Abstract
Cytokine and chemokine levels were studied in infants (<5 years) with uncomplicated (MM) and severe malaria tropica (SM), and in Plasmodium falciparum infection-free controls (NEG). Cytokine plasma levels of interleukin (IL)-10, IL-13, IL-31 and IL-33 were strongly elevated in MM and SM compared to NEG (P < 0·0001). Inversely, plasma concentrations of IL-27 were highest in NEG infants, lower in MM cases and lowest in those with SM (P < 0·0001, NEG compared to MM and SM). The levels of the chemokines macrophage inflammatory protein (MIP3)-α/C–C ligand 20 (CCL20), monokine induced by gamma interferon (MIG)/CXCL9 and CXCL16 were enhanced in those with MM and SM (P < 0·0001 compared to NEG), and MIP3-α/CCL20 and MIG/CXCL9 were correlated positively with parasite density, while that of IL-27 were correlated negatively. The levels of 6Ckine/CCL21 were similar in NEG, MM and SM. At 48–60 h post-anti-malaria treatment, the plasma concentrations of IL-10, IL-13, MIG/CXCL9, CXCL16 and MIP3-α/CCL20 were clearly diminished compared to before treatment, while IL-17F, IL-27, IL-31 and IL-33 remained unchanged. In summary, elevated levels of proinflammatory and regulatory cytokines and chemokines were generated in infants during and after acute malaria tropica. The proinflammatory type cytokines IL-31 and IL-33 were enhanced strongly while regulatory IL-27 was diminished in those with severe malaria. Similarly, MIP3-α/CCL20 and CXCL16, which may promote leucocyte migration into brain parenchyma, displayed increased levels, while CCL21, which mediates immune surveillance in central nervous system tissues, remained unchanged. The observed cytokine and chemokine production profiles and their dynamics may prove useful in evaluating either the progression or the regression of malarial disease.
Keywords: chemokine, cytokine, infant, infection, malaria, Plasmodium falciparum
Introduction
Cytokines and chemokines can act as central contributors to severe and life-threatening illness; in particular their excess production, also described as the cytokine syndrome [1], may contribute decisively to pathogenesis and the severity of malarial disease. Parallels exist between falciparum malaria and other severe illnesses such as sepsis and influenza, where inflammatory cytokines as well as chemokines are important mediators of pathogenesis [1,2]. Chemokines bridge innate and adaptive immunity [3], regulate chemotactic recruitment of inflammatory cells, leucocyte activation, angiogenesis and haematopoiesis, and in addition may also regulate host immune responses decisively during intracellular as well as intestinal protozoan parasite infections [4–8]. Recent studies have shown that the profile of chemokine expression and their serum levels varied with disease severity in children with acute Plasmodium falciparum malaria; notably, the beta-chemokines macrophage inflammatory protein (MIP)-1α/CCL3 and MIP-1β/CCL4 were elevated, while regulated upon activation normal T cell expressed and secreted (RANTES)/C–C ligand 5 (CCL5) appeared to be suppressed [9].
Resolution of P. falciparum infection requires proinflammatory immune responses that facilitate parasite clearance, while failure to regulate this inflammation leads to immune-mediated pathology, but the sequelae of disease aggravation or its resolution still require further study for a better understanding of pathogenesis as well as the prevention of malaria disease. The early production of proinflammatory T helper type 1 (Th1) cytokines, including tumour necrosis factor (TNF), interleukin (IL)-12 and possibly interferon (IFN)-γ may limit the progression from uncomplicated malaria to severe and life-threatening complications, but TNF can cause pathology if produced excessively [10–12].
Several studies support the idea that Th1 responses are important for clearance of P. falciparum malaria, and enhanced serum levels of IL-6 and IL-10 were observed in patients with severe P. falciparum malaria [13]. In young African children who presented with either mild or severe P. falciparum malaria, the acute-phase plasma IL-12 and IFN-alpha (IFN-α) levels, as well as the whole-blood production capacity of IL-12, were lower in children with severe rather than mild malaria, and IL-12 levels were correlated inversely with parasitaemia [14]. Further, TNF-α and IL-10 levels were significantly higher in those with severe malaria, being correlated positively with parasitaemia, and children with severe anaemia had the highest levels of TNF in serum [13]. The cytokine and chemokine imbalance measured in serum were suggested as useful markers for progression of cerebral malaria with fatal outcome; patients who died from malaria tropica had higher amounts of IL-6, IL-10 and TNF-α levels than those who survived; moreover, cerebral malaria (CM) was related to an inflammatory cascade characterized by dysregulation in the production of IP-10, IL-8, MIP-1β, platelet-derived growth factor (PDGF)-β, IL-1Rα, Fas-L, soluble TNF-receptor 1 (sTNF-R1) and sTNF-R2 [15].
This work addressed the levels of circulating proinflammatory and regulatory cytokines and chemokines in infants with acute P. falciparum infection, and our observations disclose clear differences associated with progression and regression of malaria tropica.
