Abstract
Concanavalin A (Con A)-induced hepatitis is a mouse model of acute autoimmune hepatitis. The aim of this study was to investigate the role of hepatic dendritic cells (DC) in the immune modulation of tissue damage. Almost all hepatic DC were plasmacytoid DC (CD11c+ I-Alow B220+); however, conventional DC were CD11c+ I-Ahigh B220–. At an early stage (3–6 h) after Con A administration, the number of DC in both the liver and spleen decreased, increasing thereafter (12–24 h) in parallel with hepatic failure. The hepatic CD11c+ DC population contained many CD11b- cells, while the majority of splenic CD11c+ DC were CD11b+. After Con A administration, the proportion of I-A+ and CD11b+ cells within the CD11c+ DC population tended to increase in the liver, but not in the spleen. Similarly, expression of the activation markers CD80, CD86 and CD40 by CD11c+ DC increased in the liver, but not in the spleen. Next, adoptive transfer of DC isolated from the liver and spleen was performed 3 h after Con A administration to examine the immunomodulatory function of DC. Only hepatic DC had the ability to suppress hepatic failure. Analysis of cytokine production and subsequent identification of the effector cells showed that hepatic DC achieved this by suppressing the production of interleukin (IL)-12 and IL-2, rather than modulating effector cell function.
Keywords: autoimmune hepatitis, dendritic cells, immunosuppression, liver, plasmacytoid DC
Introduction
It has been established recently that dendritic cells (DC) in the liver have unique properties in terms of their phenotype, morphology and function. In contrast to conventional (c) DC (CD11c+ CD11b+ B220–) present in the spleen and other organs, hepatic DC are thought to be plasmacytoid (p) DC (CD11c+ CD11b- B220+) [1–3]. Furthermore, hepatic DC are able to mediate certain immunotolerance phenomena [4,5]. These properties are quite different from those of cDC [6,7]. Thus, cDC have the potential to mediate immunostimulatory functions with regard to antigen presentation. However, a recent report shows that hepatic DC also mediate immunostimulatory function, resulting in fibrosis [8].
In light of these findings, we conducted experiments to elucidate further the characteristics of hepatic DC using a murine model of concanavalin A (Con A)-induced acute autoimmune hepatitis. The effector cells responsible for Con A-induced hepatitis are primarily CD4+ T cells [9–11], natural killer (NK) T cells [12,13] and neutrophils [14], although an association with the function of regulatory T cells (Treg) cells has also been reported [15,16]. However, the precise role played by hepatic DC in Con A-induced autoimmune hepatitis is unknown. In particular, it is unclear whether hepatic DC have an immunosuppressive or immunostimulatory function in this model. In the present study, we confirmed the phenotype and morphology of hepatic DC, and showed that hepatic DC have an immunosuppressive effect on Con A-induced hepatitis.
Materials and methods
Mice
C57BL/6(B6) mice (female; 6–12 weeks old) were purchased from Charles River Japan (Yokohama, Japan). The mice were kept in a room under constant temperature (25 ± 2°C) and humidity (50–70%) and a 12-h light–dark cycle (lights on from 08:00 to 20:00 h). They were fed under specific pathogen-free conditions in the animal facility of Niigata University (Niigata, Japan).
Induction of Con A-induced hepatitis
Con A (type IV; Sigma-Aldrich, St Louis, MO, USA) was dissolved in sterilized phosphate-buffered saline (PBS) and injected intravenously at a dose of 15 mg/kg [12]. Sera from individual mice were obtained 3, 6, 12 and 24 h after Con A injection. Serum aminotransferase [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] activity was measured using standard photometric methods. All experiments were approved by the Animal Ethics Committee of Niigata University.
Cell preparation
Mice were anaesthetized with isoflurane, perfused with 1500 IU/ml dispase II solution (Life Technologies Co., Carlsbad, CA, USA) via the portal vein, and the liver and the spleen removed. Hepatic DC were isolated using the following methods. Briefly, the liver was cut into fragments using scissors. These fragments were then incubated in dispase II solution for 15 min at 37°C in a shaking water-bath. The digested products were pressed through a 200-gauge stainless steel mesh and suspended in Eagle's minimum essential medium (MEM) supplemented with 5 mM HEPES and 10% fetal calf serum (FCS). After washing, the cell pellet was separated by 30% Percoll centrifugation to obtain mononuclear cells (including dendritic cells). The cells were collected and centrifuged in a discontinuous Percoll gradient at 960 g for 20 min. The DC-enriched fraction at the 40/50% interface was collected. Splenic dendritic cells were obtained by enzymatic digestion of the spleen and passage through a stainless steel mesh. Splenic DC were treated with 0·2% NaCl solution to remove red blood cells (RBC) and isolated using the Percoll discontinuous density gradient/centrifugation method.
