Abstract
Given the ability of erythrocytes to bind immune complexes (ICs), we postulated that they can serve a dual role during inflammatory or infectious processes. Erythrocytes could restrict stimulation of macrophages by free ICs by binding C3b-opsonized ICs via their complement receptor 1 (CR1). Conversely, IC-loaded erythrocytes could stimulate macrophages to produce proinflammatory cytokines such as tumour necrosis factor (TNF)-α. To test our hypothesis we selected 72 individuals with low, medium or high red cell CR1 expression and determined their IC binding capacity. We tested the in vitro ability of red cells to inhibit IC-mediated stimulation of TNF-α production by macrophages or to stimulate TNF-α production when loaded with ICs. Plain erythrocytes inhibited IC-induced TNF-α production by macrophages and low CR1 expressors showed the lowest inhibitory capacity. IC-loaded erythrocytes stimulated macrophages to release TNF-α, but the effect was not proportional to the CR1 level. These data support our hypothesis that erythrocytes can serve a dual role in regulation of cytokine responses in a setting of IC formation. Our findings suggest that individuals with low CR1 expression are ill-equipped to clear ICs and prevent IC-mediated stimulation of macrophages. In addition, IC-loaded red cells in areas of sluggish circulation such as in the spleen or in brain capillaries blocked by sequestered malaria-infected red cells may induce inflammation by stimulating monocytes and macrophages, the latter leading to the development of cerebral malaria.
Keywords: complement, CR1, erythrocyte, immune complexes, macrophage, malaria, TNF-α
Introduction
Complement receptor type 1 (CR1/CD35) is a complement regulatory protein found on primate red cells [1] and most leucocytes [2]. It functions as a co-factor in the factor I-mediated cleavage of C3b to C3bi and C3dg [3,4]. Although red cells have relatively few copies of CR1 (average 600) [5] compared to an average of 5000 on white cells [6], due to the fact that they are the most numerous cells in the bloodstream, they account for most of the CR1 mass in the body. Red cells, by virtue of their CR1, bind C3b-opsonized ICs which are removed by macrophages during passage through the liver and spleen [1,7]. ICs are formed when antibodies encounter their target antigens in the circulation. These antigens can be derived from infectious agents or from self, the latter as a result of autoimmune disorders. In either case, if not removed from circulation, ICs can deposit in tissues creating an inflammatory response by stimulating macrophages to release proinflammatory cytokines [8–11]. Conversely, IC-loaded red cells have been reported to interact with macrophages leading to production of the pro-inflammatory cytokine interleukin (IL)-1 [12].
The level of expression of CR1 on red cells is influenced by a variety of factors. There are known quantitative polymorphisms (H and L) that can result in low (LL), medium (HL) or high (HH) expression [5]. In addition, the level of CR1 is known to decline with the age of red cells [13,14] and can vary with the age of the host [15], as well as his/her health status [16]. For instance, individuals with certain conditions leading to formation of ICs such as malaria or systemic lupus erythematosus (SLE) tend to have lower CR1 on their red cells [15–19].
The variability in the level of red cell CR1 expression suggests that individuals at either end of the expression spectrum may suffer deleterious consequences of IC-mediated diseases. Low expressors may be less equipped to remove ICs from circulation, leading to IC deposition in tissues and the consequent inflammatory response. Conversely, high expressors may trap ICs on red cells too effectively which, under certain circumstances such as in the slow circulation of the spleen or in congested capillaries of malaria-infected individuals, may cross-link Fcγ receptors on monocyte/macrophages leading to production of proinflammatory cytokines [9–11,20].
To investigate the dual role of red cell CR1 on modulating the IC-mediated production of tumour necrosis factor (TNF)-α by macrophages and how this is affected by the CR1 expression level, we selected individuals with low, medium and high red cell CR1 expression. We then measured the ability of their red cells to enhance or inhibit TNF-α production by macrophages in vitro in the presence ICs.
Materials and methods
Study population
This study was part of a larger cross-sectional survey to study the relationship between red cell complement regulatory protein expression, age and C3b deposition [21]. It was approved by and executed in accordance with guidelines of the Human Use Research Committee of the Walter Reed Army Institute of Research and of the Kenya National Ethics Review Committee, Kenya Medical Research Institute. Informed consent was obtained from each participant or from the parent or guardian of participants under 18 years of age. The study was carried out in Kombewa Division, a malaria holoendemic region of the Lake Victoria basin in western Kenya, where most individuals are of the Luo ethnic group. The eligibility criteria and screening procedures were detailed previously [21]. Briefly, any person resident in the study area, male or female, aged 45 years or younger was eligible to participate in the study. Only healthy, malaria-negative individuals, as confirmed by a standardized physical examination and thick and thin Giemsa-stained blood smears, served as blood donors.
