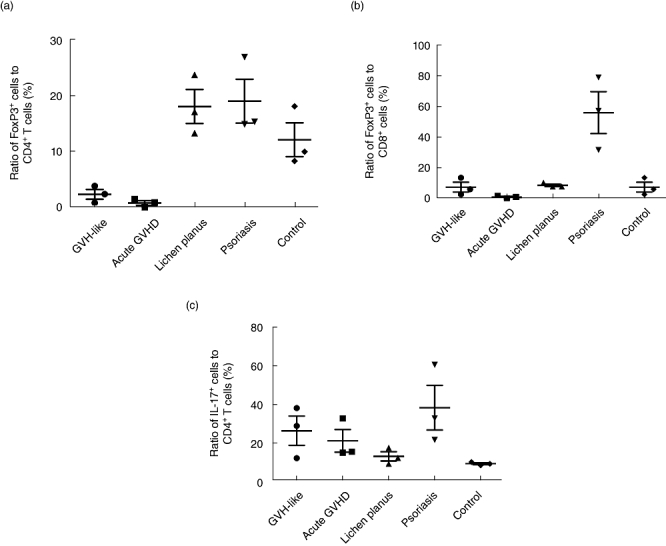
(a) In cases 1–3 and patients with acute graft-versus-host disease (aGVHD), the ratio of regulatory T cells (Tregs) to CD4+ T cells was reduced compared to that in healthy controls, while the ratio in lichen planus (LP) and psoriasis vulgaris patients increased. (b) In cases 1–3 and patients with aGVHD, the ratio of Tregs to CD8+ T cells was reduced compared to that in patients with psoriasis vulgaris. (c) In cases 1–3 and patients with aGVHD, the ratio of T helper type 17 (Th17) cells to CD4+ cells was increased as much as that seen in psoriasis vulgaris patients compared to that in either LP or healthy controls. Horizontal bars represent the mean value and the mean ± standard deviation of each group.
The scaling erythematous skin lesions seen in our three thymoma-associated multi-organ autoimmunity cases was clinically indistinguishable from patients with psoriasis vulgaris, although the histopathology was quite different between the two sets of patient materials (data not shown). Recent reports suggest that Th17 cells are key players in the induction of psoriatic skin lesions and putative targets for therapeutic intervention [19]. Therefore, we assessed skin-infiltrating Th17 cells. In thymoma-associated multi-organ autoimmunity and aGVHD patients, IL-17-producing cells infiltrated into the upper dermis, mainly into the perivascular regions. The ratio of Th17 cells per number of CD4+ cells in patients with thymoma-associated multi-organ autoimmunity and aGVHD was higher than that in LP or healthy controls, but similar to that in psoriasis vulgaris patients (Fig. 5c). Thus, skin-infiltrating T cell subsets are quite similar between patients with thymoma-associated multi-organ autoimmunity and those with aGVHD.
Discussion
In the normal thymus, immature T cells are positively selected by major histocompatibility complex peptides, depending on T cell receptor affinity [9,25,26]. Self-reactive T cells are usually depleted by medullary thymic epithelial cells. Central tolerance depends largely on the autoimmune regulator (Aire) gene, which controls the ectopic expression of a wide range of peripheral tissue-specific antigens in medullary thymic epithelial cells [27,28]. Recently, the complete lack of Aire and minimal expression of FoxP3 in intratumoural T cells were reported in patients with enterocolonopathy caused by thymoma-associated multi-organ autoimmunity [9], suggesting that self-reactive T cells, but not Tregs, might be preferentially differentiated in thymomas. In addition, self-reactive T cells might escape negative selection because professional antigen-presenting cells that ‘educate’ naive T cells in the normal thymic medulla are absent in thymoma [29]. This failure of central tolerance might cause autoimmune diseases in thymoma patients. In our study, sparse FoxP3+ Tregs in the dermis and massive CD8+ CTL infiltration in the epidermis were common features of both thymoma-associated multi-organ autoimmunity and aGVHD patients. Tregs are reduced in the skin lesions of patients with systemic sclerosis, which may be responsible for the loss of tolerance in the autoimmune skin diseases [30,31]. CD8+ CTLs are the major cellular effectors of aGVHD in either the Fas–Fas ligand or perforin/granzyme pathway [32]. We speculate that insufficient generation or skin recruitment of FoxP3+ Tregs might cause self-reactive CTL-induced cutaneous GVH-like reactions.
We found that the frequency of Th17 cells in the skin lesions of patients with thymoma-associated multi-organ autoimmunity was increased by showing an increased number of IL-17+ cells among the CD4+ population. Increased numbers of Th17 cells in the peripheral blood are correlated strongly with inflammatory processes in GVHD and have been shown previously [19]. The clinical appearances of our three cases were similar to those of psoriasis, another Th17-mediated dermatosis [33,34]. As seen in patients with aGVHD or psoriasis vulgaris, the ratio of IL-17+ cells to CD4+ T cells increased in patients with thymoma-associated multi-organ autoimmunity.
In conclusion, thymoma-associated multi-organ autoimmunity provides useful information for understanding the pathological differences and similarities between autoimmune skin diseases and GVH-like reactions, especially for the involvement of Tregs, CTLs and Th17 cells. To understand more about thymoma-associated autoimmunity, long-term observations of the T cell repertoire might be useful for monitoring effector and Tregs.
Disclosure
None.