For the adoptive transferred experiments, lymphocytes were isolated from mouse livers and spleens of DC-post injected Con A-treated mice. Hepatic lymphocytes were then prepared as described previously [13]. Briefly, the liver was pressed through a 200-gauge stainless steel mesh and suspended in Eagle's MEM supplemented with 5 mM HEPES and 2% FCS. After washing, the cells were resuspended in a 35% Percoll solution containing 100 U/ml heparin and centrifuged at 424 g for 15 min. The pellet was resuspended in RBC lysis solution [155 mM NH4Cl, 10 mM KHCO3, 1 mM ethylenediamine tetraacetic acid (EDTA)-2Na and 17 mM Tris, pH 7·3] and washed twice with medium.
Adoptive transfer of DC into mice
Purified CD11c+ cells from the liver and spleen of normal B6 mice were prepared using the magnetic bead sorting method incorporating biotin-conjugated anti-mouse CD11c monoclonal antibodies (mAb) (HL3) and streptavidin particles plus (BD Bioscience, San Diego, CA, USA) with > 90% purity. Hepatic or splenic CD11c+ cells from normal B6 mice were then transferred adoptively into Con A-injected mice (3 × 105 cells/mouse) by injection into the tail vein.
Flow cytometric analysis
The phenotype of the DC was determined by two- or three-colour immunofluorescence analysis [17]. The reagents used were anti-CD11c, anti-I-Ab (AF6-120·1), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-CD40 (3/23), anti-CD3ε (145-2C11), anti-NK1·1 (PK136) (BD Bioscience), anti-CD11b (M1/70) (e-Bioscience Inc., San Diego, CA, USA) and anti-PDCA-1 (JF05-1C2·4.I) mAbs (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). All mAbs were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE) or biotin. The biotin-conjugated reagents were developed using TRI colour-conjugated streptavidin (Caltag Laboratories, Burlingame, CA, USA). To prevent non-specific binding, anti-CD16/CD32 (2·4G2), mAb was added before staining with the labelled mAbs. The suspended mononuclear cells (MNCs; 5 × 105–106/tube) were stained with the mAbs and analysed using a fluorescence activated cell sorter (FACScan) flow cytometer (BD Bioscience). Dead cells were excluded by the use of appropriate forward-scatter, side-scatter and propidium iodide gating.
Morphological analysis
I-Ahigh CD11c+ cells and I-Alow CD11c+ cells were obtained by FACS Vantage (BC Bioscience) cell sorting and stained using the May–Grünwald–Giemsa method for morphological analysis.
Immunohistochemistry
Liver and spleen tissues were dissected from B6 mice at 6–12 weeks of age. Frozen tissue sections (3 µm in thickness) of each tissue were dried without fixation and stored at 4°C until use. For immunohistochemistry, the sections were blocked with 10% FCS in PBS for 30 min, and treated with 10-fold diluted PE-conjugated anti-mouse CD11c Armenian hamster monoclonal antibody (IgG1 λ2; BD Bioscience) and 20-fold diluted anti-mouse I-A/I-E rat monoclonal antibody (IgG2b κ; Biolegend, Inc., San Diego, CA, USA). Colour was detected by using 800-fold diluted Cy3-conjugated secondary antibodies against Armenian hamster immunoglobulin (Ig)G (Jackson Laboratories, Inc., PA, USA) and 200-fold diluted Alexa488-conjugated secondary antibodies against rat IgG (Molecular Probes Inc., Eugene, OR, USA) in PBS. As a negative control for the staining, sections were treated without primary antibodies. After washing with PBS, counterstaining was performed with Hoechst 33342 (Sigma-Aldrich) at 1 µg/ml. Serial sections were stained routinely with haematoxylin and eosin (H&E).
Intracellular forkhead box P3 (FoxP3) staining
For intracellular FoxP3 staining, MNCs were first stained with FITC-conjugated anti-CD4 (RM4-5). Cells were then fixed and permeabilized using the mouse FoxP3 buffer set (BD Bioscience). After permeabilization, the cells were stained intracellularly using an anti-FoxP3 (MF23) mAb and analysed using a FACScan.
Measurement of serum cytokine concentrations
The concentration of tumour necrosis factor (TNF)-α, interleukin (IL)-2, interferon (IFN)-γ and IL-12p70 was measured in the serum from each mouse using cytometric bead array (CBA) kits (BD Biosciences) according to the manufacturer's instructions.
Statistical analysis
Differences between the results obtained in normal mice and experimental mice were analysed using one-way analysis of variance (anova). A P-value of <0·05 was considered significant.
Results
Characterization of CD11c+ DC in the liver and spleen
First, DC in the liver and spleen of normal mice were characterized by two-colour immunofluorescence tests (Fig. 1a). These DC were detected as CD11c+. CD11c+ DC in the liver were mainly I-Alow B220+, but those in the spleen were a mixture of (CD11c+) I-Alow B220+ and (CD11c+) I-Ahigh B220–. CD11c+ I-Alow B220+ DC in the liver were characterized as hepatic pDC. In a second experiment, morphological staining was performed using DC purified by cell sorting (Fig. 1b). Irrespective of the organ of origin, I-Ahigh CD11c+ cDC were large, with a DC morphology, while I-Alow CD11c+ pDC were small, with a plasmacytoid-like morphology.