Blood collection and processing
Ethylenediamine tetraacetic acid (EDTA)-anti-coagulated blood was collected by venipuncture. Within 6 h of collection, the red cell pellet was washed in sterile phosphate-buffered saline (PBS) and the buffy coat was removed. The packed cell volume was aliquoted into several vials and cryopreserved in glycerolyte (Baxter, Deerfield, IL, USA), as described previously [22]. This method of storage is effective in preserving the level of red cell CR1 [23]. Upon thawing, the red cell pellet was washed twice and stored in Alsever's solution (114 mM dextrose, 27 mM sodium citrate, 71 mM sodium chloride, pH 6·1) at 4°C, usually within the same day. When repeat assays were required, additional aliquots were thawed.
Measurement of erythrocyte surface CR1
In preliminary experiments we observed no difference in the level of CR1 between fresh and thawed frozen samples. Red cell CR1 was measured using indirect fluorescent staining and flow cytometry. All procedures were as described previously [16].
IC preparation
The IC was prepared as described previously [23]. Rabbit anti-bovine serum albumin (BSA) and BSA (Sigma-Aldrich, St Louis, MO, USA) were made endotoxin-free by filtration through a polymyxin B column (Thermo Fisher Scientific, Inc., Waltham, MA, USA). In brief, 50 µl of 49 mg/ml rabbit anti-BSA and 3 µl of 5 mg/ml BSA were added to 950 µl of RPMI-1640 (Sigma-Aldrich).This was the point of equivalence for the antigen–antibody reaction, as determined by turbidometric assay. After 1 h incubation at 37°C, the IC was kept at 4°C overnight. The formed IC was then centrifuged at 7800 g for 10 min at 4°C and the supernatant discarded. The insoluble IC was washed three times by resuspending in sterile PBS. The protein concentration was determined by ultraviolet (UV) spectrophotometry of an aliquot solubilized in NaOH. The concentration of IC was adjusted to 700 µg/ml and the stock was stored at −70°C in 100 µl aliquots in endotoxin-free polypropylene tubes.
IC binding capacity
The IC used for IC binding capacity assays was prepared as described above, except for the use of fluorescein isothiocyanate (FITC)-labelled BSA (Accurate Chemical Corp., Westbury, NY, USA). The IC binding capacity was measured as described previously [24]. In brief, the anti-BSA : BSA-FITC IC was incubated with AB+ serum for 30 min at 37°C for opsonization. IC preparation to be used as unopsonized IC had 100 mM EDTA included in the cocktail. Opsonized and unopsonized ICs were added separately to wells containing 1 × 107 erythrocytes. The plate was covered with aluminium foil and incubated at 37°C for 30 min. The erythrocytes were washed thrice with ice-cold plain RPMI-1640. After aspiration of the supernatant, the erythrocytes were resuspended in 1% paraformaldehyde in PBS and stored at 4°C in the dark until flow cytometry performed within 24 h.
Prepararation of peripheral blood mononuclear cells (PBMCs)
A single healthy human immunodeficiency virus (HIV)-negative African adult was the source of macrophages for our experiments. Venous blood was drawn into heparinized vacutainers (Becton-Dickinson, San Diego, CA, USA). The whole blood was diluted 1:1 with sterile PBS, layered on a Histopaque cushion (Sigma-Aldrich) and centrifuged at 500 g for 30 min at room temperature. The interface between plasma and histopaque, corresponding to the PBMC fraction, was collected and washed four times with ice-cold PBS. The cells were suspended in plain RPMI-1640 media and assessed for viability using trypan blue exclusion. They were then plated at 1·5 × 105/well in 96-well flat-bottomed tissue culture plates (Costar, Cambridge, MA, USA) and incubated at 37°C in a 5% CO2 atmosphere for 1 h to allow the macrophages to adhere. The plate was then washed three times with sterile PBS to remove non-adherent cells.
