Abstract
Visceral sensory afferents during disease or following injury often produce vague, diffuse body sensations and pain referred to somatic targets. Alternatively, injury due to trauma or disease of somatic nerve targets can also lead to referred pain in visceral targets via a somatovisceral reflex. Both phenomenons are thought to be due to convergence of visceral and somatic afferents within the spinal cord. To investigate a potential peripheral influence for referred pain in visceral targets following somatic nerve injury, we examined whether a sciatic nerve injury known to produce known to produce hindpaw tactile hyperalgesia alters the frequency of micturition and the sensitivity of bladder-associated sensory neurons to pro-nociceptive chemokines. Adult female Sprague-Dawley rats received injections of cholera toxin b subunit conjugated to 555 into urinary bladder wall to retrogradely label visceral primary afferent neurons. Seven days later, the right sciatic nerve of these animals was subjected to a lysophosphatidylcholine (LPC)-induced focal demyelination injury. Pre- and post-injury tactile sensitivity in the hind paw and micturition frequency were assayed. Animals were allowed to survive for 14–28 days. Lumbosacral and lumbar dorsal root ganglia (DRG) ipsilateral to the nerve injury were acutely dissociated from sham and nerve injured animals. Bladder wall-associated sensory neurons identified via the retrograde marker were assayed for fluxes in intracellular calcium following administration of pro-nociceptive chemokines. The assayed chemokines included monocyte chemoattractant protein-1 (MCP1/CCL2) and stromal cell derived factor-1 alpha (SDF1/CXCL12). LPC nerve injured animals exhibited tactile hyperalgesia and increased micturition frequency for at least 28 days. Focal demyelination of the sciatic nerve also increased the number of injured L4L5 and non-injured L6-S2 bladder-associated sensory neurons that responded to MCP1 and SDF1 when compared with sensory neurons derived from uninjured naïve and sham-injured control animals. Taken together, this data suggests that some visceral hypersensitivity states may have a somatic origin. More importantly, nociceptive somatovisceral sensation may be mediated by upregulation of chemokine signaling in visceral sensory neurons.
Keywords: somatic nerve injury, chemokine, sensory neuron, bladder
Introduction
Chronic pelvic pain (CPP) is defined as non-menstrual or non-cyclic pelvic pain with duration of at least 6 months. The disorder can affect women and men, but is more common in women. CPP is characterized by visceral hypersensitivity, expanded dermatomes of referred sensation, and somatic hyperalgesia including the presence of tenderness on examination of the musculature of the pelvic floor and hip girdle (Fitzgerald et al., 2005, Powell-Boone et al., 2005). No pathological basis has been agreed upon for the visceral hyperalgesia present in CPP. It is possible that the clinically apparent tenderness of pelvic musculature is secondary to primary visceral (bladder) abnormalities and thus represents a form of ‘referred pain’. Alternatively, abnormalities of pelvic musculature could be a primary abnormality that is associated with secondary sensitization of both somatic and visceral peripheral nerves, producing altered (increased) bladder sensitivity (Ustinova et al., 2010). A number of studies support the notion that somatic injury or stressors can impact visceral sensitivity (Miranda et al., 2004, Cameron et al., 2008, Robbins and Ness, 2008, Montenegro et al., 2009).
Recently, animal models of somatic nerve injury have demonstrated that de novo production of neuronal chemokines/receptors and related functional signaling in both injured and adjacent, uninjured dorsal root ganglion (DRG) nociceptive neurons may be important in the maintenance of neuropathic pain behaviors as these factors produce potent excitatory effects (Oh et al., 2001, White et al., 2005, Sun et al., 2006, Dansereau et al., 2008, Gao et al., 2009, Jung et al., 2009, Thacker et al., 2009). Pro-inflammatory chemokines associated with sensory neuron hyperexcitability in rodents include monocyte chemoattractant protein-1 (MCP1) and stromal-derived factor-1 (SDF1) signaling through the chemokine receptors, CCR2 and CXCR4, respectively (Oh et al., 2001, Bhangoo et al., 2007a, Bhangoo et al., 2007b, Jung et al., 2009, Wilson et al., 2010). MCP1, in particular, is known to be colocalized with nociceptive neuropeptides (Qin et al., 2005, Jung et al., 2008) and can sensitize nociceptive sensory neurons via transactivation of the transient receptor potential channel transient receptor potential vanilloid type 1 (TRPV1) (Oh et al., 2001, Jung et al., 2008). Perhaps more importantly, studies using rodent pain models have shown that genetic deletion, small molecules and antibodies directed at the chemokine receptors, CCR2 and CXCR4, can transiently or permanently reverse both injury and drug-induced pain behavior in rodents (Abbadie et al., 2003, Bhangoo et al., 2007a, Bhangoo et al., 2007b, Dansereau et al., 2008, Bhangoo et al., 2009, Jung et al., 2009, Thacker et al., 2009, Dubovy et al., 2010, Wilson et al., 2010).