Fig. 1.
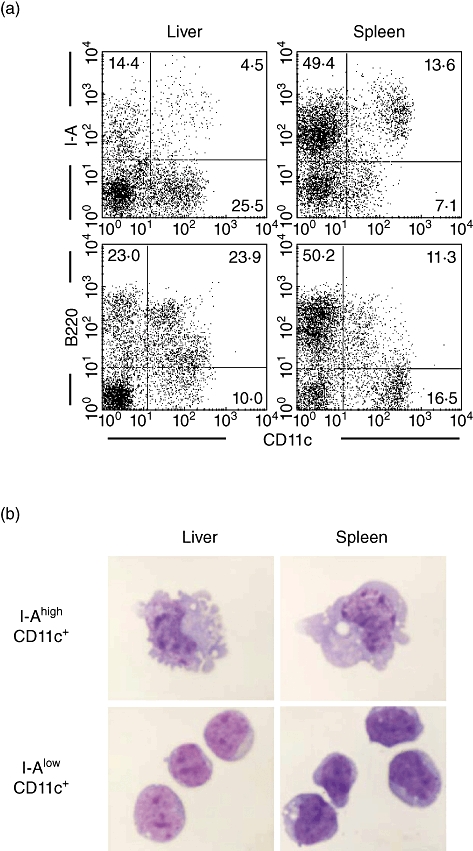
Characterization of dendritic cells (DC) in the liver and spleen. (a) Phenotype and (b) morphology. DC phenotype was characterized by immunofluorescence analysis. The expression of I-A and B220 on CD11c+ DC was examined. I-Ahigh CD11c+ and I-Alow CD11c+cells were purified using a cell sorter and then stained using the Giemsa method. Representative data of four experiments are depicted.
Con A-induced hepatitis and the number of intrahepatic DC
AST and ALT levels in the sera of Con A-treated mice increased after 12–24 h (Fig. 2a). During this time, DC were also identified in the immunofluorescence experiments (Fig. 2b). DC were identified as CD11c+, irrespective of the organ of origin. During the early stages (3–6 h after administration), the absolute number of DC in the liver decreased; however, the number increased during the later stages (12–24 h). A similar pattern was observed for splenic DC.
Fig. 2.
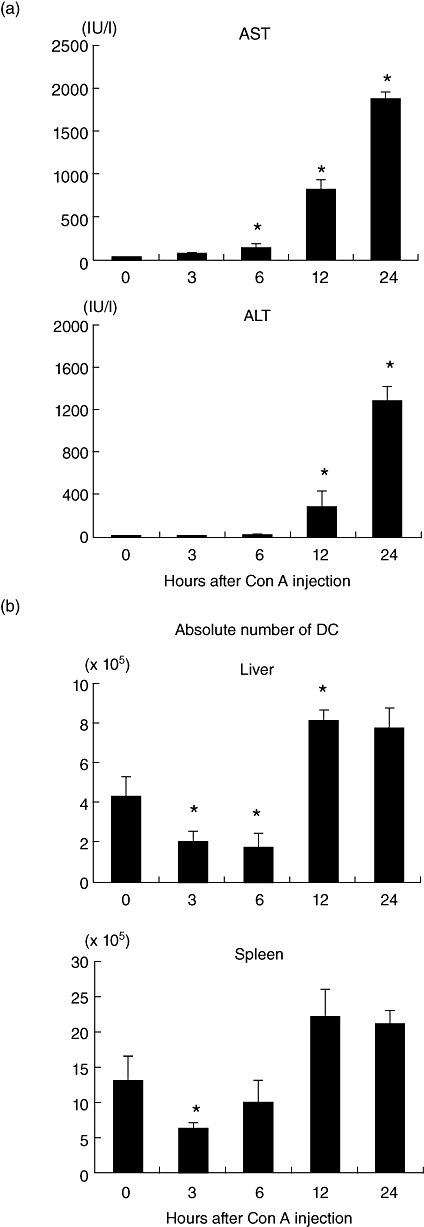
Kinetics of tissue damage and the number of dendritic cells (DC) after concanavalin A administration. (a) The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in sera. (b) The absolute numbers of CD11c+ DC cells in the liver and spleen. The mean ± 1 standard error were produced by five experiments.
Data showing the identification of DC are presented in Fig. 3. There were few CD11c+ I-Ahigh DC in the liver, but were abundant in the spleen. However, the results clearly show that the number of DC decreased in the early stages (3–6 h) after Con A administration.
Fig. 3.
