Abstract
Ovarian serous borderline tumors (SBTs) are presumed to originate in the ovarian cortex or peritoneal surface. The pathogenetic role of the fallopian tube (FT) is unclear but recently, secretory cell outgrowths (SCOUTs) lacking PAX2 expression have been described in benign FTs. This study addressed 1) the differentiation characteristics of SBTs, 2) the frequency of SCOUTs lacking PAX2 expression in the fallopian tubes of patients with SBTs, and compared 3) SCOUT morphology and 4) PAX2 expression with SBTs. SBTs and FT epithelium shared both ciliated (p73) and secretory (HMFG2) differentiation. PAX2-null SCOUT frequency in FT cross sections from patients with SBTs was 0.27 (110/398) versus 0.09 in benign hysterectomies and nearly zero in pediatric and post partum sterilization specimens. (p=<0.001). When adjusted for age, the differences narrowed but remained significant (p = 0.010). SCOUTs were heterogenous, some displaying ciliated differentiation and papillary architecture. Two cases of discrete multi-focal papillary SCOUTs in the fallopian tube were associated with SBTs. All SBTs had heterogeneous PAX2 staining with areas of PAX2 loss. This study shows for the first time that PAX2-null SCOUTs are more common in the oviducts of women with SBTs and that loss of PAX2 expression occurs in most SBTs. These discoveries link both morphologic and functional gene (PAX2) alterations in the oviduct to SBTs, similar to that reported in high grade serous carcinoma. Further study is warranted to clarify the relationship of the oviduct to serous neoplasia.
Introduction
Serous borderline tumors (SBTs) of the ovary are predominantly clonal neoplasms and fall between benign serous cystadenoma and high-grade serous carcinoma in the spectrum of ovarian serous tumors (21). Serous tumors have traditionally been thought to arise from the ovarian surface epithelium or from Müllerian epithelium within the ovary (8,21). Recently, the fallopian tube has been implicated as a primary site for some high-grade serous carcinomas, and a spectrum of changes ranging from frank serous tubal intraepithelial carcinoma to a precursor condition (p53 signature) has been described, all linked by mutations in the tumor suppressor gene p53 (2,7,9,15). More recently, the spectrum of potential precursors in the fallopian tube has been expanded to include more generic secretory cell outgrowths (SCOUTs) which are characterized by loss of PAX2 expression (PAX2-null). The latter have been linked to high-grade serous carcinoma, both by their greater association with this malignancy relative to controls and the loss of PAX2 expression (2).
Epithelial hyperplasia has also been noted in the fallopian tubes of patients with serous borderline tumors, but its significance is as of yet unknown (16). We previously identified two cases with unique multi-focal proliferations (SCOUTs) in the benign fallopian tubes from two women with serous cystadenomas with borderline features. The purpose of this study was to describe these two cases and to examine the relationship between SCOUTs and SBTs. Thus, we explored fallopian tubes with the intent to determine the frequency of PAX2-null SCOUTs in the fallopian tubes of patients with SBTs as compared to controls, examine SCOUT morphology in more detail, describe the differentiation characteristics of SBTs, and compare PAX2 expression patterns in SCOUTs and SBTs.
Materials and Methods
Study material
This study was approved by the Institutional Review Board at Brigham & Women's Hospital and Children's Hospital. Consecutive serous borderline tumors (SBTs) received between 2007 and 2010 comprised the cases (n = 48), with fallopian tubes available for review in 33 of these cases. Additional SBTs (n = 14) from archival material were evaluated for selected immunohistochemical studies. Controls consisted of three populations: fallopian tubes from pediatric autopsies (n = 20), post-partum sterilizations (n = 25) and salpingo-oophorectomies associated with benign hysterectomies from women without ovarian pathology (e.g. leiomyomata, n = 20). Age-specific data was obtained from the adults, but data on age were not available from the pediatric group.
Two unique cases of proliferations in the fallopian tubes associated with SBT were studied separately.
Immunohistochemistry
The following antibodies were used, with methods as previously described. p73 (Courtesy Frank McKeon): A gene with homology to p53, but without a clear tumor suppressor function, which is expressed in the nuclei of ciliated cells of the respiratory tract and fallopian tube, as well as several foci within the central nervous system (25). Cases were scored as positive by the presence of nuclear staining, which contrasts with stromal and secretory cells (9).