Materials and methods
Study population
This work was conducted at the Centre Hospitalier Regional (CHR) in Sokodé in the Central Region of Togo. The study was approved by the Comite de Bioethique pour la Recherche en Sante (CBRS) in Togo, and by the Ethikkommission at University Clinics of Tübingen, Germany. Informed written consent was obtained from all parents for the participation of their children in this study.
Infants of less than 5 years of age were recruited, and classification of malaria was performed according to previously published criteria [14], with severe malaria (SM) characterized by parasitaemia of higher than 250 000parasites/µl and/or the presence of severe anaemia with haemoglobin concentrations of lower than 5 g/dl. Matched uncomplicated malaria (MM) patients were defined by parasitaemia of lower than 250 000 parasites/µl and haemoglobin concentrations equal to or higher than 5 g/dl and the absence of any signs or symptoms of severe malaria [13]. P. falciparum-exposed infants negative for parasites in thick blood film, and negative in rapid detection test kits for P. falciparum (Paracheck-Pf, Orchid, Biomedical Systems, Goa, India; OptiMAL-IT; Biorad, Marnes la Coquette, France), were defined as participants with previous malaria episode(s) and the actual absence of illness due to malaria within the last 2 weeks. Blood samples were obtained prior to treatment with anti-malarials and/or anti-pyretics, and immediately following primary diagnosis all P. falciparum-positive infants received anti-malarial and appropriate supportive therapy as required and recommended by the Guidelines for Malaria Treatment indicated by the Ministry of Health in Togo. Infants with MM were treated with Coartem and Artemeter or Artesunate, and for SM, quinine perfusion or injectable Artemeter were applied as recommended. All hospitalized uncomplicated as well as severe malaria cases were followed until discharge from the hospital paediatric ward.
Chemokine and cytokine enzyme-linked immunosorbent assays (ELISAs)
Quantitative enzyme-linked immunosorbent assay (ELISA) was performed with commercially available assays to determine plasma levels of the cytokines IL-10, IL-13, IL-17F, IL-27, IL-31 and IL-33, as well as of the chemokines MIP3-α/CCL20, monokine induced by gamma interferon (MIG)/CXCL19, 6Ckine/CCL21 and CXCL16 (Duo-Set; R&D Minneapolis, MN, USA). Sample concentrations of each cytokine and chemokine were quantified from standard curves generated with recombinant chemokines/cytokines, and the lower limit for their detection was 50 pg/ml.
Statistical data analyses
For data analyses the statistical package jmp version 5·0.1·2 was used. For the cytokine and chemokine analyses, differences between groups were determined after logarithmic transformation to stabilize the variance of data [log (pg/ml + 1)]. The level of significance was adjusted according to Bonferroni–Holm (α = 0·0025). Paired data from patients were evaluated by t-test and unpaired data of patient groups were compared using Wilcoxon's rank sum test.
Results
Infant patient groups
A total of 392 infants 0·2–4·8 years of age were included in this investigation and Table 1 shows the characteristics of the infant patient groups; the endemic control group (NEG) were infants in whom P. falciparum was not detectable by means of thick blood smear and rapid antigen detection kits. The infant group with severe malaria (SM: >250 000 parasites/µl; <5 g/dl haemoglobulin) was significantly younger and had higher leucocyte counts than NEGs and uncomplicated malaria cases (MM: <250 000 parasites/µl; ≥5 g/dl), and in both malaria patient groups haemoglobin levels were significantly lower compared to the levels in NEG infants (P < 0·0001).
Table 1.
Characteristics of patient groups
| Group | n | Male/female | Age (years) (min/max) | mean HB (g/dl) (min/max) | Parasite density (Pf/µl blood) | Leucocytes (n/µl) (min/max) |
|---|---|---|---|---|---|---|
| NEG | 81 | 33/48 | 2·7 | 11·61 | 0 | 6 597 |
| (0·2/4·8) | (8·2/17·3) | (3 500/28 000) | ||||
| MM | 184 | 99/85 | 2·1* | 8·56** | 44 987 | 10 059** |
| (0·2/4·5) | (5/15·4) | (50/250 000) | (1 002/210 100) | |||
| SM | 127 | 70/57 | 1·8** | 4·22** | 165 187 | 18 020** |
| (0·1/4·5) | (1·4/10·8) | (50/2 100 000) | (1 100/121 000) |
Demographic data, leucocyte counts, haemoglobin concentrations and parasite densities in infant patient groups and controls. Infants with severe malaria (SM) were characterized by parasite densities >250 000parasites/µl blood (mean; min/max) and/or haemoglobin concentrations <5 g/dl (mean; min/max). Uncomplicated malaria (MM) patients had <250 000 parasites/µl and haemoglobin concentrations ≥5 g/dl and no signs or symptoms of severe malaria. Plasmodium falciparum-exposed infants negative for parasites in thick blood film, and negative in rapid detection test kits for P. falciparum were defined as participants (NEG) with previous malaria episode(s) and the actual absence of illness due to malaria within the last 2 weeks. Significant differences (*P = 0·0002; **P < 0·0001) between NEG and uncomplicated malaria (MM) or severe malaria (SM) patients are indicated. HB: haemoglobin.