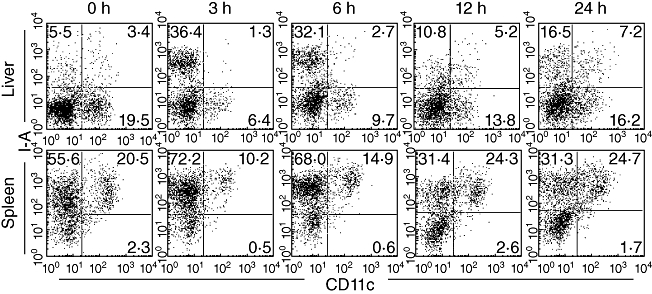
Variations in the proportion of I-A+ CD11c+ dendritic cells (DC) in the liver and spleen after concanavalin A administration. Two-colour staining for CD11c and I-A was conducted. Data are representative of three independent experiments.
Characterization of DC in the liver and spleen
Next, the phenotypes of the DC isolated from the liver and spleen were compared (Fig. 4) after two-colour staining for CD11c and I-A (or CD11b or PDCA-1). The expression of I-A (or CD11b or PDCA-1) on CD11c DC was identified using gated analysis. The expression of I-A on liver DC increased from 8·3% to 29·5% (Fig. 4a). The expression of CD11b also increased after administration of Con A (from 15·2% to 50·9%). In contrast, the expression of PDCA-1 decreased.
Fig. 4.
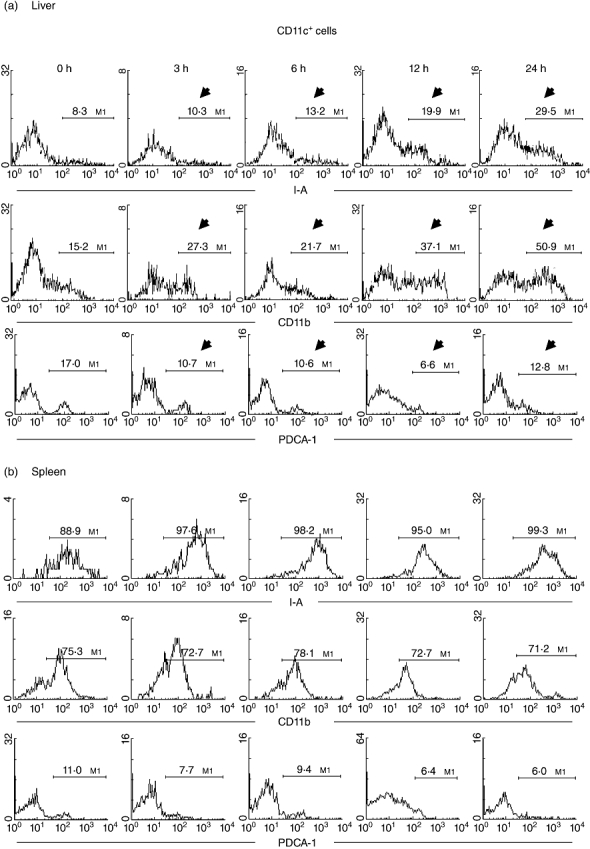
Further characterization of dendritic cell (DC) phenotype in (a) liver and (b) spleen. Two-colour staining for CD11c and I-A (or CD11b or PDCA-1) was conducted. Gated analysis was used to examine the proportion of DC expressing I-A, CD11b or PDCA-1. Significant changes are indicated by arrows. Representative data of five experiments are depicted.
The same experiments were performed using spleen DC (Fig. 4b). A striking difference was seen in the expression of I-A and CD11b on splenic DC, which showed high expression of both molecules (I-A+, 88·9%; CD11b+, 75·3%). This high I-A expression increased with time (up to 24 h), but CD11b levels remained static. The expression of PDCA-1 was very low in splenic DC, and tended to decrease with time.
Next, the costimulatory molecules expressed by DC were characterized further (Fig. 5) using two-colour staining for CD11c and CD80 (or CD86 or CD40). The expression of CD80, CD86 and CD40 by hepatic CD11c+ DC was determined both before and after Con A administration (Fig. 5a). In the late stages (12–24 h), the expression of all these markers was increased (indicated by arrows).
Fig. 5.