HMFG2
A monoclonal antibody which binds to a particular glycoform of the high-molecular-weight transmembrane glycoprotein MUC1. MUC1 is expressed on the apical aspect of simple epithelial cells, including those of the female genital tract and more specifically, the fallopian tube (19), where it is a marker for secretory cells (4). A positive reaction was cytoplasmic staining that was absent in the subjacent stromal and ciliated cells (9).
PAX2
A member of the paired box transcription factor gene family which is critical in the embro-and organogenesis of the central nervous system, eye, ear, and the genitourinary tract. PAX2 is expressed throughout the female genital tract, including in the endometrial epithelium, and in particular, the secretory cells of the fallopian tube epithelium. (Invitrogen, Cat# 71–6000) (2, 23). Nuclear immuno-localization parallels that of PAX8.
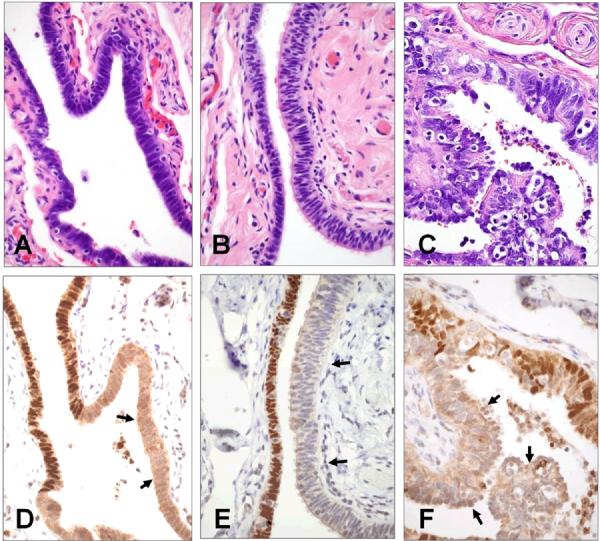
Fourteen archival SBTs were stained for evidence of ciliated (p73) and secretory (HMFG2) differentiation (Figure 1). Fallopian tubes and ovarian tumors from 33 patients with serous borderline tumors were stained with PAX2, the former to ascertain the frequency of PAX2-null SCOUTs and the latter to determine the distribution of PAX2 in the tumors. PAX2-null SCOUTs were defined as at least 30 consecutive horizontally arranged cells that contained predominantly secretory cells and were devoid of nuclear PAX2 staining (Figure 2). The number of SCOUTs and number of FT cross sections were recorded and expressed as a ratio (frequency) of PAX2-null SCOUTs per section. The differentiation characteristics (secretory cells only vs. secretory with ciliated cells) of each SCOUT were also recorded.
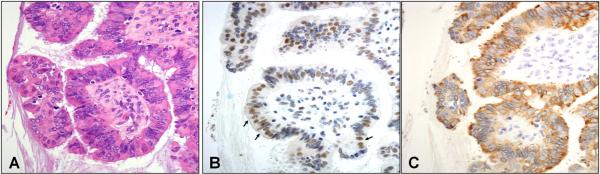
Figure 1.
Immunophenotype of serous borderline tumor (A), displaying ciliated (p73 staining nuclei, panel B) and secretory cell (HFMG2 staining cytoplasm, panel C) differentiation.
Figure 2.
Secretory cell outgrowths (SCOUTs) of the fallopian tube composed of low columnar secretory cells (A), pseudostratified epithelium (B) and prominent ciliated cell differentiation (C). Corresponding PAX2-null areas are denoted by arrows in panels D, E, and F.
Data analysis
The data were analyzed to compare: 1) histopathology and immunophenotype of PAX2-null SCOUTs with normal salpingeal mucosa and SBTs; and 2) the frequencies of PAX2-null SCOUTs per tubal cross section in cases (SBTs) and controls (pediatric autopsies, post partum sterilizations and hysterectomies for benign ovarian disorders). Differences in the aggregate data (PAX2-null SCOUTs per cross section in each group) were analyzed by chi-square. To account for age differences and the varying numbers of sections examined for each subject, the data were modeled on the assumption that the number of SCOUTS in each section follows a Poisson distribution, with Poisson rate depending on group and age and number of sections examined. The data were adjusted for age in the model, using 10 year groupings (25–35, 35–45, etc). The Poisson probability distribution is commonly used to model count data; it is a one-parameter distribution and it fixes the variance to be equal to the mean. As this is often overly restrictive, it may be extended by assuming variability of the Poisson parameter across subjects, which yields a negative binomial model.