Cytokine levels in infants with uncomplicated and severe malaria
Plasma levels of IL-10, IL-13, IL-17F, IL-27, IL-31 and IL-33 were quantified by specific ELISA in NEG, MM and SM infants (Fig. 1). In those negative for P. falciparum (NEG) the mean plasma IL-10 concentration was 120 pg/ml; with P. falciparum parasite presence it enhanced to 1030 pg/ml in MM and 1600 pg/ml in SM patients, significantly higher (for both P < 0·0001) when compared to NEG. The mean plasma concentrations of IL-13 were 230 pg/ml in MM and 380 pg/ml in SM. The mean levels of IL-17F were 2070 pg/ml, 3150 pg/ml and 2950 pg/ml in NEG, MM and SM infants, with differences (P = 0·007) between NEG and MM or SM groups, respectively. Plasma levels of IL-27 ranged between 1370 and 48 540 pg/ml, with mean concentrations greatly exceeding those of IL-10, IL-17F, IL-31 and IL-33 and, in contrast to the aforementioned measured cytokines, IL-27 concentrations were highest in NEG infants (23 320 pg/ml), lower in cases with uncomplicated malaria (MM: 15 530 pg/ml) and lowest in those children with severe malaria (SM: 10 850 pg/ml) (P < 0·0001, NEG compared to MM and SM). Mean levels of IL-31 and IL-33 in infants with MM were above those of the NEG group, and clearly higher (P < 0·0001) in SM infants compared to NEG. The concentrations of IL-31 were 1580 pg/ml in NEG, 2740 pg/ml in MM and 5940 pg/ml in SM. In all infant groups, IL-33 levels were considerably lower than those for IL-31, with IL-33 plasma concentrations at 90 pg/ml in parasite-free controls (NEG) which rose to 200 pg/ml in MM, reaching 310 pg/ml in SM cases (SM versus NEG; P < 0·0001).
Fig. 1.
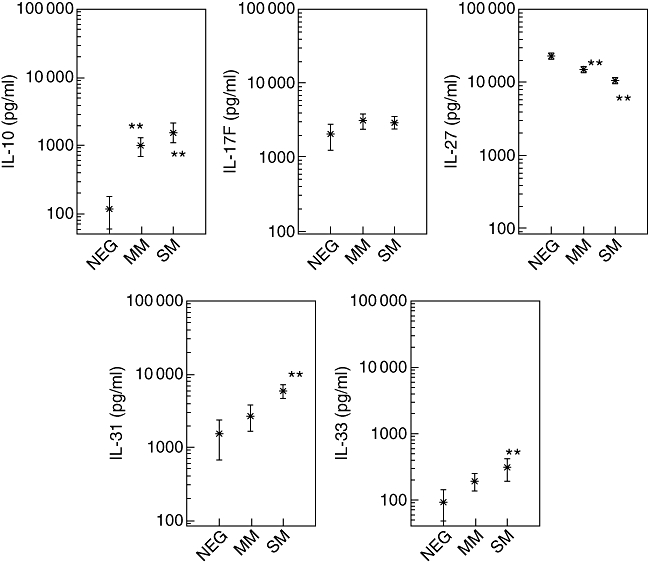
Plasma concentrations of cytokines interleukin (IL)-10, IL-17F, IL-27, IL-31 and IL-33 were quantified in Plasmodium falciparum-infected infants (<5 years) and in non-infected endemic controls (<5 years). Cytokine concentrations are shown as means in pg/ml with the 95% lower and upper confidence interval. Infants with severe malaria (SM) were characterized by parasitaemias of higher than 250 000 parasites/µl and/or haemoglobin concentrations of less than 5 g/dl. Uncomplicated malaria (MM) patients were defined by parasitaemias of lower than 250 000 parasites/µl and haemoglobin concentrations equal or higher than 5 g/dl and the absence of any signs or symptoms of severe malaria. P. falciparum-exposed infants negative for parasites in thick blood film, and negative in rapid detection test kits for P. falciparum were defined as participants with previous malaria episode(s) and the actual absence of illness due to malaria within the last 2 weeks. Significant differences (**P < 0·0001) between infection-free controls (NEG) and MM or SM patients are indicated. **P < 0·0001 compared to NEG.
Chemokine levels in infants with mild and severe malaria
Plasma levels of MIP3-α/CCL20, MIG/CXCL9, the lymphoid and homeostatic chemokine 6Ckine/CCL21 and the inflammation-associated chemokine CXCL16 were quantified in NEG, MM and SM infants (Fig. 2). Concentrations of CCL20, CXCL16 and CXCL19 were enhanced in those with P. falciparum, while CCL21 remained at around 320 ± 5 pg/ml in NEG, MM and SM infants. The mean levels of CCL20 were 90 pg/ml in NEG infants, and were significantly higher (P < 0·001) in MM (550 pg/ml) and SM (900 pg/ml), with no difference between the MM and SM groups. For MIG/CXCL9, the concentrations were 720 pg/ml in NEG, clearly enhanced (P < 0·0001) in MM (2180 pg/ml) and SM (3170 pg/ml) infants with no differences between the P. falciparum-infected groups.
Fig. 2.