In order to explore the possibility that somatic injury may directly alter visceral sensory neuron responses to pro-inflammatory chemokines, we sought to demonstrate that there is increased sensitivity of both injured and adjacent uninjured rat primary sensory neurons following a lysophosphatidylcholine (LPC; also known as lysolecithin)-induced focal demyelination injury (Bhangoo et al., 2007a, Jung et al., 2009). LPC is a major plasma component that is produced following tissue injury as it is converted to lysophosphatidic acid by autotaxin and results in nerve demyelination and neuropathic pain by an unknown mechanism (Wallace et al., 2003, Inoue et al., 2008, Nagai et al., 2010). We now show that functional chemokine signaling is present de novo in bladder-associated DRG neurons following LPC-induced nerve injury. Given that there is evidence of increased chemokine-dependent interganglion excitability following peripheral nerve injury (White et al., 2005, Bhangoo et al., 2007a, Jung et al., 2009), activation of bladder-associated nociceptive afferent neurons by these pro-nociceptive factors may contribute to the development of visceral hypersensitivity.
Materials and Methods
Animals
Pathogen-free, adult female Sprague-Dawley rats (150–200 g; Harlan Laboratories, Madison, WI) were housed in temperature (23 ± 3°C) and light (12-h light:12-h dark cycle; lights on at 07:00 h) controlled rooms with standard rodent chow and water available ad libitum. Experiments were performed during the light cycle. Animals were randomly assigned to either LPC nerve injury (n=40), sham injury (n=32) and naïve (n=32) and all animals were subjected to retrograde tracer injections into the bladder wall. These experiments were approved by the Institutional Animal Care and Use Committee of Loyola University, Chicago. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain. Care and handling of animals were in accordance with institutional guidelines and approved by the Loyola University IACUC.
Retrograde Labeling of Bladder Afferents
At least seven days prior to sciatic nerve injury, DRG neurons were labeled by retrograde axonal transport of cholera toxin subunit B conjugated to a fluorescent chromagen (CTB-Alexa Fluor 555 [CTB-555]; Invitrogen). CTB-555 is known to retrogradely label small, medium and large neurons in the DRG affiliated with the bladder and other visceral organs (Wang et al., 1998, Dworkin et al., 2002). Under isoflurane anesthesia, a laparotomy incision was made and the pelvic viscera were exposed. Using a syringe (30-gauge), subserosal injections of CTB-555 (total injection volume was 8–10 μl) were made on both the left and right side of the bladder in multiple locations. At each injection site, the needle was kept in place for 10–20 seconds and any leakage of tracer was removed by application of a sterile cotton swab. Immediately after the injections, the bladder surface was thoroughly cleaned with sterile saline to remove any excess of tracers. Prior to closing, the peritoneal cavity was lavaged with warm sterile saline. Thereafter, the wound was closed and animal left to recover.
Foot withdrawal to punctate mechanical indentation
Following bladder injection, rodents were subjected to behavioral testing (n=8 per each of three treatment conditions). The incidence of foot withdrawal was measured in response to mechanical indentation of the plantar surface of each hind paw with sharp, Von Frey–type nylon filaments. Mechanical stimuli were applied with seven filaments, each differing in the bending force delivered (10, 20, 40, 60, 80, 100, and 120 mN), but each fitted with the same metal cylinder with a flat tip and a fixed diameter of 0.2 mm (Bhangoo et al., 2007b). In each behavioral testing sequence, the operator was blinded to the animal treatment condition.
The rat was placed on a metal mesh floor and covered with a transparent plastic dome. Typically, the animals rest quietly in this situation after an initial few minutes of exploration. Animals were habituated to this testing apparatus for 15 minutes a day, two days prior to the behavioral assays. Following acclimation each filament was applied to six spots spaced across the paw. The filaments were tested in order of ascending force, with each filament delivered in sequence from the 1st to the 6th spot alternately from one paw to the other. In the absence of withdrawal, the duration of each stimulus was 1 second and the interstimulus interval was 10–15 seconds. A cutoff value of 120 mN was used; animals that did not respond at 120 mN were assigned that value (Ma et al., 2003, Bhangoo et al., 2007b).
The incidence of foot withdrawal was expressed as a percentage of the six applications of each filament as a function of force. A Hill equation was fitted to the function (Origin version 6.0, Microcal Software, Northhampton MA) relating the percentage of indentations eliciting a withdrawal to the force of indentation. From this equation, the threshold force was obtained and defined as the force corresponding to a 50% withdrawal. A threshold that exhibits at least a −20 mN difference from the baseline threshold of testing in a given animal is representative of neuropathic pain (Ma et al., 2003).
Tactile behavioral measurements were taken on two successive days before surgery. Postoperative testing was performed on days 7–28 following nerve injury. Threshold values were statistically analyzed for each foot separately. The experimenter was blinded to the injury condition of the animal in all behavioral trials.
Micturition frequency
All treatment conditions (retrograde tracer only, retrograde tracer plus sham injury, and retrograde tracer plus LPC sciatic nerve injury; n=12 per condition) were habituated to metabolic cages (Nalgene, Rochester, New York) for 3 days prior to testing procedure. Animals were given free access to water during both habituation and testing phases. On two of the days of habituation, we measured total water consumption during a 24 h period. Water intake was measured during a 24 h period once a week following peripheral nerve injury or sham treatment.