Results
Immunostaining of SBTs for evidence of ciliated and secretory differentiation
Fourteen cases of SBT were immunostained for both secretory (HMFG2) and ciliated (p73) differentiation. In all, there was evidence of both immuno-phenotypes (Figure 1).. However, despite the immunophenotypic parallels between borderline serous tumors and normal salpingeal epithelium, there was a wider range of cellular morphology in the borderline tumors. Nuclear p73 immunostaining could be appreciated in both conspicuous ciliated cells as well as cells with apical eosinophilia suggestive of terminal bar preservation. The latter were interpreted as evidence of the ciliated phenotype and were more typical of borderline tumors in contrast to normal salpingeal epithelium. Cortical inclusion cysts were also immunostained and exhibited a similar immunophenotype (not shown).
Fallopian tubes with marked epithelial proliferation associated with SBT of the ovary
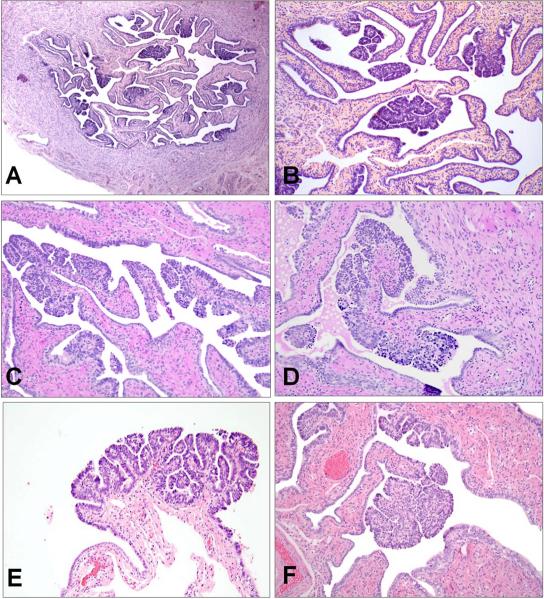
The histologic findings in these two cases were very similar. In each case, multiple discrete papillary structures were evident on low power, distributed throughout the cross sections of fallopian tube and evenly distributed throughout the oviduct (Figure 3A–B). These structures had focally complex branching architecture and were lined by an epithelium which appeared lush in comparison to the background tubal epithelium (Figure 3C–F). The epithelium was pseudostratified, focally tufted, and composed of both secretory and ciliated cells.
Figure 3.
Multifocal oviductal papillary SCOUTs from two cases, associated with SBTs; Case 1 (A, B) and Case 2 (C–F).
Differentiation and immunophenotype of PAX2-null SCOUTs
PAX2-null SCOUT morphology varied and three different patterns were identified. The first consisted of almost exclusively secretory-type cells with elongated nuclei arranged in a single or mildly pseudostratified layer (Figure 2A). The second consisted of a multilayered epithelium with scattered inflammatory cells (Figure 2B). The appearance suggested a mixed secretory and ciliated phenotype; however, conspicuous cilia were not present. The third, and one that was least commonly encountered, contained a conspicuous ciliated component, often with some papillary architecture and scattered inflammatory cells (Figure 2C). In all three types, there was loss of nuclear PAX2 staining (Figure 2D–2F).