Macrophage stimulation
All the reagents used were found to contain less than 0·01 EU/ml of endotoxin with the Limulus amoebocyte lysate (BioWhittaker Inc., Walkersville, MD, USA). To test the ability of erythrocytes to inhibit the IC-mediated stimulation of macrophages, ICs were added to 108 erythrocytes in 10% AB+ serum to a final concentration of 35 µg/ml in 150 µl and incubated at 37°C for 30 min. A separate set of negative control cells had either RPMI-1640 medium only or were incubated with 35 µg/ml purified rabbit IgG. The erythrocytes were then added to duplicate wells of a 96-well culture plate containing attached macrophages and 10 µg/ml of polymyxin B sulphate (Sigma-Aldrich). Positive control wells contained ICs without erythrocytes or LPS (Sigma-Aldrich) at a concentration of 7 µg/ml. To test the ability of IC-loaded red cells to stimulate macrophages, red cells were incubated with ICs as above, but following incubation they were washed three times with plain RPMI-1640 and added to duplicate wells containing macrophages as above. Negative control wells contained erythrocytes that were not loaded with ICs. To block IC-mediated stimulation of macrophages, some macrophage wells were pretreated for 30 min at 37°C with 20 µg/ml of endotoxin-free purified rabbit IgG Fc fragments (Jackson Immunoresearch, West Grove, PA, USA). The plates were incubated for 8 h at 37°C in a 5% CO2 atmosphere. At the end of the incubation, the supernatants were harvested and stored at −70°C.
TNF-α enzyme-linked immunosorbent assay (ELISA)
All incubations were performed at room temperature and all washes were performed at least three times. Immulon HB 96-well plates (Thermo Labsystems, Helsinki, Finland) were coated overnight with 6 µg/ml anti-TNF-α monoclonal antibody (Thermo Fisher Scientific). The wells were then blocked with 200 µl of blocking buffer (PBS, 1% Tween 20, 0·5% boiled casein) for 2 h and washed in wash buffer (PBS–0·05% Tween). One hundred µl of macrophage culture supernatant, diluted 1:1 in dilution buffer (0·025% Tween–0·5% boiled casein), was added to each well followed by a 2-h incubation. A standard curve was prepared by making serial dilutions of a known sample of human recombinant TNF-α (Thermo Fisher Scientific). The plates were washed again and incubated for 1 h with a 1:400 dilution of biotinylated rabbit anti-TNF-α (Thermo Fisher Scientific). After further washing, 100 µl/well of a 1:5000 dilution of horseradish peroxidase (HRP)-conjugated streptavidin (KPL Inc., Gaithersburg, MD, USA) was added. Following a 30-min incubation, the plates were washed and 100 µl/well of ABTS substrate [2,2′-azino-bis-(3-benzthiazoline-6-sulphonic acid)] (KPL) was added. Colour development was stopped after 30 min by the addition of 50 µl/well of 1% sodium dodecyl sulphate (SDS) (Sigma-Aldrich). The light absorption at 415 nm was measured with a Bioassay HTS 7000 plate reader (PerkinElmer, Waltham, MA, USA).
Statistical analysis
Data analysis was perormed with spss version 11·5 (SPSS Inc., Chicago, IL, USA). Analysis of variance with Tukey's post-hoc test was used to detect differences in continuous variables across groups controlling for assay date. Pearson's correlation coefficient was used to study the relationship between numeric variables. The t-test or the non-parametric Mann–Whitney rank sum test were used to test for differences between the means of two groups. Differences were considered statistically significant if P < 0·05. All tests were two-tailed.
Results
Erythrocyte CR1 levels and IC binding capacity
Of 344 individuals recruited in the cross-sectional study we selected 72 individuals with either low (between 253–388 copies/red cell), medium (443–579 copies per red cell) or high (581–1125 copies per red cell) red cell CR1 expression (Fig. 1a). Because the red cell CR1 level determines the IC binding capacity, we measured this parameter in each individual. There was no significant difference in the IC binding capacity between low and medium CR1 expressors (Fig. 1b). However, the IC binding capacity correlated well with the CR1 level (Fig. 1c).
Fig. 1.