References
- 1.Kornacki S, Hansen FC, III, Lazenby A. Graft-versus-host-like colitis associated with malignant thymoma. Am J Surg Pathol. 1995;19:224–8. doi: 10.1097/00000478-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Holder J, North J, Bourke J, et al. Thymoma-associated cutaneous graft-versus-host-like reaction. Clin Exp Dermatol. 1997;22:287–90. [PubMed] [Google Scholar]
- 3.Wang MH, Wong JM, Wang CY. Graft-versus-host disease-like syndrome in malignant thymoma. Scand J Gastroenterol. 2000;35:667–70. doi: 10.1080/003655200750023660. [DOI] [PubMed] [Google Scholar]
- 4.Sader C, Sharma S, Edwards MG. Graft-versus-host disease-type colitis: an unusual association of malignant thymoma. Ann Thorac Surg. 2002;73:1947–8. doi: 10.1016/s0003-4975(01)03505-6. [DOI] [PubMed] [Google Scholar]
- 5.Lowry PW, Myers JD, Geller A, Bostwick DG, Clain JE. Graft-versus-host-like colitis and malignant thymoma. Dig Dis Sci. 2002;47:1998–2001. doi: 10.1023/a:1019656425332. [DOI] [PubMed] [Google Scholar]
- 6.Sleijfer S, Kaptein A, Versteegh MI, Hegt VN, Snels DG, van Tilburg AJ. Full-blown graft-versus-host disease presenting with skin manifestations, jaundice and diarrhoea: an unusual paraneoplastic phenomenon of a thymoma. Eur J Gastroenterol Hepatol. 2003;15:565–9. doi: 10.1097/01.meg.0000059131.68845.65. [DOI] [PubMed] [Google Scholar]
- 7.Nakagiri T, Okumura M, Inoue M, et al. Thymoma-associated graft-versus-host disease-like erythroderma. J Thorac Oncol. 2007;2:1130–2. doi: 10.1097/JTO.0b013e31815ba23a. [DOI] [PubMed] [Google Scholar]
- 8.Wadhera A, Maverakis E, Mitsiades N, Lara PN, Fung MA, Lynch PJ. Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease. J Am Acad Dermatol. 2007;57:683–9. doi: 10.1016/j.jaad.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Offerhaus GJ, Schipper ME, Lazenby AJ, et al. Graft-versus-host-like disease complicating thymoma: lack of AIRE expression as a cause of non-hereditary autoimmunity? Immunol Lett. 2007;114:31–7. doi: 10.1016/j.imlet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56:12–16. doi: 10.1136/jcp.56.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanafusa T, Umegaki N, Yamaguchi Y, Katayama I. Good's syndrome (hypogammaglobulinemia with thymoma) presenting intractable opportunistic infections and hyperkeratotic lichen planus. J Dermatol. 2010;37:171–4. doi: 10.1111/j.1346-8138.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–93. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 16.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 17.Stanzani M, Martins SL, Saliba RM, et al. CD25 expression on donor CD4+ or CD8+ T cells is associated with an increased risk for graft-versus-host disease after HLA-identical stem cell transplantation in humans. Blood. 2004;103:1140–6. doi: 10.1182/blood-2003-06-2085. [DOI] [PubMed] [Google Scholar]
- 18.Fondi C, Nozzoli C, Benemei S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15:938–47. doi: 10.1016/j.bbmt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Dander E, Balduzzi A, Zappa G, et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009;88:1261–72. doi: 10.1097/TP.0b013e3181bc267e. [DOI] [PubMed] [Google Scholar]
- 20.Moran CA, Suster S. The World Health Organization (WHO) histologic classification of thymomas: a reanalysis. Curr Treat Options Oncol. 2008;9:288–99. doi: 10.1007/s11864-009-0084-6. [DOI] [PubMed] [Google Scholar]
- 21.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 22.Rensing-Ehl A, Gaus B, Bruckner-Tuderman L, Martin SF. Frequency, function and CLA expression of CD4+CD25+FOXP3+ regulatory T cells in bullous pemphigoid. Exp Dermatol. 2007;16:13–21. doi: 10.1111/j.1600-0625.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GF, Merot Y, Tong AK, Smith B, Mihm MC., Jr Depletion and repopulation of epidermal dendritic cells after allogeneic bone marrow transplantation in humans. J Invest Dermatol. 1985;84:210–14. doi: 10.1111/1523-1747.ep12265149. [DOI] [PubMed] [Google Scholar]
- 24.Perreault C, Pelletier M, Landry D, Gyger M. Study of Langerhans cells after allogeneic bone marrow transplantation. Blood. 1984;63:807–11. [PubMed] [Google Scholar]
- 25.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–82. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 26.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 27.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 29.Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg. 2008;56:143–50. doi: 10.1007/s11748-007-0185-8. [DOI] [PubMed] [Google Scholar]
- 30.Klein S, Kretz CC, Ruland V, et al. Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma. Ann Rheum Dis. 2010;70:1475–81. doi: 10.1136/ard.2009.116525. [DOI] [PubMed] [Google Scholar]
- 31.Antiga E, Quaglino P, Bellandi S, et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol. 2010;162:1056–63. doi: 10.1111/j.1365-2133.2010.09633.x. [DOI] [PubMed] [Google Scholar]
- 32.Maeda Y, Levy RB, Reddy P, et al. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood. 2005;105:2023–7. doi: 10.1182/blood-2004-08-3036. [DOI] [PubMed] [Google Scholar]
- 33.Asarch A, Barak O, Loo DS, Gottlieb AB. Th17 cells: a new paradigm for cutaneous inflammation. J Dermatol Treat. 2008;19:259–66. doi: 10.1080/09546630802206686. [DOI] [PubMed] [Google Scholar]
- 34.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]