The frequency of micturition was determined over a 150 minute period between the hours of 8:30AM and 12:00PM. Habituated animals were placed into metabolic cages with fluid collecting apparatus removed. Every 15 minutes during the test period, blotting paper placed underneath each metabolic cage was monitored for wet spots (Randich et al., 2006, DeBerry et al., 2010). If a wet spot was found in the 15 minute time period, the investigator would replace the paper with a new sheet and record the occurrence. At the end of the test period, the number of wet spots was tabulated to reach a total number of micturition events for the 150 minutes. Micturition frequency for all conditions was performed weekly until 28 days following surgery.
Somatic Nerve Injury
Animals previously injected with CTB-555 were anesthetized with 4% isoflurane and maintained on 2% isoflurane (Halocarbon, River Edge, NJ) in O2. For all demyelination experiments, lysophosphatidylcholine (LPC), (type V, 99% pure; Sigma-Aldrich, St. Louis, MO) was dissolved in buffered sterile saline (pH 7.2) to give a final concentration of 10 mg/ml. The right sciatic nerve of the rat was exposed at the mid-thigh level under sterile conditions. A sterile polyvinyl acetal (PVAc) sponge (Ivalon, San Diego, CA), 2-mm × 2-mm soaked in 7 μl of LPC, was placed adjacent to the sciatic nerve and left in place as the dermal incision site was closed with 5.0 prolene suture. Sham control animals were prepared as described above, but buffered sterile saline was used in place of LPC plus saline.
Preparation of acutely dissociated dorsal root ganglion neurons
Lumbosacral DRG (L6–S2) and lumbar DRG (L4/L5) were harvested from animals subjected to CTB-555 bladder labeling only, CTB-555 bladder labeling plus sham vehicle injury on post-operative day 14–21 and CTB-555 bladder labeling in combination with LPC treatment on post-operative day 14–21. The DRGs were maintained as seperate groups i) L6–S2 and ii) L4/L5. The excised tissues was allowed to digest in HBSS containing collagenase A and collagenase D for 20 minutes (1 mg/ml; Roche Applied Science, Indianapolis, IN). DRGs were then treated with HBSS containing papain (30 units/ml, Worthington Biochemical, Lakewood, NJ), .5 mM EDTA, and cysteine at 35°C. The tissue was then washed with DMEM. The cells were dissociated via mechanical titration in culture media (10% fetal bovine serum, 100 mg/ml and 100 U/ml penicillin and streptomycin, and N2 from Life Technologies in Ham’s F12) containing 1 mg/ml bovine serum albumin and trypsin inhibitor (1 mg/ml, Sigma, St. Louis MO). The dissociated cells were plated on coverslips coated with Poly-L-Lysine and 1 mg/ml laminin and incubated for 2 hours at 37°C and 5 % CO2 prior to being fed with more culture media. Plated cells were then incubated for 12–15 hours before use for calcium imaging experiments. At least 8 animals were used for each treatment condition.
Calcium imaging
The AM form of fura-2 (Invitrogen) was used as the fluorescent Ca2+ indicator. The dissociated DRG cells were loaded with fura-2 AM in a balanced salt solution (BSS) [NaCl (140 mM), Hepes (10 mM), CaCl2 (2 mM), MgCl2 (1 mM), glucose (10 mM), KCl (50 mM)] for 20 minutes at room temperature. The cells were then washed three times with BSS. Intracellular calcium was measured by digital video microfluorometry with an intensified CCD camera coupled to a microscope and MetaFluor software (Molecular Devices Corporation, Downington, PA). 1 ml each of MCP1 and SDF1 (R & D Systems, Minneapolis, MN; 100 nM in BSS), were separately applied directly onto the dissociated cells in random order and in 5 minute intervals. The cells were continuously perfused with BSS for all experiments except during chemokine application during which cells were exposed to the chemokine solution for 1 minute. High K+, capsaicin and adenosine-5′-triphosphate (ATP) were added to further assess the identity and viability of the assayed cells. A response to high K+ stimulation indicates the presence of voltage-dependent Ca2+ channels, which is indicative of neurons. Capsaicin was added to determine whether the neuron was a TRPV1 nociceptive neuron or a non-TRPV1 neuron. Administration of ATP determined whether the neuron or non-neuronal cell was viable. the The concentrations of chemokines used in these experiments were all supramaximal to ensure activation of any expressed receptors (Bhangoo et al., 2007a). At least 50 CTB-555+ neurons were assayed for each experimental data point.
Statistical Analyses
Data is presented as the mean ± SEM, unless otherwise noted. GraphPad Software (LaJolla, CA) was used to statistically evaluate all data. Frequency of micturition differences between treatment conditions was determined by two-way ANOVA with Bonferroni’s post-hoc test for animal urinary and tactile behavior groups. A difference of p<0.05 was considered significant. Statistical significance of differences in calcium response among sham and treatment groups was determined using Chi-square test with Yates correction with p<0.05 set as statistical significance.