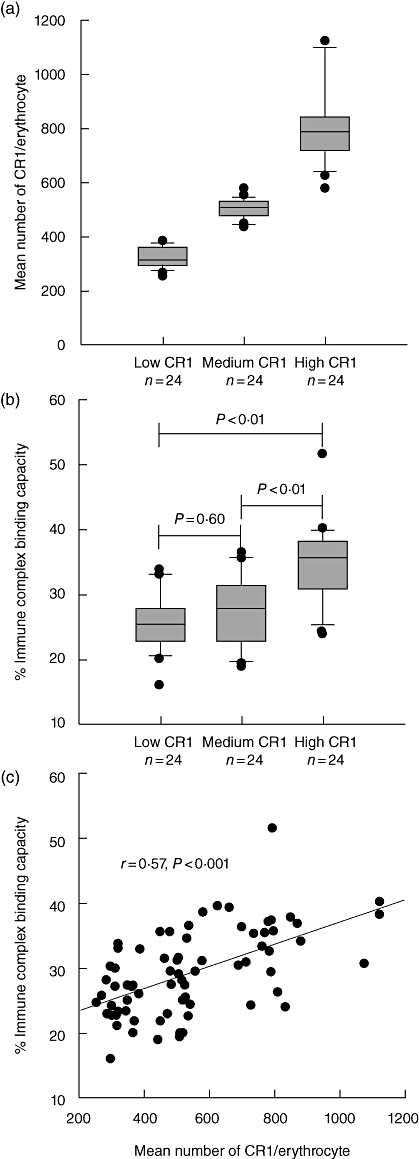
Erythrocyte complement receptor 1 (CR1) levels and immune complex (IC) binding capacity. (a) Erythrocyte CR1 levels in low, medium and high CR1 expressors. (b) IC binding capacity of erythrocytes from low, medium and high CR1 expressors. (c) Correlation between erythrocyte IC binding capacity and CR1 level. The box plots indicate the values between the 25th and 75th percentiles, a line within the box marks the median, and the whiskers indicate the 90th and 10th percentiles. The outliers are represented as dots.
Stimulation of macrophages with ICs
We confirmed that IC-dependent TNF-α production by macrophages is inhibited by Fc fragments, and therefore it is dependent on Fcγ receptors (Fig. 2a). We then set out to investigate whether binding of free opsonized ICs to erythrocytes leads to inhibition of the IC-mediated stimulation of macrophages and whether, conversely, IC-loaded erythrocytes can stimulate macrophages to release TNF-α. As can be seen in Fig. 2b, incubation of red cells with opsonized ICs inhibited the production of TNF-α by the macrophages (P < 0·001) and IC-loaded erythrocytes stimulated production of TNF-α compared to non-IC bearing erythrocytes (P < 0·001).
Fig. 2.
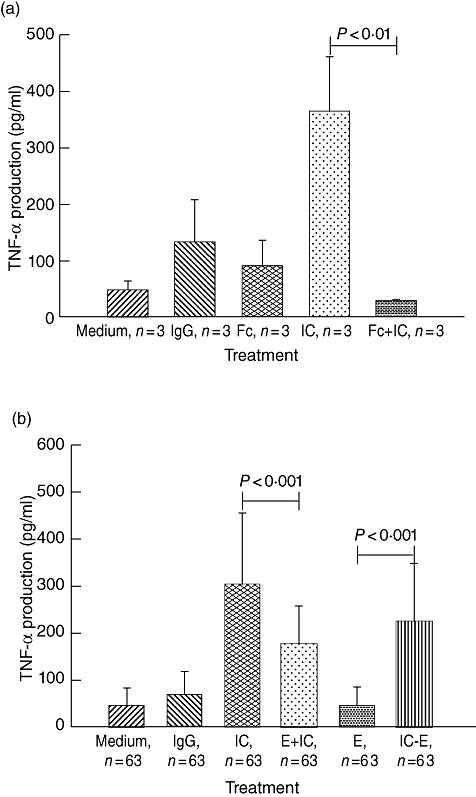
Stimulation and inhibition of tumour necrosis factor (TNF)-α production by macrophages. (a) Immune complex (IC)-mediated stimulation of TNF-α production by macrophages was inhibited by rabbit Fc fragments (Fc). The data represent mean ± standard deviation (s.d.) of duplicate wells from three different experiments. (b) Macrophages were stimulated with opsonized ICs, a cocktail of ICs and erythrocytes (E + ICs) and IC-loaded erythrocytes (lC-E). Negative controls included medium containing human AB+ serum from a healthy donor (medium), rabbit anti-human immunoglobulin (Ig)G and plain erythrocytes (E). The data represent mean ± s.d. of duplicate wells from 63 different individuals.