PAX2-null SCOUT frequency in the fallopian tubes of patients with serous borderline tumors relative to controls
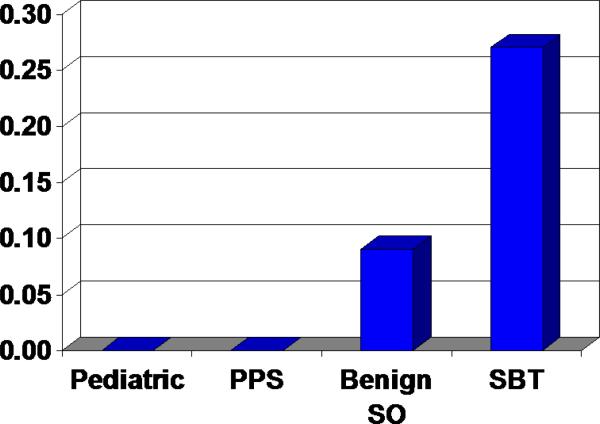
For each case a frequency distribution of SCOUTs expressed as a fraction of total tubal cross-sections examined was compiled. The distribution of individual frequencies for cases and controls is summarized in Table 1. Overall, the average frequency of SCOUTs per cross section in 33 SBTs was 0.27(110/398). This was in contrast to essentially zero per cross section examined in both 20 pediatric autopsies (0/35) and 25 post-partum sterilizations (1/190) and 0.091 in 20 benign salpingo-oophorectomies (8/88) (Figure 4 and Table 1). Comparing the aggregate data for each group, these differences were highly significant (p = <0.001) by chi square analysis. When adjusted for age group (in 10 year intervals) and for number of sections (from the negative binomial regression model) the difference in frequency of SCOUTs between SBT and control-associated fallopian tubes remained significant (p=0.010).
Table 1
| Source | No. | Mean Age | Sections | PAX2-null Foci | Percent |
|---|---|---|---|---|---|
| Child and adolescent | 20 | NS | 35 | 0 | 0.0 |
| Post partum tubal ligation | 25 | 35 | 190 | 1 | 0.5 |
| Benign Hysterectomy | 20 | 53 | 161 | 18 | 11.2 |
| Serous Borderline Tumors | 33 | 51 | 398 | 110 | 27.6 |
| Total | 98 | 784 | 129 | 16 |
Figure 4.
Graphical depiction of average PAX2-null SCOUT frequency per tubal section in pediatric autopsies, postpartum sterilization specimens (PPS), benign salpingooophorectomies (SO) and serous borderline tumors (SBTs).
PAX2 immunostaining in serous borderline tumors
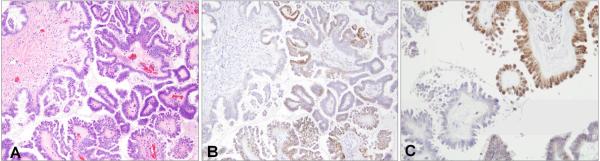
Because SCOUTs have a high frequency of loss of PAX2 staining, we analyzed 33 SBTs to determine the range of immunostaining in these tumors. All of the SBTs were heterogeneous for PAX2 (Figure 5). Thirty-five percent showed greater than 80% positivity, 56% were 20–80% positive, and in 9% less than 20% of the cells stained for PAX2. This was compared to a recent study by Roh et al of high-grade Müllerian carcinomas of the pelvis (17). In that study 72% of cases were PAX2-null in greater than 90% of the tumor cells; the remaining 28% were either PAX2 positive or heterogeneous for PAX2 staining.
Figure 5.
A SBTs (A) with heterogeneous PAX2 immunostaining (B); at higher power showing positive (upper right) and negative (lower left) negative staining in a single field (C).
Discussion
There is remarkable similarity between serous ovarian tumors and salpingeal epithelium, both in morphology and gene expression (11,16). An intriguing finding is the coexistence of both ciliated and secretory cells in SBTs, similar to the normal oviduct. One difference is the apparent broader range of p73 immunostaining, which can be seen in cells without conspicuous cilia, a feature more commonly encountered in the SBTs. This mixed phenotype is also characteristic of Müllerian inclusions in the ovarian cortex, which were also present in the cases immunostained and presumably give rise to SBTs (5,12,14,21). Whether this benign ovarian “precursor” condition is derived from the fallopian tube or via mesothelial-to-Müllerian trans-differentiation of the ovarian surface lining is controversial (1). However, irrespective of the mechanism, ovarian cortical inclusions are a de facto representation of the salpingeal phenotype in the ovarian cortex. Another intriguing aspect of this mixed epithelial differentiation seen in SBTs is the suggestion based on a recent study that it emanates from a single clone of transformed progenitor cells (22). This possibility takes on added significance with the identification of SCOUTs in the fallopian tube – a common occurrence (Figure 2), and more rarely more conspicuous micropapillary proliferations (Figure 3) with both a secretory and ciliated cell phenotype. Together these observations present compelling evidence for the capacity of the oviductal epithelium to both expansion and at the same time, bidirectional (secretory and ciliated) differentiation.