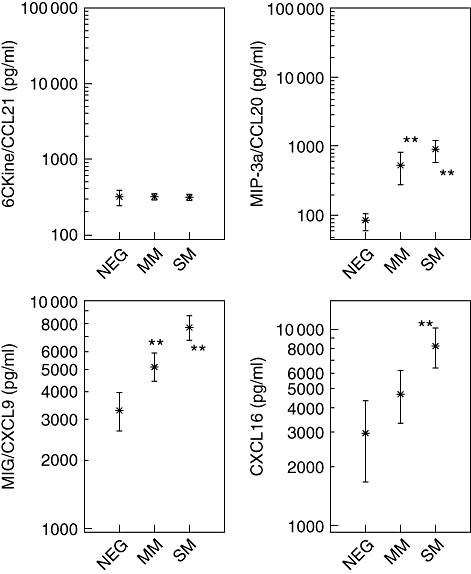
Plasma concentrations of chemokines 6Ckine/CCL21, macrophage inflammatory protein (MIP)3-α/C–C ligand 20 (CCL20), CXCL16 and MIG/CXCL9 were quantified in Plasmodium falciparum-infected infants (<5 years) and in non-infected endemic controls (<5 years). Chemokine concentrations are shown as means in pg/ml with the 95% lower and upper confidence interval. Infants with severe malaria (SM) were characterized by parasitaemias of higher than 250 000 parasites/µl and/or haemoglobin concentrations of less that 5 g/dl. Uncomplicated malaria (MM) patients were defined by parasitaemias of lower than 250 000 parasites/µl and haemoglobin concentrations equal or higher than 5 g/dl and the absence of any signs or symptoms of severe malaria. P. falciparum exposed infants negative for parasites in thick blood film, and negative in rapid detection test kits for P. falciparum were defined as participants with previous malaria episode(s) and the actual absence of illness due to malaria within the last 2 weeks. Significant differences (**P < 0·0001) between infection-free controls (NEG) and MM or SM patients are indicated.
Plasma concentrations of CXCL16 in NEG patients were 2930 pg/ml (mean) and the levels were enhanced in those with P. falciparum, to 5160 pg/ml in MM and 8840 pg/ml in SM cases. CXCL9 and CXCL16 levels were clearly higher (P < 0·0001) in SM than in NEG, and CXCL9 levels in SM were higher than those of MM patients (P < 0·0001).
Cytokine and chemokine changes post-anti-malarial treatment
At 48–60 h post-anti-malarial treatment (Fig. 3), significantly diminished cytokine concentrations were detected for IL-10, IL-13 and the chemokines MIG/CXCL9, CXCL16 and MIP-3α/CCL20 (not shown). The mean levels of IL-17F, IL-27, IL-31 and IL-33 did not change at 48–60 h post-anti-malaria treatment and with reduced parasitaemia.
Fig. 3.
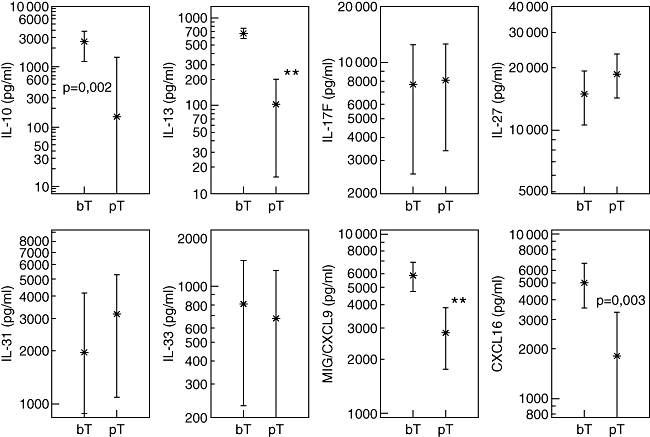
Plasma concentrations of cytokines interleukin (IL)-10, IL-13, IL-17F, IL-27, IL-31, IL-33 and chemokines monokine induced by gamma interferon (MIG)/C–X–C ligand 9 (CXCL9) and CXCL16 were quantified in Plasmodium falciparum-infected infants (<5 years) before treatment (bT) with anti-malarials and/or anti-pyretics and at 48–60 h post-treatment (pT). Chemokine concentrations are shown as means in pg/ml with the 95% lower and upper confidence interval. Significant differences (**P < 0·0001, paired observations) between bT and pT are indicated.
Cytokine and chemokine correlations
In P. falciparum-infected infants, the levels of MIP3-α/CCL20 (r2 = 0·28; P = 0·0002) and MIG/CXCL9 (r2 = 0·33, P = 0·0005) were correlated positively with parasite density, while IL-27 displayed a weak negative correlation (r2 = −0·17; P = 0·01).