Influence of erythrocyte CR1 level on the stimulation of macrophages by ICs
To understand the influence of red cell CR1 expression level on their inhibitory and stimulatory capacity we analysed the above data by CR1 expression level. Medium and high CR1-expressing red cells were more effective at inhibiting the IC-mediated stimulation of macrophages than low CR1-expressing erythrocytes (Fig. 3a). However, there was no significant difference between medium and high CR1-expressing erythrocytes. We observed no significant difference in the ability of IC-loaded erythrocytes with different CR1 expression level to stimulate TNF-α production from macrophages (Fig. 3b).
Fig. 3.

Effect of erythrocyte complement receptor 1 (CR1) expression level on the inhibition and stimulation of macrophages. (a) Inhibition of immune complex (IC)-mediated stimulation of macrophages by erythrocytes with different CR1 expression levels. (b) Stimulation of tumour necrosis factor (TNF)-α production by macrophages with IC-loaded erythrocytes with different CR1 expression levels. Data are presented as box plots where the boundary of the box indicates the values between the 25th and 75th percentiles, a line within the box marks the median and the whiskers indicates the 90th and 10th percentiles. The outliers are represented as dots. The P-values for comparisons between the cohorts are based on analysis of variance using Tukey's post-hoc test.
Discussion
We set out to investigate whether CR1 present on the surface of erythrocytes could play a dual role in inhibiting and promoting the IC-induced production of TNF-α by macrophages. Our experiments showed clearly that this is possible. Red cells were able to inhibit the IC-mediated stimulation of macrophages. Conversely, IC-loaded red cells were able to stimulate TNF-α production by macrophages in the absence of free ICs. Although the pro-inflammatory [12] and anti-inflammatory potential [8] of erythrocytes have been recognized separately, in this study we highlight how the two can occur simultaneously, and explore their relationship to the CR1 level.
We hypothesized that the ability of erythrocytes to serve as inhibitors of IC-mediated production of TNF-α by macrophages varies with the level of CR1 expression. For this purpose we selected donors on the basis of their red cell CR1 expression as low, medium or high expressors. Because the IC binding capacity is the critical factor in determining the buffering capacity of the red cell, we also measured this parameter. Surprisingly, the IC binding capacity did not show a good relationship with the inhibitory capacity of red cells. We observed that medium and high expressor red cells were able to inhibit IC-mediated macrophage stimulation equally effectively, despite their having clearly different IC binding capacities. Conversely, low expressors inhibited less effectively than medium expressors, despite the two groups having a similar IC binding capacity. One possible explanation for these results is that both medium and high expressor red cells were capable of binding most of the free opsonized ICs available, despite having different IC binding capacities. Closer examination of Fig. 1b shows that medium expressors had a slightly higher IC binding capacity than low expressors. Therefore, it is likely that our assay for IC binding capacity lacked the sensitivity to detect differences in CR1-mediated IC binding at the lower end of the spectrum. Although the data did not show a straightforward relationship between the IC binding capacity and the inhibitory ability of the red cells, the CR1 level showed a better relationship with the medium and high expressors, being more effective inhibitors than the low expressors.
Lastly, we demonstrated that IC-loaded red cells are effective stimulators of TNF-α from macrophages. This is in agreement with a previous observation that IC-loaded red cells induce production of IL-1 when they interact with macrophages [12], although the mechanism was not clearly recognized at that time. Surprisingly, there was no difference in the stimulatory capacity in relation to the CR1 level of expression. One possible explanation is that even the lowest level of CR1 when saturated with ICs is able to maximally stimulate macrophages by cross-linking their Fcγ receptors.
Our findings have several important clinical implications. A number of infectious and autoimmune disorders such as malaria, SLE, hepatitis B and HIV are characterized by the production of ICs [16–18,25–28]. If not removed from circulation, these ICs can deposit in tissues or interact with phagocytes leading to activation of the inflammatory response [8,10,11]. Our data suggest that individuals with low erythrocyte CR1 are less equipped to mop up these ICs than individuals with high erythrocyte CR1 and are more likely to develop complications as a result. This is complicated further by the fact that individuals afflicted by some of these diseases develop low CR1 levels as a result of the infection [16,17,24]. In addition, we have reported that the level of CR1 can vary with age, and young children aged from 6 to 24 months have the lowest levels of CR1 [15,21]. This population is at greatest risk from complications due to Plasmodium falciparum infection [29]. Young children are known to produce more TNF-α during malaria infection than older children, regardless of the level of parasitaemia [30], and differential capacity to remove ICs during malaria infection may be one potential explanation.