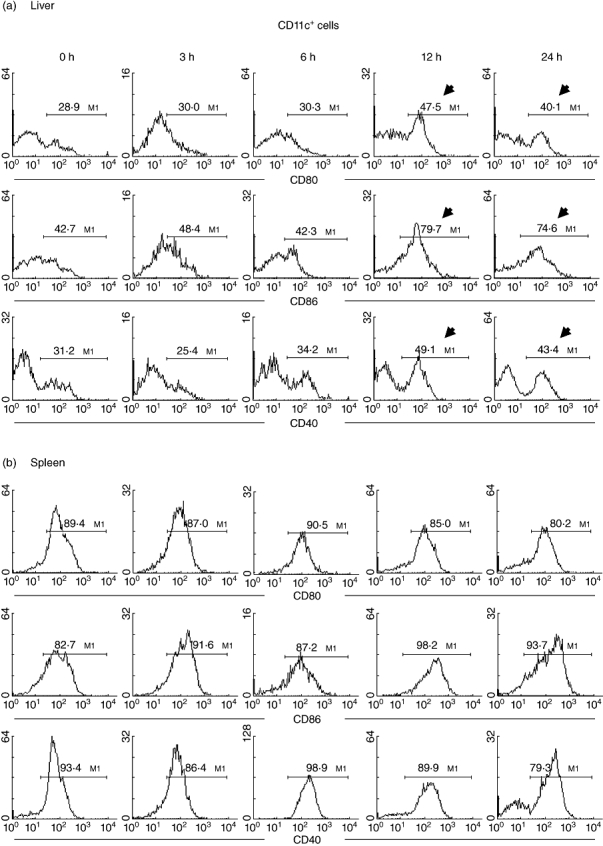
Further characterization of dendritic cell (DC) phenotype in (a) liver and (b) spleen. Two-colour staining for CD11c and CD80 (or CD86 or CD40) was conducted. Gated analysis was used to examine the proportion of DC expressing CD80, CD86 and CD40. Representative data of five experiments are depicted. Significant changes are indicated by arrows.
The expression of CD80, CD86 and CD40 on spleen DC was high compared with that on liver DC (89·4%, 82·7% and 93·4%, respectively), and remained constant after Con A administration.
Adoptive transfer of DC into mice with Con A-induced hepatitis
The immunomodulatory function of hepatic or splenic DC was then investigated (Fig. 6). CD11c+ DC were isolated by magnetic cell sorting and injected intravenously into mice 3 h after Con A administration (Fig. 6a). Purified CD11c+ DC in the liver contained > 60% pDC. However, those in the spleen contained no pDC (data not shown). The indicated parameters were then examined after 18 h. Figure 6b shows that hepatic DC, but not splenic DC, suppressed hepatic failure.
Fig. 6.
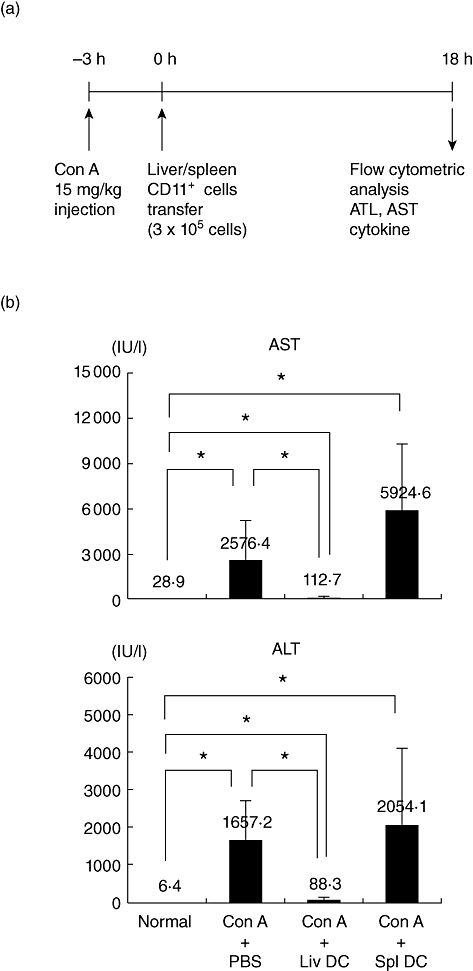
Adoptive transfer of dendritic cells (DC). (a) Time schedule and (b) modulation of tissue damage by adoptive transfer of DC. DC were transferred 3 h after concanavalin A administration. Mean ± 1 standard error were produced by three experiments. *P < 0·05.
Histological examination of the liver and immunohistochemical staining of hepatic DC was then conducted (Fig. 7). The administration of Con A + PBS (at 18 h) induced inflammatory cell infiltration into the liver and haemorrhage (Fig. 7b). However, this was attenuated by the adoptive transfer of hepatic DC (Fig. 7c).
Fig. 7.