RESULTS
Fluorescent retrograde labeling of lumbosacral dorsal root ganglion with cholera toxin B subunit
Bladder afferent neurons were labeled in the L6 S2 DRG when CTB-555 was injected into the wall of the bladder. CTB-555 positive cells were observed in L6 S2 DRG (Fig. 1B). No CTB-555 positive cells were observed in any of the L4/L5 DRG (Fig. 1A). The distribution of CTB-555 positive cells is largely in accordance with previous descriptions of lumbosacral innervation in the rat bladder (Janig and McLachlan, 1987, Sengupta and Gebhart, 1994).
Figure 1.
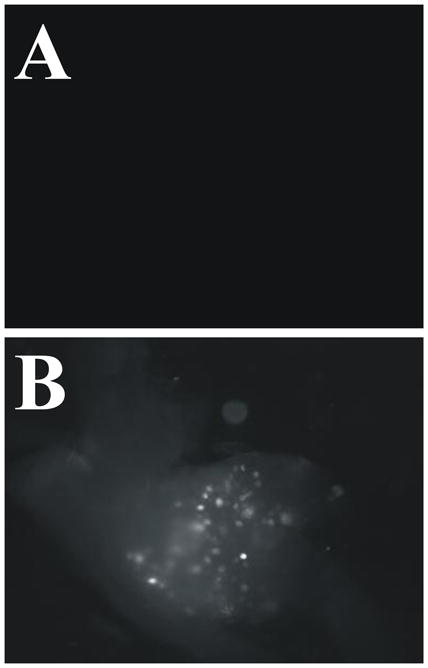
Wholemount dorsal root ganglia (DRG) exhibit fluorescent sensory neurons following bladder wall injections of cholera toxin β subunit-conjugated to Alexa Fluor 555 (CTB-555). Three days after bladder wall injections, CTB-555 was observed in both lumbar (B) S1 DRG. CTB-555 was absent in L4 DRG (A).
Mechanical stimuli elicit tactile hyperalgesia following LPC- induced sciatic nerve demyelination
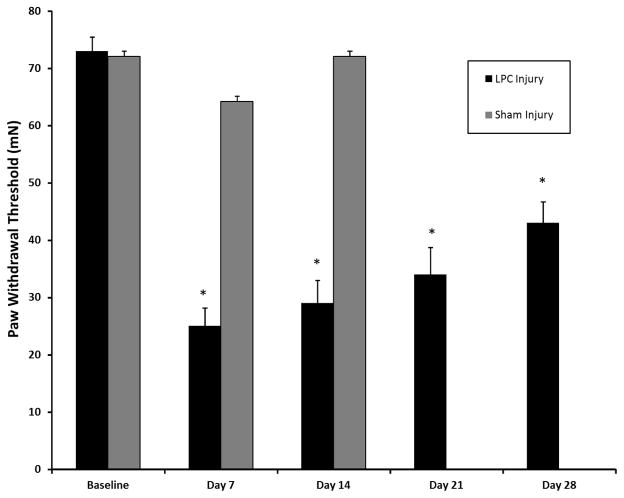
Similar to previous findings using the focal demyelination of the sciatic nerve injury model (Wallace et al., 2003, Bhangoo et al., 2007a, Jung et al., 2009), we observed alterations in the threshold force necessary for eliciting a flexion hindpaw withdrawal reflex in the rat. Neither naïve nor sham-injured rodents in combination with bladder wall injections of CTB-555 exhibited a paw withdrawal threshold decrease that was significant at any timepoint up to and including post-operative day14 (data not shown). In contrast, the force required to elicit paw withdrawal was diminished by post-operative day 14–28 in animals subjected to a combination of LPC demyelination and CTB-555 bladder wall injection (Fig. 2; p<0.001).
Figure 2.
Mean threshold force required for paw withdrawal to von Frey stimulation at 7, 14, 21, and 28 days following lysophosphatidylcholine (LPC)-induced focal nerve demyelination. Each data point is the mean threshold (± SE) force on the hindpaw ipsilateral (right foot; gray bar) or contralateral (left foot; black bar) to the focal nerve injury site eliciting a withdrawal response. Thresholds for both hindpaw were significantly different from pre-injury baseline for at least 28 days. Analysis was performed using two-way ANOVA followed by the Bonferroni post-hoc pairwise comparisons (*p < 0.01).
Injury-induced changes in frequency of micturition
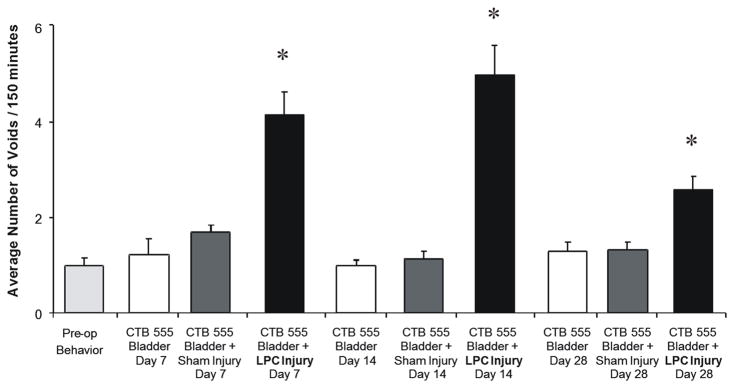
The frequency of micturition events were performed in treatment groups that had been subjected to CTB-555 bladder injections, CTB-555 bladder injections in combination with sham injury to the sciatic nerve injury and CTB-555 bladder injections in combination with LPC-induced focal demyelination injury (n=8–10 per condition). Mean frequency of micturition at baseline was 1.2±0.14 per 150 minutes (Fig 3). Following LPC-induced peripheral nerve injury, the frequency of micturition increased to 4.16±0.48, 5.00±0.64 and 3.17±0.31, at day 7, 14 and 28, respectively. All LPC injury- induced changes in frequency of micturition were significantly different from pre-operative events (Two-way ANOVA F=20.61, p<0.01 vs baseline). In comparison, sham injury affiliated changes in frequency of micturition at day 7 (1.6±0.61), 14 (1.33±0.42), or 28 (1.27±0.31) did not differ from pre-operative levels at any time point. Animals subjected to CTB-555 alone exhibited a micturition frequency of 1.3±0.75, 1.0±0.43, and 1.20±0.28 at 7, 14 and 28 days post injury, respectively.