Discussion
Naturally acquired protective immunity against malaria requires subclass-specific antibody responses [16–18], and the secretion of cytokines, chemokines and further immune mediators is essential for the regulation both of cellular effector mechanisms against P. falciparum blood-stage parasites and of organ-specific inflammation and pathogenesis [19,20]. In MM and SM infants substantial cytokine and chemokine levels were detected, which disclosed both innate and memory immune responsiveness. The first parasite encounter and sensitization to P. falciparum antigens may already occur prenatally and continue in infants shortly after birth [21]. P. falciparum infection during pregnancy is a major health problem in our study area [22,23], and prenatal and early life contact with plasmodial antigens has to be considered as a regularity. In infants, antibody responses and pronounced parasite-specific IL-10 production were found to be associated with faster P. falciparum parasite clearance [24], and the higher longevity of regulatory T cell (Treg)-type IL-10 compared to Th1-type IFN-γ responses [25] suggested that prenatal and early postnatal sensitization with P. falciparum antigens has occurred [26,27]. It is noteworthy that parasite-specific IL-10 responses were observed frequently and of high magnitude in umbilical cord blood cells from newborns of infected mothers [21–23,28]. In the present work, plasma IL-10 levels were not correlated with parasite densities or the infants' age, and this further supported early life P. falciparum-specific immune sensitization and IL-10 induction. The role of IL-10 in malaria pathogenesis is controversial. High IL-10 levels were associated with cerebral malaria [13], with high parasite density and severe disease in children [29,30], while lower plasma concentrations of IL-10 occurred in those with severe malarial anaemia [13,30]. IL-10 will modulate Th1-type responses to Plasmodium antigens by depressing proinflammatory TNF and also production of IFN-γ by dampening the release of IL-12p70 [14]; however, in response to P. falciparum infection, cytokine profiles and their relative balance, not single pro- and anti-inflammatory T helper and T regulatory cytokines, may mediate protective immunity and disease severity [31].
With regard to the regulatory type IL-10, the Th2-type anti-inflammatory cytokine IL-13 disclosed similar levels and dynamics; it was enhanced in MM and SM infants and declined rapidly with parasite clearance following treatment. In 1–4-year-old children with acute uncomplicated P. falciparum malaria, increased IL-13 levels were found [32], which decreased up to day 2 post-treatment. IL-13 provides protection from LPS-induced lethal endotoxaemia similar to but independent from IL-10, and IL-13 can be considered as an immune modulator which might be beneficial in the treatment of septic shock [33]. As revealed recently, IL-13 mediated phagocytosis of P. falciparum-parasitized erythrocytes by alternative activated monocytes [34], and resistance to severe malaria through altered IL-13 production may be associated with a single nucleotide polymorphism in the IL-13 promoter [35].
As a cytokine with dual regulatory capacity, IL-27 will first initiate Th1-type IFN-γ responses and promote IL-10 synthesis by regulatory T cells, then attenuate inflammatory Th2 and Th17 cells [36] and depress proinflammatory cytokines and chemokines [37]. IL-27R-deficient mice infected with Toxoplasma gondii, Trypanosoma cruzi or Leishmania donovani first controlled parasite replication, but then developed lethal proinflammatory cytokine responses and succumbed to infection [38], and such mice infected with the intestinal helminth Trichuris muris developed an increased production of Th2-associated cytokines and were able to clear intestinal worms very early [39]. IL-27R-deficient mice were susceptible to P. berghei infection and developed Th1-mediated immune responses which, despite efficient parasite clearance, led to severe liver pathology [40]. The regulatory function of IL-27 via the induction of IL-10 and suppression of IL-17 secretion may help to prevented early manifestations of malarial disease, but IL-27 alone may not suffice to prevent chronic infection and severe malaria.
The capacity of IL-27 in suppressing Th17-type responses may be critical for pathology prevention; IL-17F levels were similarly high in MM, SM and NEG infants, and the unchanged IL-17F levels post-parasite clearance suggested that IL-17F may not be implicated in malaria progression or regression. Enhanced levels of Th17-associated cytokines have been detected in psoriasis, arthritis, asthma and bacterial and fungal infections [41], and Th17 cells might breach the blood–brain barrier and infiltrate the central nervous system (CNS) parenchyma [42], thereby inducing the production of other proinflammatory cytokines and chemokines which will attract effector cells and provoke tissue inflammation. However, IL-17 was not found to be associated with development of cerebral malaria in P. berghei-infected mice [43], and in Ghanaian children cerebral malaria mortality was not associated with IL-17 [15].
While IL-17F levels were similar in NEG, MM and SM infants, the cytokine IL-31, which has comparable effects to IL-17 [44], was highest in SM patients. IL-17 and IL-31 both have additive effects on secretion of cytokines and chemokines [44,45], and IL-31, a member of the gp130/IL-6 cytokine family [45], may recruit polymorphonuclear cells, monocytes and T cells to an inflammatory site in vivo[46]. IL-31 will induce the genes of inflammatory chemokines MIP-1β, MIP-3α, MIP-3β[47,48] and proinflammatory cytokines IL-6, IL-8, IL-16 and IL-32 [44,45]. IL-31-receptor-deficiency in mice injected with Schistosoma mansoni eggs resulted in severe pulmonary inflammation, enlarged granuloma and significantly more IL-4, IL-5 and IL-13 than in wild-type mice [48]. In allergic asthma patients, serum levels of IL-31 were elevated above controls [49], a further suggestion that the IL-31/IL-31R signalling pathway will regulate type 2 inflammations [48]. Another key player promoting Th2 type responses, the cytokine IL-33, is considered a mediator of pathology with allergies and septic shock [50–52]; IL-33 was suggested to function as an alarmin [53], to alert after endothelial or epithelial cell damage during trauma, stress or infection [53]. IL-33 levels were enhanced in infants with MM and SM, clearly above NEG, correlated positively with parasite densities, and diminished strongly following parasite clearance. Sequestration of P. falciparum-infected erythrocytes or the release of merozoites may have amplified IL-33 production by endothelial cells, and additional cytokines augmented by IL-33 are IL-5, IL-13, TNF and IL-3 [54]. Furthermore, IL-33 will promote splenomegaly, blood eosinophilia and epithelial hyperplasia, massive mucus production in lungs and pulmonary inflammation [55]. To what extent the enhanced production of IL-31 and IL-33 may contribute to pathogenesis of acute P. falciparum infection to cerebral inflammation and vascular obstructions should be investigated further.