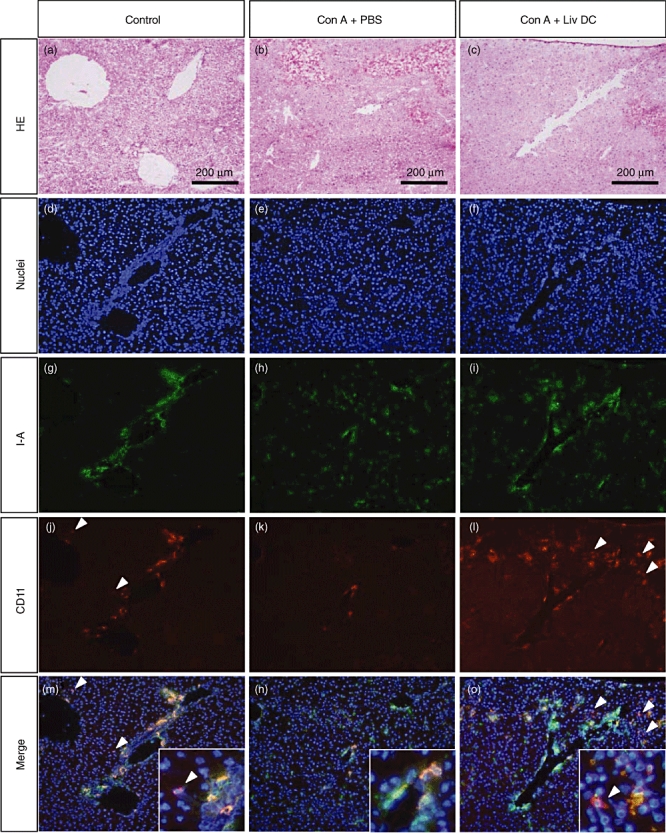
Histology and immunohistochemical staining of the liver. (a, d, g, j, m) Control mice. (b, e, h, k, n) Mice treated with concanavalin A (Con A). (c, f, i, l, o) Mice treated with Con A plus adoptive transfer of liver dendritic cells (DC). (a–c) Haematoxylin and eosin staining. (d–o) Immunohistochemical staining. White arrowheads indicate the CD11c single positive cells. They were only seen in the control liver and the DC-post injected Con A-treated liver, but not in the Con A-treated liver. Representative data of three experiments are depicted.
Staining for I-A and CD11c showed that CD11c+ DC in the liver were primarily I-A- (Fig. 7g,j,m). These CD11c+ I-A- DC disappeared after the administration of Con A (Fig. 7h,k,n). After adoptive transfer of hepatic DC, CD11c+ I-A- DC accumulated in the liver (Fig. 7i,l,o). These results showed that adoptively transferred DC infiltrated into the liver and were able to suppress hepatic failure.
Further characterization of cytokines and effector cells
The serum levels of proinflammatory cytokines (TNF-α and IL-12) and T helper type 1 (Th1)-type cytokines (IFN-γ and IL-2) were measured in mouse serum 18 h after the adoptive transfer of hepatic DC (Fig. 8). The levels of TNF-α were comparable before and after adoptive transfer of DC, but IL-12p70 levels decreased after the transfer of hepatic DC. IFN-γ levels were also comparable, but IL-2 levels tended to decrease.
Fig. 8.
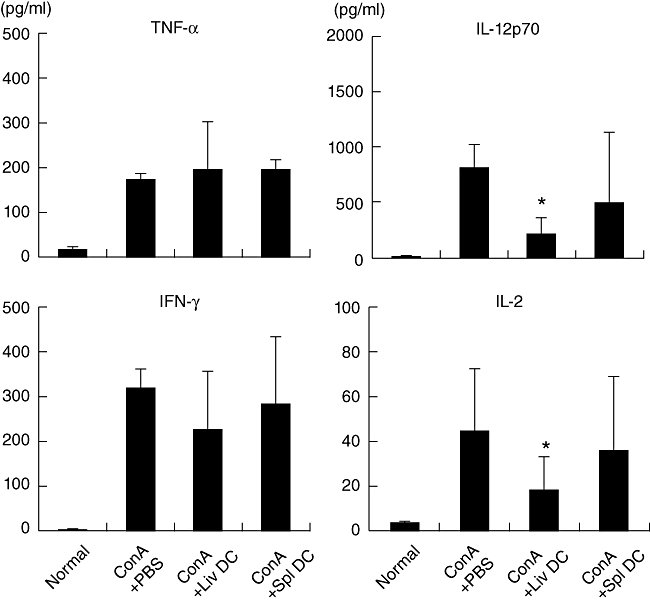
Cytokine production after adoptive transfer of dendritic cells (DC). The level of cytokines was determined 24 h after concanavalin A administration. Data represent the mean ± 1 standard error from four experiments. *P < 0·05.
The major effector cells involved in hepatic damage during Con A-induced hepatitis are NK T cells. Therefore, the proportion of NK T cells was analysed 18 h after adoptive transfer (Fig. 9). NK T cells were abundant in the liver of normal mice (12·4%), but their numbers were reduced after Con A administration (2·7%). This did not change after the adoptive transfer of hepatic or splenic DC. CD4+ T cells are also involved in Con A-induced hepatitis, and respond directly to Con A. The proportion of CD4+ T cells was also down-regulated after Con A administration (from 20·4% to 13·0%). This was not changed by the adoptive transfer of hepatic DC or splenic DC.
Fig. 9.