Figure 3.
Lysophosphatidylcholine (LPC)-induced focal demyelination of the sciatic nerve induced a substantial increase in the frequency of micturition. The micturition frequency was monitored for each animal (2.5 hours) before and after random assignment to one of three treatment conditions. Micturition frequency was significantly different from pre-operative averages from 7–28 days in animals subjected to a combination of cholera toxin β subunit conjugated to a fluorescent tracer (CTB-555) injected into the wall of the bladder and LPC-induced focal nerve demyelination. Control conditions including animals subjected to either bladder wall injections of CTB-555 only or sham-injury in combination with CTB555 did not reach significance by day 7. All treatment conditions were subjected to bladder wall injections of CTB-555 at least 7 days prior to micturition frequency assays. Analysis was performed using two-way ANOVA followed by the Bonferroni post-hoc pairwise comparisons (*p < 0.01).
The observed increase in the frequency of micturition events did not appear to be due to increased water consumption as we failed to observe differences in the volume of water intake between the treatment groups throughout the testing period (data not shown).
Chemokines increase intracellular Ca2+ in somatic and bladder-associated DRG neurons following sciatic nerve injury
Affirmation that somatic sensory neurons exhibit functional chemokine receptors after LPC injury was first determined in cells dissociated from DRGs directly affiliated with the sciatic nerve (L4/L5 DRG). The numbers of chemokine responsive neurons associated with the LPC treated sciatic nerve were significantly increased when compared with L4/L5 sensory neurons derived from the sham injured animals (see Table 1).
Table 1.
Table illustrates the responsiveness of acutely isolated sensory neurons derived from different sets of ganglia to chemokines and capsaicin in fura-2-based Ca2+ imaging experiments.
| Chemokine/Receptor | L4/L5 sham (n=56) | L4/L5 LPC (n=66) | L6-S2 CTB+ sham (n=63) | L6-S2 CTB+ LPC (n=51) |
|---|---|---|---|---|
| SDF1/CXCR4 | 5.3% | 35.1%* | 4.6% | 26%* |
| MCP1/CCR2 | 5.3% | 22.6%* | 6.0% | 20%* |
| Capsaicin | 42% | 45% | 35% | 40% |
The value in the parentheses indicates the number of DRG neurons in which [Ca2+]i transients were induced after single additions of each substance/the total numbers of DRG neurons tested (*= p<0.05; Chi-square test with Yates correction; each treatment condition compared to sham controls). Following chemokine application, capsaicin was used to characterize the identity of responsive neurons as either non-nociceptive or nociceptive and is based on the total number of assayed cells.
As noted in a previous publication (Bhangoo et al., 2007a), nearly half of the assayed L4/L5 sensory neurons derived from both sham injured and animals subjected to LPC exhibited capsaicin-induced intracellular Ca2+ changes. The percentages of capsaicin-responsive neurons did not differ between the treatment conditions.
Discussion
Previous studies have demonstrated the important role of chemokine signaling in neuropathic pain models. In the present study, we demonstrated that LPC-induced demyelination of the sciatic nerve effectively increased the number of chemokine-responsive bladder-associated L6-S2 sensory neurons in addition to sensory neurons directly affiliated with the LPC nerve injury (L4L5). Notably, many of these chemokine responsive neurons were likely nociceptors based on capsaicin-induced calcium fluxes. Concurrent with the increased numbers of bladder-associated L6-S2 sensory neurons that responded to chemokines following LPC-induced demyelination, we also observed an increase in the frequency of micturition events. Taken together, L4L5 and L6-S2 chemokine-responsive sensory neurons may serve to respectively alter tactile hyperalgesia and bladder sensibility in the peripheral nerve injured rodent.