For the development of cerebral malaria, an important role has been attributed to cytokines and chemokines [56,57]. With severe P. falciparum infection an increased production of MCP-1/CCL2, MIP-1α and MIP-1β, and also IL-8/CXCL8, has been observed [9,13], and the mortality risk with cerebral malaria (CM) was associated independently with the serum concentration of IP-10/CXCL10 [15]. The chemokines IP-10/CXCL10 and MIG/CXCL9, together with their common receptor CXCR3, are required for the development of murine CM [58]. MIG/CXCL9 and its receptor are expressed predominantly in Th1 cells, and MIG/CXCL9 is considered to be a predictive marker for antigen-specific IFN-γ-secreting peripheral blood mononuclear cells (PBMCs) in volunteers immunized with irradiated P. falciparum sporozoites [59]. In the present work, MIG/CXCL9 levels were highest in SM infants and lessened rapidly with parasite clearance after anti-malarial therapy, suggesting that MIG/CXCL9 may be an indicator for parasite multiplication or diminution, and possibly also for the sequestration of P. falciparum-infected erythrocytes (Pf-IRBC) in blood vessels of the CNS. MIP-3α/CCL20 will stimulate the migration, homing and maturation of leucocytes, and CCL20 together with CXCL1, CXCL2, IL-6 and IL-8 increased more than 100-fold in blood–brain barrier endothelial cells during Pf-IRBC contact, which suggests its participation in cellular defence during Pf-IRBC sequestration [60]. Astrocytes which line parenchymal blood vessels will respond in a pathogen-specific way to infection and release MIP-3α/CCL20 and CXCL16 [61]; both chemokines will promote Th1-type responses by enhancing IFN-γ and TNF-α release, and CXCL16 may attract neutrophil granulocytes across the blood–brain barrier into the cerebrospinal fluid [62,63]. Both CCL20 and CXCL16 were elevated substantially in SM and MM infants; CCL20 correlated positively with parasite densities, and therefore CCL20 and CXCL16 should be investigated further as to what extent they contribute to the manifestation of CM. The chemokines 6Ckine/CCL21 and CCL19 are both involved in T lymphocyte migration into CNS tissues during immune surveillance and inflammation [64–66], and expression of their common receptor CCR4 is required for protective immune responses during acute T. gondii infection [67,68]. The abrogation of CCL21 function in mice with L. major infection resulted in failure to clear parasites from infected skin [68]. In the present work, 6Ckine/CCL21 plasma levels were similar in NEG, MM and SM infants, suggesting that with malaria progression or regression 6Ckine/CCL21, which may promote immune surveillance against intracellular parasite in CNS tissues, has not been activated or remained suppressed.
In summary, proinflammatory and regulatory cytokine and chemokines were generated in infants during progression and regression of acute malaria tropica. Proinflammatory type cytokines IL-31 and IL-33 were strongly enhanced, while regulatory IL-27 was lowered with severe malaria. Similarly, the chemokines CCL20 and CXCL16 which promote leucocyte migration into brain parenchyma increased while CCL21, which mediates immune surveillance in CNS tissues, remained unchanged. These cytokine and chemokine production profiles and their dynamics could be considered for evaluating the progression or regression of malarial disease.
Acknowledgments
We kindly thank all parents who participated in the present work. For their competent assistance we thank the medical assistants and nurses at the Paediatric Ward at the Centre Hospitalier Regional (CHR) in Sokodé in Togo.
Disclosure
The authors declare that no conflict of interest exists.