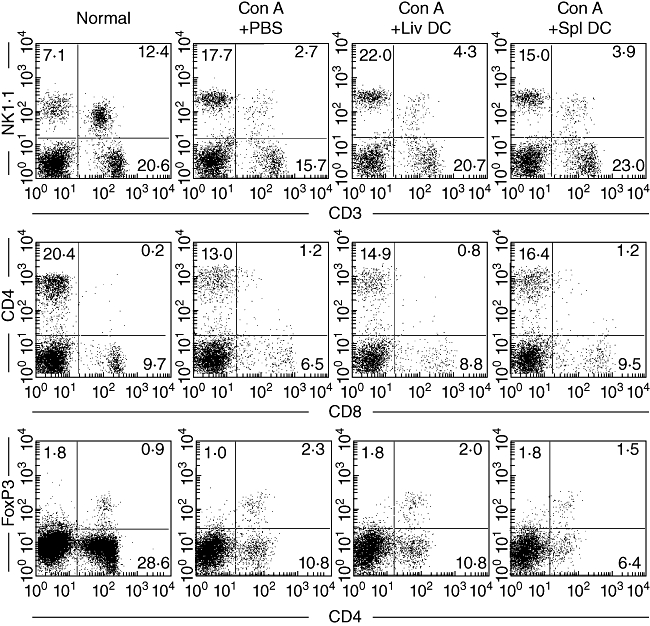
Identification of lymphocyte subsets by immunofluorescence analysis. Natural killer (NK) T cells were NK1·1+ CD3int. CD4+ T cells were CD4+ CD8–. Regulatory T cells were CD4+ forkhead box P3+. Data are representative of three independent experiments.
Finally, Treg cells (CD4+ FoxP3+) were examined (Fig. 9, bottom panel). The proportion of Treg cells did not change significantly after the adoptive transfer of hepatic DC or splenic DC compared with that in the controls (Con A + PBS).
Discussion
In the present study, we first characterized the phenotype and morphology of DC in the livers of normal mice. Almost all CD11c+ DC in the liver were I-Alow B220+. This phenotype was quite different from that of splenic DC (I-Ahigh B220–). In other words, hepatic DC had a phenotype of pDC, which sometimes show immunosuppressive functions [4,5]. We then purified I-Ahigh CD11c+ cells and I-Alow CD11c+ by cell sorting and compared their morphology. Irrespective of the organ of origin, I-Ahigh CD11c+ cells were large and highly dendritic in nature, whereas I-Alow CD11c+ cells were small and less dendritic in nature. These results suggest that hepatic DC comprise pDC rather than cDC, which is in agreement with earlier studies [1–3].
An acute Con A-induced autoimmune hepatitis model was used to examine the function of hepatic DC. First, the variation in the number and proportion of DC in the liver and spleen was examined. The level of transaminases in mouse serum indicated that hepatic damage occurred as early as 3 h after Con A administration. At this early stage (3–6 h), the number of DC in the liver and spleen tended to decrease. However, at a later stage (12–24 h), the numbers increased. A similar pattern was seen when the proportion (rather than discrete numbers) of DC in the liver and spleen was assessed.
The expression of I-A and CD11b on CD11c+ DC in the livers of normal mice was low, but increased after Con A administration. The level of I-A and CD11b was extremely high in splenic DC, independent of Con A administration. The expression of the pDC marker, PDCA-1 [18], was also assessed. The proportion of PDCA-1+ cells within the hepatic DC population was slightly higher (17%) than in the splenic DC population (11%) in normal mice, although expression tended to decrease in hepatic DC after Con A administration. pDC are relatively abundant in mouse liver [4] and produce IFN-α[19]. They have also been implicated in hepatic tolerance [20]. The costimulatory molecules CD80, CD86 and CD40, expressed by DC, are associated with antigen presentation and activation. The results of this study showed that the expression of costimulatory molecules by CD11c+ DC in the liver increased 12–24 h after Con A administration. Activated DC in the liver also produced large amounts of the proinflammatory TNF-α, IL-6 and macrophage inflammatory protein (MIP)-1α (data not shown). Thus, it was speculated that activated hepatic DC modulate liver damage by secreting certain cytokines.
Adoptive transfer experiments were conducted to determine directly the function of hepatic DC. Cell transfer was performed 3 h after Con A administration. The results showed that that only hepatic DC had the potential to suppress hepatic damage induced by Con A. All these phenomena were also confirmed by histology and immunohistochemical staining. Because hepatic damage is mediated by killer molecules, the levels of cytokines with killer activity, including TNF-α, IL-12 and IFN-γ, were examined. The most prominent change observed after the transfer of hepatic DC was a reduction in IL-12p70 levels. Transfer of splenic DC did not evoke such an effect. In other words, the ability of hepatic DC to suppress liver damage may be related directly to their ability to suppress IL-12 secretion. IL-12 exerts its biological activity mainly by promoting a Th1 immune response, and its detrimental effect during Con A-induced hepatitis occurs via this mechanism [21–24]. IL-12 promotes increased IL-4 and IFN-γ production by invariant NK T cells in the livers of mice with Con A-induced hepatitis and aggravates the disease. In other words, a reduction in IL-12 levels suppresses the activation of NK T cells and effector T cells in the liver, and reduces the level of damage induced by Con A administration. The results of the present study support this mechanism, at least in part. The levels of TNF-α and IFN-γ remained unchanged even after the transfer of hepatic DC.