The increased numbers of chemokine-responsive sensory neurons observed in the L4L5 DRG between post-operative day 14–28 largely agrees with a previous peripheral nerve injury study (Bhangoo et al., 2007a). Of greater interest is the increased number of bladder-associated sensory neurons that became responsive to chemokines following LPC injury to the sciatic nerve. As recent studies demonstrating that reversal of tactile hyperalgesia following the systemic administration of a stereospecific CCR2 receptor antagonist isomer or inhibition of CXCR4 by AMD3100 suggests that the de novo chemokine/receptor signaling in bladder-associated sensory neurons may serve to sensitize nociceptive sensory neurons (Bhangoo et al., 2007a, Bhangoo et al., 2007b, Bhangoo et al., 2009, Jung et al., 2009, Dubovy et al., 2010, Wilson et al., 2010). Moreover, the effectiveness of chemokine receptor antagonists are likely not limited to somatovisceral sensibilities as neuronal CXCL12/CXCR4 signaling in both normal micturition and with bladder hyperreflexia following bladder inflammation are altered following the administration of AMD3100 (Arms et al., 2009).
That the prolonged functional chemokine receptor expression of CCR2 and CXCR4 in bladder-associated sensory neurons may be a significant contributor to chronic pain syndromes following peripheral nerve injury, there is a necessity to define the mechanisms that underlie upregulation of the injury-induced chemokine expression in adjacent, uninjured sensory neurons (L6-S2) as they may represent novel targets for therapeutic intervention. Numerous studies to date clearly demonstrate that distal nerve injury and axon degeneration of motor and/or sensory fibers associated with the L5 DRG are capable of influencing neighboring L4 nerve fibers and induce functional changes in the L4 DRG sensory neurons (Ma et al., 2003, Obata et al., 2004, He et al., 2010). Somewhat similar injury circumstances are also known to alter function of both L6 DRG neurons including the de novo synthesis of the chemokine MCP1 and its cognate receptor, CCR2 (White et al., 2005).
Considering that both the pelvis and distal extremities are innervated by an overlapping complex system of somatic, sympathetic, and parasympathetic nerves (Pinter and Szolcsanyi, 1995, Wesselmann and Lai, 1997), our present findings may further serve to suggest that somatic nerve injury directly or indirectly influence the sensitivity of bladder-associated afferents to pro-nociceptive chemokines; a type of somatovisceral hypersensitivity. How this action may occur is unclear. One possibility is that inter- or intraganglion excitability may serve to alter information derived from somato- or viscerotopic sensory territories. Alternatively, there is the potential for dichotomizing fibers of neurons associated with the bladder and sciatic nerve subserving a somatic nerve-visceral nerve convergence (Bahr et al., 1981, McNeill and Burden, 1986, Dawson et al., 1992, Wesselmann and Lai, 1997). Evidence to support either of these concepts would necessitate that chemokine-positive neurons with distal axons in the sciatic nerve (e.g. L6 DRG) be juxtaposed with bladder-associated sensory neurons expressing either CCR2 or CXCR4. More importantly, there must be evidence that chemokines are both expressed and released by sensory neurons.
To date it is known that chemokines can be upregulated in a number of sensory neurons following peripheral nerve injury or inflammation (Qin et al., 2005, White et al., 2005, Bhangoo et al., 2007a, Bhangoo et al., 2007b, Yang et al., 2007, Jung et al., 2008, Bhangoo et al., 2009, Jeon et al., 2009, Jung et al., 2009, Dubovy et al., 2010, Wang et al., 2010, Wilson et al., 2010). There is also direct evidence that adjacent, uninjured ganglia undergo similar changes following injury to the peripheral nervous system (White et al., 2005). As evidence of the possible neuronal release of MCP1, recent studies have demonstrated that this chemokine is processed through the secretory pathway into large dense-core vesicles and released from the somata of cultured neurons by Ca2+-dependent exocytosis (Jung et al., 2008). There is also evidence that F11 neuroblastoma-sensory neuron hybrid cells transfected to produce SDF releases the chemokine in an activity-independent manner (Wilson et al., 2010). The possibility that MCP-1/CCR2 signaling participates in cell to cell communication within the ganglion potentially affecting both somatic and visceral associated sensory neurons is supported by a number of studies (Bhangoo et al., 2007a, Bhangoo et al., 2007b, Jung et al., 2008, Bhangoo et al., 2009, Jung et al., 2009). Similar events may be present with regard to SDF-1/CXCR4 signaling between sensory neurons (Wilson et al., 2010).
The possibility that the aforementioned intraganglionic cell to cell communication via chemokine signaling present in injured ganglia may subserve the possibility of somatovisceral cross-sensitization (hind paw and bladder) is supported by the potentially maladaptive influence of neurogenic inflammation in target tissues such as the bladder (Bjorling et al., 2003, Noronha et al., 2007, Rudick et al., 2007, Merriam et al., 2010). The somatic nerve injury may induce neurogenic inflammation and the subsequent release of Substance P or calcitonin gene-related peptide (CGRP) in distal somatic or visceral targets. If the bladder is the associated visceral target associated with neurogenic inflammation, one might presuppose that capsaicin sensitive unmyelinated bladder afferent C-fibers may mediate changes in some urodynamic properties, including the voiding reflex (Maggi and Conte, 1990).
Data to support the possibility that intraganglionic mixing of efferent signals might impact both somatic and visceral targets was elegantly demonstrated by Pinter and Szolcsanyi (1995). These investigators demonstrated that antidromic stimulation of lumbosacral dorsal roots could elicit plasma extravasation in both the plantar skin of the hind paw and urinary bladder simultaneously (Pinter and Szolcsanyi, 1995).