We have provided evidence for a unique role of red cells in the stimulation of TNF-α production by presenting ICs and cross-linking Fcγ receptors on macrophages. This phenomenon may be important whenever slow circulation allows close contact between erythrocytes and monocyte/macrophages, such as in the liver and the spleen, leading to local production of proinflammatory cytokines. In the setting of P. falciparum malaria, this could also happen in capillaries of the brain and other tissues where infected erythrocytes tend to adhere to the endothelium and sequester, slowing down the circulation. This is the pathognomonic feature of cerebral malaria, one of the deadliest complications of this infection. In these capillaries, local production of TNF-α has been documented by immunohistochemistry [31]. We propose that presentation of ICs to monocytes/macrophages by red cells is one possible mechanism for the localized production of proinflammatory cytokines in sequestered capillaries. In addition, IC-loaded red cells in microhaemorrhages of patients with CM could stimulate microglial cells, resident macrophages that express Fcγ receptors [32]. Differential expression of CR1 on red cells is an appealing explanation for the increased susceptibility to cerebral malaria of older children compared to young children [16]. However, our data do not support that differences in CR1 expression level can lead to differences in the ability of red cells to stimulate macrophages.
In conclusion, we have demonstrated that erythrocytes can play a dual role in immune regulation, removing ICs from circulation to prevent inflammation and at the same time being capable of stimulating an inflammatory response by presenting ICs to macrophages. Our findings justify further exploration of the role of these mechanisms in the pathology of IC-mediated diseases such as malaria.
Acknowledgments
This work was supported by NIH grant HL71502 (Principle Investigator José A. Stoute). We are grateful to individuals who participated in the study. We acknowledge the staff of clinicians, nurses, drivers and fieldworkers whose efforts made the study possible.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte–immune complex-clearing mechanism. J Clin Invest. 1983;71:236–47. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss L, Fischer E, Haeffner-Cavaillon N, et al. The human C3b receptor (CR1) Adv Nephrol Necker Hosp. 1989;18:249–69. [PubMed] [Google Scholar]
- 3.Medof ME, Iida K, Mold C, Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982;156:1739–54. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JG, Wong WW, Schur PH, Fearon DT. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982;307:981–6. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- 6.Fearon DT, Collins LA. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983;130:370–5. [PubMed] [Google Scholar]
- 7.Davies KA, Hird V, Stewart S, et al. A study of in vivo immune complex formation and clearance in man. J Immunol. 1990;144:4613–20. [PubMed] [Google Scholar]
- 8.Beynon HL, Davies KA, Haskard DO, Walport MJ. Erythrocyte complement receptor type 1 and interactions between immune complexes, neutrophils, and endothelium. J Immunol. 1994;153:3160–7. [PubMed] [Google Scholar]
- 9.Debets JM, van der Linden CJ, Dieteren IE, Leeuwenberg JF, Buurman WA. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988;141:1197–201. [PubMed] [Google Scholar]
- 10.Jarvis JN, Wang W, Moore HT, Zhao L, Xu C. In vitro induction of proinflammatory cytokine secretion by juvenile rheumatoid arthritis synovial fluid immune complexes. Arthritis Rheum. 1997;40:2039–46. doi: 10.1002/art.1780401117. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian N, Berman JW, Casadevall A. Immune complexes increase nitric oxide production by interferon-gamma-stimulated murine macrophage-like J774.16 cells. J Leukoc Biol. 1995;57:657–62. doi: 10.1002/jlb.57.4.657. [DOI] [PubMed] [Google Scholar]
- 12.Chou YK, Sherwood T, Virella G. Erythrocyte-bound immune complexes trigger the release of interleukin-1 from human monocytes. Cell Immunol. 1985;91:308–14. doi: 10.1016/0008-8749(85)90054-1. [DOI] [PubMed] [Google Scholar]
- 13.Lutz HU, Stammler P, Fasler S, Ingold M, Fehr J. Density separation of human red blood cells on self forming Percoll gradients: correlation with cell age. Biochim Biophys Acta. 1992;1116:1–10. doi: 10.1016/0304-4165(92)90120-j. [DOI] [PubMed] [Google Scholar]