Other experiments also showed that the transfer of hepatic DC suppressed IL-2 production. There is a possibility that the growth of some effector cells, which respond to IL-2, might be suppressed by the transfer of hepatic DC into mice treated with Con A. Therefore, the effector cells responsible were identified in further experiments.
It is well established that several different effector cells are associated with hepatic damage during Con A-induced hepatitis. These are primarily CD4+ T cells [9–11] and NK T cells [12,13]. However, we did not observe suppression of CD4+ T cell or NK T cell numbers after the adoptive transfer of hepatic DC.
Finally, experiments were conducted to identify the suppressor cells involved. Treg cells and NK T cells both have an immunosuppressive function. Treg cells regulate autoreactive T cells, and their reduction or dysfunction causes certain autoimmune diseases in both animals and humans [25,26]. Treg cells produce IL-10 and transforming growth factor (TGF)-β, and these cytokines suppress T cell effector function and inflammatory disease [27,28]. Treg cells also express cytotoxic T lymphocyte antigen 4 (CTLA-4), which inhibits effector T cell proliferation via cell–cell interactions and also inhibits IL-2 production [29]. NK T cells also suppress certain autoimmune diseases, and their absence is associated with autoimmune phenomena [30–33]. There is a possibility that the transfer of hepatic DC might influence the induction of suppressor cells, rather than that of effector cells [15,16]. However, we did not detect any increase in Treg cells or NK T cell numbers after the transfer of hepatic DC.
These results suggest that immunosuppression by hepatic DC was not due to their effects on effector cells or suppressor cells. Therefore, it appears that functional modulation of killer molecules may be the main mechanism by which hepatic DC suppress liver damage. In parallel with the assay of several cytokines, the level of IL-10 was also examined in sera. The level of IL-10 was not detectable with or without the transfer of hepatic DC in Con A-induced hepatitis (data not shown). We have to consider that some factors other than IL-10 might be used for the immunosuppression (down-regulation of IL-12 and IL-2) by hepatic DC in Con A-induced hepatitis.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) (no. 19790394) from the Japan Society for the Promotion of Science (JSPS), Japan (C.T.). The authors thank Mrs Yuko Kaneko for preparation of the manuscript.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Jomantaite I, Dikopoulos N, Kröger A, et al. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–65. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 2.Lau AH, Thomson AW. Dendritic cells and immune regulation in the liver. Gut. 2003;52:307–14. doi: 10.1136/gut.52.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–54. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 4.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 5.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-α production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–32. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmieg J, Yang G, Franck RW, Rooijen NV, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci USA. 2005;102:1127–32. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Wang Y-H, Wang Y, et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–4. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 8.Connolly MK, Bedrosian AS, Clair JM, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest. 2009;119:3213–25. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuhara H, O'Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529–37. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casini A, Ricci OE, Paoletti F, Surrenti C. Immune mechanisms for hepatic fibrogenesis: T-lymphocyte-mediated stimulation of fibroblast collagen production in chronic active hepatitis. Liver. 1985;5:134–41. doi: 10.1111/j.1600-0676.1985.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 12.Toyabe S, Seki S, Iiai T, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–42. [PubMed] [Google Scholar]
- 13.Saito T, Okumura A, Watanabe H, et al. Increase in hepatic NKT cells in leukocyte cell-derived chemotaxin 2-deficient mice contributes to severe concanavalin A-induced hepatitis. J Immunol. 2004;173:579–85. doi: 10.4049/jimmunol.173.1.579. [DOI] [PubMed] [Google Scholar]
- 14.Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Abe M, Akbar SK, Hasebe A, Horike N, Onji M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J Gastroenterol. 2003;38:962–7. doi: 10.1007/s00535-003-1179-7. [DOI] [PubMed] [Google Scholar]
- 16.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–85. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, Miyaji C, Kawachi Y, et al. Relationships between intermediate TCR cells and NK1.1T cells in various immune organs. NK1.1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 18.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naïve mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–5. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 19.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 20.Goubier A, Dubois B, Gheit H, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 22.Smyth MJ, Crowe NY, Pellicci DG, et al. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for antimetastatic effect of α-galactosylceramide. Blood. 2002;99:1259–66. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti F, Di Marco R, Zaccone P, et al. Murine concanavalin A-induced hepatitis is prevented by interleukin-12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-γ-dependent mechanism. Hepatology. 2000;32:728–33. doi: 10.1053/jhep.2000.17701. [DOI] [PubMed] [Google Scholar]
- 24.Habu Y, Uchida T, Inui T, Nakashima H, Fukasawa M, Seki S. Enhancement of synthetic ligand-mediated function of liver NK1.1 Ag+ T cells in mice by interleukin-12 pretreatment. Immunology. 2004;113:35–43. doi: 10.1111/j.1365-2567.2004.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 26.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 27.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell response and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Kitani A, Fuss I, et al. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T-cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T-cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–81. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 31.Godfery DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro M, Almeida CF, Caridade M, et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-β. J Immunol. 2010;185:2157–63. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]