Subsequently, it could be inferred that the intraganglionic cell to cell communication via chemokine signaling present in L6-S2 DRG may also impart changes in distal and central targets such as the increased frequency of micturition observed in the injured rodent. Alternatively, the mechanism responsible for somatovisceral cross-sensitization may be similar to cross-convergence between pelvic organs such as the bladder and the colon which may be partially responsible for viscerovisceral referred pain in patients with gastrointestinal and genitourinary disorders (Malykhina, 2007). This mechanism is dependent on dichotomizing primary afferents that innervate two distinct structures such as distal colon and bladder and suggests that increased nociceptive input from one organ can sensitize the affiliated organ by neurogenic inflammatory processes (Christianson et al., 2007). Viscerosomatic sensitization may also be responsible as inflamed visceral afferents can trigger secondary somatic hyperalgesia (referred to the hindpaw) (Jaggar et al., 1999, Miranda et al., 2004, Robbins and Ness, 2008). However, the consensus is that this effect is largely due to the convergence of visceral afferents onto the same spinal segments as somatic afferents (viscerosomatic convergence) (Cameron et al., 2008). In addition to complex mechanisms in the spinal cord, the development of visceral hyperalgesia in the pelvic area likely involves supraspinal neural pathways and descending pain pathways (Brumovsky and Gebhart, 2009).
The recognition that somatic abnormalities can give rise to secondary visceral effects is important, and may offer new treatment paradigms. We believe it is likely that many patients with urogenital pain syndromes may have a underlying primary somatic problem: hence their symptoms are unlikely to resolve unless their somatic abnormality is addressed. Notably, individuals with painful bladder syndromes commonly exhibit tension and tenderness of the pelvic floor musculature (Weiss, 2001, Peters et al., 2007) and the beneficial effect of myofascial physical therapy for individuals suffering from urological chronic pelvic pain syndromes has recently been described in a NIH-sponsored multicenter feasibility study (FitzGerald et al., 2009). Moreover, recognition that chemokine pathways may be integral to the maintenance of chronic pain in a number of clinical conditions may provide some hope as many of these injury paradigms are reversible with the use of chemokine receptor antagonists (Bhangoo et al., 2007a, Bhangoo et al., 2007b, Arms et al., 2009, Bhangoo et al., 2009, Jung et al., 2009).
In conclusion, the results obtained in the present study indicate that primary afferent neurons associated with the rat bladder exhibit increased chemokine receptor signaling following sciatic nerve injury. These results suggest a role for either MCP1/CCR2 and/or SDF1/CXCR4 signaling in bladder-associate pain syndromes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Risha Foster, Department of Urology, Loyola University Health System, Maywood, IL.
Jeanette Jung, Department of Cell Biology, Neurobiology & Anatomy, Loyola University, Chicago, Maywood, IL.
Ahmer Farooq, Urology, Loyola University Health System, Maywood, IL.
Christopher McClung, Department of Urology, Loyola University Health System, Maywood, IL.
Matthew S. Ripsch, Department of Cell Biology, Neurobiology & Anatomy, Loyola University, Chicago, Maywood, IL
Mary P. Fitzgerald, Division of Female Pelvic Medicine & Reconstructive Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Maywood, IL
Fletcher A. White, Department of Cell Biology, Neurobiology & Anatomy and Anesthesiology, Loyola University, Chicago, Maywood, IL.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms L, Girard BM, Vizzard MA. Expression and Function of CXCL12/CXCR4 in Rat Urinary Bladder with Cyclophosphamide (CYP) - Induced Cystitis. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr R, Blumberg H, Janig W. Do dichotomizing afferent fibers exist which supply visceral organs as well as somatic structures? A contribution to the problem or referred pain. Neurosci Lett. 1981;24:25–28. doi: 10.1016/0304-3940(81)90353-0. [DOI] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007a;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007b;21:581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol Pain. 2009;5:48. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorling DE, Beckman M, Saban R. Neurogenic inflammation of the bladder. Adv Exp Med Biol. 2003;539:551–583. doi: 10.1007/978-1-4419-8889-8_37. [DOI] [PubMed] [Google Scholar]
- Cameron DM, Brennan TJ, Gebhart GF. Hind paw incision in the rat produces long-lasting colon hypersensitivity. J Pain. 2008;9:246–253. doi: 10.1016/j.jpain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008;106:757–769. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- Dawson NJ, Schmid H, Pierau FK. Pre-spinal convergence between thoracic and visceral nerves of the rat. Neurosci Lett. 1992;138:149–152. doi: 10.1016/0304-3940(92)90493-q. [DOI] [PubMed] [Google Scholar]
- DeBerry J, Randich A, Shaffer AD, Robbins MT, Ness TJ. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain. 2010;11:247–255. doi: 10.1016/j.jpain.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P, Klusakova I, Svizenska I, Brazda V. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol. 2010;133:323–337. doi: 10.1007/s00418-010-0675-0. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, Turner JA, Mancl L, Wilson L, Massoth D, Huggins KH, LeResche L, Truelove E. A randomized clinical trial of a tailored comprehensive care treatment program for temporomandibular disorders. J Orofac Pain. 2002;16:259–276. [PubMed] [Google Scholar]