References
- 1.Clark IA, Budd AC, Alleva LM, et al. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85–117. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark IA, Alleva LM, Mills AC, et al. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–39. doi: 10.1128/CMR.17.3.509-539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–35. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 4.Sharma M, Vohra H, Bhasin D. Enhanced pro-inflammatory chemokine/cytokine response triggered by pathogenic Entamoeba histolytica: a basis of invasive disease. Parasitology. 2005;131:783–96. doi: 10.1017/S0031182005008541. [DOI] [PubMed] [Google Scholar]
- 5.Benevides L, Milanezi CM, Yamauchi LM, et al. CCR2 receptor is essential to activate microbicidal mechanisms to control Toxoplasma gondii infection in the central nervous system. Am J Pathol. 2008;173:741–51. doi: 10.2353/ajpath.2008.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma M, Bhasin D, Vohra H. Differential induction of immunoregulatory circuits of phagocytic cells by Gal/Gal NAc lectin from pathogenic and nonpathogenic Entamoeba. Clin Immunol. 2008;28:542–57. doi: 10.1007/s10875-008-9184-5. [DOI] [PubMed] [Google Scholar]
- 7.Roffe E, Oliviera F, Souza AL, et al. Role of CCL3/MIP-1alpha and CCL5/Rantes during acute Trypanosoma cruzi infection in rats. Microbes Infect. 2010;12:669–76. doi: 10.1016/j.micinf.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oghumu S, Lezama-Dávila CM, Isaac-Márquez AP, et al. Role of chemokines in regulation of immunity against leishmaniasis. Exp Parasitol. 2010;126:389–96. doi: 10.1016/j.exppara.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochiel DO, Awandara GA, Keller CC, et al. Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect Immun. 2005;73:4190–7. doi: 10.1128/IAI.73.7.4190-4197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlmann P, Perlmann H, ElGhazali G, Blomberg MT. IgE and tumor necrosis factor in malaria infection. Immunol Lett. 1999;65:29–33. doi: 10.1016/s0165-2478(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 11.Perlmann P, Troye-Blomberg M. Malaria blood-stage infection and its control by the immune system. Folia Biol (Praha) 2000;46:210–18. [PubMed] [Google Scholar]
- 12.Torre D, Giola M, Speranza F, Matteelli A, Basilico C, Biondi G. Serum levels of interleukin-18 in patients with uncomplicated Plasmodium falciparum malaria. Eur Cytokine Netw. 2001;12:361–4. [PubMed] [Google Scholar]
- 13.Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the pro-inflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–7. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luty AJ, Perkins DJ, Lell B, et al. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun. 2000;68:3909–15. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armah HB, Wilson NO, Sarfo BY, et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147–64. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druilhe P, Pérignon JL. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol Lett. 1994;41:115–20. doi: 10.1016/0165-2478(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Stanisic DI, Richards JS, McCallum FJ, et al. Imunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roussilhon C, Oeuvray C, Müller-Graf C, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good MF, Xu H, Wykes M, Engwerda CR. Development and regulation of cell-mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu Rev Immunol. 2005;23:69–99. doi: 10.1146/annurev.immunol.23.021704.115638. [DOI] [PubMed] [Google Scholar]
- 20.Schofield L. Intravascular infiltrates and organ-specific inflammation in malaria pathogenesis. Immunol Cell Biol. 2007;85:130–7. doi: 10.1038/sj.icb.7100040. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra I, Mungai P, Muchiri E, et al. Distinct Th1- and Th2-type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun. 2005;73:3462–70. doi: 10.1128/IAI.73.6.3462-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirch AK, Agossou A, Banla M, et al. Parasite-specific antibody and cytokine profiles in newborns from Plasmodium falciparum- and Entamoeba histolytica/dispar-infected mothers. Pediatr Allergy Immunol. 2004;15:133–41. doi: 10.1046/j.1399-3038.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 23.Kocherscheidt L, Agossou A, Gantin R, et al. Cytokine and chemokine responses in adults newborns and children exposed to Entamoeba histolytica/Entamoeba dispar, Onchocerca volvulus and Plasmodium falciparum. Pediatr Allergy Immunol. 2010;21:e756–63. doi: 10.1111/j.1399-3038.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 24.Luty AJ, Lell B, Schmidt-Ott R, et al. Parasite antigen-specific interleukin-10 and antibody responses predict accelerated parasite clearance in Plasmodium falciparum malaria. Eur Cytokine Netw. 1998;9:639–46. [PubMed] [Google Scholar]
- 25.Wipasa J, Okell L, Sakkhachornphop S, et al. Short lived IFN-γ effector responses, but long-lived IL-10 memory responses, to malaria in an area of low malaria endemicity. PLoS Pathog. 2011;7:e1001281. doi: 10.1371/journal.ppat.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brustoski K, Möller U, Kramer M, et al. IFN-γ and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J Immunol. 2005;1:1738–45. doi: 10.4049/jimmunol.174.3.1738. [DOI] [PubMed] [Google Scholar]
- 27.Broen K, Brustoski K, Engelmann I, et al. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol Biochem Parasitol. 2007;151:1–8. doi: 10.1016/j.molbiopara.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Soboslay PT, Geiger SM, Drabner B, et al. Prenatal immune priming in onchocerciasis –Onchocerca volvulus-specific cellular responsiveness and cytokine production in newborns from infected mothers. Clin Exp Immunol. 1999;117:130–7. doi: 10.1046/j.1365-2249.1999.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirghani HA, Eltahir HG, A-Elgadir TM, et al. Cytokine profiles in children with severe Plasmodium falciparum malaria in an area of unstable malaria transmission in central Sudan. J Trop Pediatr. 2010 doi: 10.1093/tropej/fmq109. 30 November. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Kurtzhals JA, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin 10 in severe malarial anemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–72. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 31.Sinha S, Qidwai T, Kanchan K, et al. Distinct cytokine profiles define clinical immune response to falciparum malaria in regions of high or low disease transmission. Eur Cytokine Netw. 2010;21:232–40. doi: 10.1684/ecn.2010.0208. [DOI] [PubMed] [Google Scholar]