- 14.Ripoche J, Sim RB. Loss of complement receptor type 1 (CR1) on ageing of erythrocytes. Studies of proteolytic release of the receptor. Biochem J. 1986;235:815–21. doi: 10.1042/bj2350815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waitumbi JN, Donvito B, Kisserli A, Cohen JH, Stoute JA. Age-related changes in red blood cell complement regulatory proteins and susceptibility to severe malaria. J Infect Dis. 2004;190:1183–91. doi: 10.1086/423140. [DOI] [PubMed] [Google Scholar]
- 16.Ross GD, Yount WJ, Walport MJ, et al. Disease-associated loss of erythrocyte complement receptors (CR1, C3b receptors) in patients with systemic lupus erythematosus and other diseases involving autoantibodies and/or complement activation. J Immunol. 1985;135:2005–14. [PubMed] [Google Scholar]
- 17.Iida K, Mornaghi R, Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982;155:1427–38. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoute JA, Odindo AO, Owuor BO, Mibei EK, Opollo MO, Waitumbi JN. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J Infect Dis. 2003;187:522–5. doi: 10.1086/367712. [DOI] [PubMed] [Google Scholar]
- 19.Waitumbi JN, Opollo MO, Muga RO, Misore AO, Stoute JA. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood. 2000;95:1481–6. [PubMed] [Google Scholar]
- 20.Abrahams VM, Cambridge G, Lydyard PM, Edwards JC. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000;43:608–16. doi: 10.1002/1529-0131(200003)43:3<608::AID-ANR18>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Odhiambo CO, Otieno W, Adhiambo C, Odera MM, Stoute JA. Increased deposition of C3b on red cells with low CR1 and CD55 in a malaria-endemic region of western Kenya: implications for the development of severe anemia. BMC Med. 2008;6:23. doi: 10.1186/1741-7015-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Normark J. In: Freezing of patient isolates and strains with glycerolyte. Moll K, Ljungstrom I, Perlmann H, Scherf A, Wahlgren M, editors. Manassas: MR4/ATCC; 2008. Methods in malaria mesearch. [Google Scholar]
- 23.Cockburn IA, Donvito B, Cohen JH, Rowe JA. A simple method for accurate quantification of complement receptor 1 on erythrocytes preserved by fixing or freezing. J Immunol Methods. 2002;271:59–64. doi: 10.1016/s0022-1759(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 24.Owuor BO, Odhiambo CO, Otieno WO, Adhiambo C, Makawiti DW, Stoute JA. Reduced immune complex binding capacity and increased complement susceptibility of red cells from children with severe malaria-associated anemia. Mol Med. 2008;14:89–97. doi: 10.2119/2007-00093.Owuor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madi N, Steiger G, Estreicher J, Schifferli JA. Immune adherence and clearance of hepatitis B surface Ag/Ab complexes is abnormal in patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1991;85:373–8. doi: 10.1111/j.1365-2249.1991.tb05734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madi N, Steiger G, Estreicher J, Schifferli JA. Abnormal immune adherence and elimination of hepatitis B surface antigen/antibody complexes in patients with AIDS. J Immunol. 1992;148:723–8. [PubMed] [Google Scholar]
- 27.Mibei EK, Orago AS, Stoute JA. Immune complex levels in children with severe Plasmodium falciparum malaria. Am J Trop Med Hyg. 2005;72:593–9. [PubMed] [Google Scholar]
- 28.Mibei EK, Otieno WO, Orago AS, Stoute JA. Distinct pattern of class and subclass antibodies in immune complexes of children with cerebral malaria and severe malarial anaemia. Parasite Immunol. 2008;31:61–3. doi: 10.1111/j.1365-3024.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 29.Reyburn H, Mbatia R, Drakeley C, et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461–70. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 30.Nussenblatt V, Mukasa G, Metzger A, Ndeezi G, Garrett E, Semba RD. Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute, uncomplicated Plasmodium falciparum malaria. Clin Diagn Lab Immunol. 2001;8:1164–70. doi: 10.1128/CDLI.8.6.1164-1170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udomsangpetch R, Chivapat S, Viriyavejakul P, et al. Involvement of cytokines in the histopathology of cerebral malaria. Am J Trop Med Hyg. 1997;57:501–6. doi: 10.4269/ajtmh.1997.57.501. [DOI] [PubMed] [Google Scholar]
- 32.Komine-Kobayashi M, Chou N, Mochizuki H, Nakao A, Mizuno Y, Urabe T. Dual role of Fcgamma receptor in transient focal cerebral ischemia in mice. Stroke. 2004;35:958–63. doi: 10.1161/01.STR.0000120321.30916.8E. [DOI] [PubMed] [Google Scholar]