This study cannot determine with certainty whether SBTs are directly derived from SCOUTs. Nevertheless, there appears to be a relationship between these discrete changes in the oviduct and SBTs. We have previously shown that SCOUTs exist in the fallopian tube, occur throughout the entire length of the organ, are increased in frequency in cases of high-grade serous cancer, and frequently are PAX2-null (2). The current analysis was intended to explore the possibility that PAX2-null SCOUTs were more frequent in SBTs. As shown in Figure 2 and Table 1, there is a range of PAX2-null SCOUTs in these tubes and as a group they are highly significantly associated with the presence of SBT. The relationship of PAX2 staining to both SCOUTs and their associated SBTs is less clear but there is evidence of loss of PAX2 expression in the latter (Figure 5). The contrast between complete loss of PAX2 expression in SCOUTs and heterogeneous staining in SBTs might seem paradoxical. However, similar contrasts in staining frequency can be seen between SCOUTs (including p53 signatures) and high-grade serous carcinomas (2,17). One explanation is that the emergence of SCOUTs is associated with a consistent down-regulation of certain genes (PAX2 and others), while the onset of malignancy, accompanied by a presumably broader range of gene dysregulations, may reverse this process in portions of the tumor (17). The observation of both secretory and ciliated cell differentiation in putative clonal (SBTs) neoplasms may have a parallel in PAX2-null SCOUTs (22). The latter have yet to be established as clonal, but could be explained by alteration of a cell with capacity to differentiated into both cell types, similar to SBTs.
In this study, an effort was made to standardize as much as possible the tabulation of SCOUT frequency by computing it as a function of number of cross sections examined. This revealed significant differences in frequency between controls and SBTs. However, the controls varied considerably in terms of frequency of PAX2-null SCOUTs. SCOUTs were virtually absent from the fallopian tubes of pediatric autopsies and post partum sterilization specimens. It is important to emphasize that both increasing age and number of sections examined appear to influence the number of SCOUTs detected. Thus, SCOUTs may not signify as much a precursor as a reflection of a more global dysregulation of genes in oviductal secretory epithelium that occurs with increasing age. This phenomenon may have significance beyond its association with SBT and bears further analysis in the context of multiple variables.
The distribution of SBTs is not unlike that of high grade serous carcinomas, given the involvement of ovaries and peritoneal surfaces. Based on the findings in this study, it is conceivable that a precursor condition originating in the FT in a field of multifocal gene dysregulation might gain a growth advantage over pelvic/ovarian surface mesothelium, leading to SBT. Alternatively, the factors influencing the development of PAX2-null SCOUTs in the tube could also be in play in the ovarian cortex within stromal-epithelial interactions. Confirmation of either model could be germane to the origins of both low and high grade pelvic serous neoplasms.
The more pronounced multifocal micropapillary lesions seen two individuals and illustrated in Figure 3 are similar to SCOUTs albeit more striking in their level of architectural development, and raise the intriguing possibility that the affected individuals have a unique susceptibility to epithelial outgrowths in the oviduct. Parallels can be seen to germline BRCA1 or BRCA2 mutations, reportedly associated with a significantly higher index of proliferation (and malignancy)(3,13,15), fallopian tubes from women with Li Fraumeni syndrome, which have multiple p53 signatures (24), and other germline mutation-related syndromes with multifocal neoplasia (6,10,20). The described tubal proliferations, including SCOUTs, bear some resemblance to microadenomas described in colonic carcinogenesis (18). Whether they have a similar significance remains to be determined, but their study may uncover novel pathways that intersect with those involved in the genesis of serous neoplasia.