- FitzGerald MP, Anderson RU, Potts J, Payne CK, Peters KM, Clemens JQ, Kotarinos R, Fraser L, Cosby A, Fortman C, Neville C, Badillo S, Odabachian L, Sanfield A, O’Dougherty B, Halle-Podell R, Cen L, Chuai S, Landis JR, Mickelberg K, Barrell T, Kusek JW, Nyberg LM. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2009;182:570–580. doi: 10.1016/j.juro.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24:627–632. doi: 10.1002/nau.20178. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, Wei XH, Li YY, Xin WJ, Qin ZH, Liu XG. TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010;151:266–279. doi: 10.1016/j.pain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 2008;152:296–298. doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- Janig W, McLachlan EM. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987;67:1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Lee KM, Cho HJ. Expression of monocyte chemoattractant protein-1 in rat dorsal root ganglia and spinal cord in experimental models of neuropathic pain. Brain Res. 2009;1251:103–111. doi: 10.1016/j.brainres.2008.11.046. [DOI] [PubMed] [Google Scholar]
- Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar Electrophysiological Changes in Axotomized and Neighboring Intact Dorsal Root Ganglion Neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- McNeill DL, Burden HW. Convergence of sensory processes from the heart and left ulnar nerve onto a single afferent perikaryon: a neuroanatomical study in the rat employing fluorescent tracers. Anat Rec. 1986;214:441–444. 396–447. doi: 10.1002/ar.1092140416. [DOI] [PubMed] [Google Scholar]
- Merriam FV, Wang ZY, Hillard CJ, Stuhr KL, Bjorling DE. Inhibition of fatty acid amide hydrolase suppresses referred hyperalgesia induced by bladder inflammation. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Montenegro ML, Gomide LB, Mateus-Vasconcelos EL, Rosa-e-Silva JC, Candido-dos-Reis FJ, Nogueira AA, Poli-Neto OB. Abdominal myofascial pain syndrome must be considered in the differential diagnosis of chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2009;147:21–24. doi: 10.1016/j.ejogrb.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Nagai J, Uchida H, Matsushita Y, Yano R, Ueda M, Niwa M, Aoki J, Chun J, Ueda H. Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol Pain. 2010;6:78. doi: 10.1186/1744-8069-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha R, Akbarali H, Malykhina A, Foreman RD, Greenwood-Van Meerveld B. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol. 2007;293:F1461–1467. doi: 10.1152/ajprenal.00311.2007. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KM, Carrico DJ, Kalinowski SE, Ibrahim IA, Diokno AC. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16–18. doi: 10.1016/j.urology.2007.02.067. [DOI] [PubMed] [Google Scholar]
- Pinter E, Szolcsanyi J. Plasma extravasation in the skin and pelvic organs evoked by antidromic stimulation of the lumbosacral dorsal roots of the rat. Neuroscience. 1995;68:603–614. doi: 10.1016/0306-4522(95)00104-q. [DOI] [PubMed] [Google Scholar]
- Powell-Boone T, Ness TJ, Cannon R, Lloyd LK, Weigent DA, Fillingim RB. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832–1836. doi: 10.1097/01.ju.0000176747.40242.3d. [DOI] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotropin-releasing factor receptors. J Pain. 2008;9:991–998. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1191–1198. doi: 10.1152/ajpregu.00411.2007. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29:77–81. doi: 10.1002/nau.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VCJ, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal Lysolecithin-Induced Demyelination of Peripheral Afferents Results in Neuropathic Pain Behavior That Is Attenuated by Cannabinoids. J Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Zou LJ, Zhang YL, Jiao YF, Sun JH. The excitatory effects of the chemokine CCL2 on DRG somata are greater after an injury of the ganglion than after an injury of the spinal or peripheral nerve. Neurosci Lett. 2010;475:48–52. doi: 10.1016/j.neulet.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Wang HF, Shortland P, Park MJ, Grant G. Retrograde and transganglionic transport of horseradish peroxidase-conjugated cholera toxin B subunit, wheatgerm agglutinin and isolectin B4 from Griffonia simplicifolia I in primary afferent neurons innervating the rat urinary bladder. Neuroscience. 1998;87:275–288. doi: 10.1016/s0306-4522(98)00061-x. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J Urol. 2001;166:2226–2231. doi: 10.1016/s0022-5347(05)65539-5. [DOI] [PubMed] [Google Scholar]
- Wesselmann U, Lai J. Mechanisms of referred visceral pain: uterine inflammation in the adult virgin rat results in neurogenic plasma extravasation in the skin. Pain. 1997;73:309–317. doi: 10.1016/S0304-3959(97)00112-7. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NM, Jung H, Ripsch MS, Miller RJ, White FA. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.12.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Mitchell K, Keller JM, Iadarola MJ. Peripheral inflammation increases Scya2 expression in sensory ganglia and cytokine and endothelial related gene expression in inflamed tissue. J Neurochem. 2007;103:1628–1643. doi: 10.1111/j.1471-4159.2007.04874.x. [DOI] [PubMed] [Google Scholar]