- 32.Hugosson E, Montgomery SM, Premji Z, et al. Relationship between antipyretics effects and cytokine levels in uncomplicated falciparum malaria during different treatment regimes. Acta Trop. 2006;99:77–82. doi: 10.1016/j.actatropica.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Muchamuel T, Menon S, Pisacan P, et al. IL-13 protects mice from lipopolysaccharide-induced lethal endotoxemia: correlation with down-modulation of TNF-α, IFN-γ, and IL-12 production. J Immunol. 1997;158:2896–903. [PubMed] [Google Scholar]
- 34.Berry A, Balard P, Costa A, et al. IL-13 induces expression of CD36 in human monocytes through PPAR-gamma activation. Eur J Immunol. 2007;37:1642–52. doi: 10.1002/eji.200636625. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi J, Naka I, Patarapotikul J, et al. A single nucleotide substitution from C to T at position −1055 in the IL-13 promoter is associated with protection from severe malaria in Thailand. Genes Immun. 2003;4:528–31. doi: 10.1038/sj.gene.6364010. [DOI] [PubMed] [Google Scholar]
- 36.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–43. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturmhofer SJ, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–30. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–14. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: a key role in induction of chronic intestinal nematode infection. J Immunol. 2004;172:7635–41. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 40.Findlay EG, Greig R, Stumhofer JS, et al. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J Immunol. 2010;185:2482–92. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- 41.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 42.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–75. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida H, Matsuzaki-Moriya C, Imai T, et al. Development of experimental cerebral malaria is independent of IL-23 and IL-17. Biochem Biophys Res Commun. 2010;402:790–5. doi: 10.1016/j.bbrc.2010.10.114. [DOI] [PubMed] [Google Scholar]
- 44.Yagi Y, Andoh A, Nishida A, et al. Interleukin-31 stimulates production of inflammatory mediators from human colonic subepithelial myofibroblasts. Int J Mol Med. 2007;19:941–6. [PubMed] [Google Scholar]
- 45.Heinrich PC, Behrmann I, Muller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Puthetib P, Zhoub Q, Liua Q, Gaob W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–56. doi: 10.1016/j.cytogfr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 48.Perrigoue JG, Li J, Zaph C, et al. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007;204:481–7. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei Z, Liu G, Huang Q, et al. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–32. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 50.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–10. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 51.Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–2. doi: 10.1016/j.immuni.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Humphreys NE, Xu D, Hepworth MR, et al. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–9. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 53.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–60. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 56.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–9. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 57.Armah H, Dodoo AK, Wiredu EK, et al. High-level cerebellar expression of cytokines and adhesion molecules in fatal, paediatric, cerebral malaria. Ann Trop Med Parasitol. 2005;99:629–47. doi: 10.1179/136485905X51508. [DOI] [PubMed] [Google Scholar]
- 58.Campanella GS, Tager AM, Houry JK, et al. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci USA. 2008;105:4814–19. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berthoud TK, Dunachie SJ, Todryk S, Hill AVS, Fletcher HA. MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J Immunol Methods. 2009;340:33–41. doi: 10.1016/j.jim.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114:4243–52. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKimmie CS, Graham GJ. Astrocytes modulate the chemokines network in a pathogen-specific manner. Biochem Biophys Res Commun. 2010;394:1006–11. doi: 10.1016/j.bbrc.2010.03.111. [DOI] [PubMed] [Google Scholar]
- 62.Fahy OL, Townley SL, Coates NJ, Clark-Lewis I, McColl SR. Control of Salmonella dissemination in vivo by macrophage inflammatory protein (MIP)-3alpha/CCL20. Lab Invest. 2004;84:1501–11. doi: 10.1038/labinvest.3700176. [DOI] [PubMed] [Google Scholar]
- 63.Woehrl B, Klein M, Rupprecht TA, et al. CXCL16 contributes to neutrophil recruitment to cerebrospinal fluid in pneumococcal meningitis. J Infect Dis. 2010;202:1389–96. doi: 10.1086/656532. [DOI] [PubMed] [Google Scholar]
- 64.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL21 (SLC) at the blood–brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunl. 2002;32:2133–44. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 65.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood–brain barrier. J Neural Transm. 2006;113:477–85. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 66.Ploix CC, Noor S, Cran J, et al. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav Immun. 2011;25:883–96. doi: 10.1016/j.bbi.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noor S, Habashy AS, Nance JP, et al. CCR7-dependent immunity during acute Toxoplasma gondii infection. Infect Immun. 2010;78:2257–63. doi: 10.1128/IAI.01314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Unsoeld H, Mueller K, Schleicher U, et al. Abrogation of CCL21 chemokine function by transgenic over-expression impairs T cell immunity to local infections. Int Immunol. 2007;19:1281–9. doi: 10.1093/intimm/dxm098. [DOI] [PubMed] [Google Scholar]