Footnotes
Disclosures: The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Auersperg N. The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol. 2011;30:12–21. doi: 10.1097/PGP.0b013e3181f45f3e. [DOI] [PubMed] [Google Scholar]
- 2.Chen EY, Mehra K, Mehrad M, Ning G, Miron A, Mutter GL, Monte N, Quade BJ, McKeon FD, Yassin Y, Xian W, Crum CP. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–6. doi: 10.1002/path.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colgan TJ, Murphy J, Cole DE, et al. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod. 1998 Nov;13(11):3114–20. doi: 10.1093/humrep/13.11.3114. [DOI] [PubMed] [Google Scholar]
- 5.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001 Feb;38(2):87–95. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 6.Gerstenblith MR, Goldstein AM, Tucker MA. Hereditary genodermatoses with cancer predisposition. Hematol Oncol Clin North Am. 2010;24:885–906. doi: 10.1016/j.hoc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal oviduct. J. Pathol. 2006;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 10.Lynch HT, Casey MJ, Snyder CL, Bewtra C, Lynch JF, Butts M, Godwin AK. Hereditary ovarian carcinoma: heterogeneity, molecular genetics, pathology, and management. Mol Oncol. 2009;3:97–137. doi: 10.1016/j.molonc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, Gershenson DM, Mills GB, Bast RC, Jr, Lu KH. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 12.Mulligan RM. A survey of epithelial inclusions in the ovarian cortex of 470 patients. J Surg Oncol. 1976;8(1):61–6. doi: 10.1002/jso.2930080110. [DOI] [PubMed] [Google Scholar]
- 13.Norquist BM, Garcia RL, Allison KH, Jokinen CH, Kernochan LE, Pizzi CC, Barrow BJ, Goff BA, Swisher EM. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116:5261–71. doi: 10.1002/cncr.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura H, Katabuchi H. Detailed morphology of human ovarian surface epithelium focusing on its metaplastic and neoplastic capability. Ital J Anat Embryol. 2001;106(2 Suppl 2):263–76. [PubMed] [Google Scholar]
- 15.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 16.Robey SR, Silva EG. Epithelial Hyperplasia of the Fallopian Tube. Int J Gynecol Pathol. 1990;9:382–3. doi: 10.1097/00004347-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Roh MH, Yassin Y, Miron A, Mehra KK, Mehrad M, Monte NM, Mutter GL, Nucci MR, Ning G, Mckeon FD, Hirsch MS, Wa X, Crum CP. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod Pathol. 2010;23:1316–24. doi: 10.1038/modpathol.2010.119. [DOI] [PubMed] [Google Scholar]
- 18.Roncucci L, Stamp D, Medline A, Cullen JB, Bruce WR. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol. 1991;22:287–94. doi: 10.1016/0046-8177(91)90163-j. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai J, Hattori N, Nakajima M, Moriya T, Suzuki T, Yokoyama A, Kohno N. Differential expression of the glycosylated forms of MUC1 during lung development. Eur J Histochem. 2007;51:95–102. [PubMed] [Google Scholar]
- 20.Saran A. Basal cell carcinoma and the carcinogenic role of aberrant Hedgehog signaling. Future Oncol. 2010;6:1003–14. doi: 10.2217/fon.10.49. [DOI] [PubMed] [Google Scholar]
- 21.Scully RE. Pathology of ovarian cancer precursors. J Cell Biochem Suppl. 1995;23:208–18. doi: 10.1002/jcb.240590928. [DOI] [PubMed] [Google Scholar]
- 22.Sieben NL, Roemen GM, Oosting J, Fleuren GJ, van Engeland M, Prat J. Clonal analysis favours a monoclonal origin for serous borderline tumours with peritoneal implants. J Pathol. 2006;210:405–11. doi: 10.1002/path.2074. [DOI] [PubMed] [Google Scholar]
- 23.Tong GX, Chiriboga L, Hamele-Bena D, Borczuk AC. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Müllerian system origin? Mod Pathol. 2007;20:856–63. doi: 10.1038/modpathol.3800827. [DOI] [PubMed] [Google Scholar]
- 24.Xian W, Miron A, Roh M, Semmel DR, Yassin Y, Garber J, Oliva E, Goodman A, Mehra K, Berkowitz RS, Crum CP, Quade BJ. The Li-Fraumeni syndrome (LFS): a model for the initiation of p53 signatures in the distal Fallopian tube. J Pathol. 2010;220:17–23. doi: 10.1002/path.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, McKeon F, Caput D. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]