Abstract
OBJECTIVE
Recent work has shown that insulin stimulates its own secretion in insulin-sensitive humans, suggesting that insulin resistance in the β-cell could cause β-cell dysfunction. We have tested whether insulin exposure and insulin sensitivity modulate β-cell function in subjects with normal glucose tolerance (NGT) and whether they contribute to dysglycemia in impaired glucose regulation (IGR).
RESEARCH DESIGN AND METHODS
Insulin sensitivity (by euglycemic clamp), insulin-induced secretory response at isoglycemia (IISR) (as C-peptide percent change from basal during the clamp), glucose-induced secretory response (GISR) to an intravenous glucose bolus, and β-cell glucose sensitivity (β-GS) (by oral glucose tolerance test [OGTT] modeling) were measured in 1,151 NGT and 163 IGR subjects from the RISC (Relationship between Insulin Sensitivity and Cardiovascular Disease) study.
RESULTS
In NGT, IISR was related to both insulin sensitivity and antecedent insulin exposure; GISR was related to insulin exposure. IISR was positively, if weakly, related to β-GS (r= 0.16, P < 0.0001). Both IISR (−23 [39] vs. −9 [2]%, median [interquartile range], P < 0.03) and β-GS (69 [47] vs. 118 [83] pmol ⋅ min–1 ⋅ m–2 ⋅ mmol–1 ⋅ L, P < 0.0001) were decreased in IGR compared with NGT. Insulin sensitivity and β-GS were the major determinants of mean OGTT glucose in both NGT and IGR, with a minor role for IISR. In a multivariate logistic model, IGR was predicted by β-GS (odds ratio 4.84 [95% CI 2.89–8.09]) and insulin sensitivity (3.06 [2.19–4.27]) but not by IISR (1.11 [0.77–1.61]).
CONCLUSIONS
Pre-exposure to physiological hyperinsulinemia stimulates insulin secretion to a degree that depends on insulin sensitivity. However, this phenomenon has limited impact on β-cell dysfunction and dysglycemia.
β-Cells are richly endowed with insulin receptors and their intracellular signaling machinery (1); the role of insulin signaling in the regulation of β-cell function is, however, still debated. Whereas historically insulin has been thought to “exert a negative feedback on β-cells, recent data provide evidence for a positive role of insulin in transcription, translation, ion flux, insulin secretion, proliferation, and β-cell survival” (rev. in 2). A recent report in healthy volunteers has shown that, in young insulin-sensitive volunteers, exposure to euglycemic hyperinsulinemia for 4 h causes a greater increment in glucose-stimulated insulin secretion compared with a saline infusion (3). This finding has led to the attractive proposal that the same defect in insulin signaling may underlie both the insulin resistance and the β-cell dysfunction that characterize glucose intolerance. By this paradigm, insulin-sensitive subjects should have an enhanced β-cell function compared with insulin-resistant subjects. This prediction seems at odds with the concept of a reciprocal relationship between insulin secretion and insulin sensitivity (4), which originates from the observation that high insulin sensitivity is associated with lower acute insulin response to an intravenous glucose bolus. However, we have previously shown that some modes of response of the β-cell compensate for insulin resistance while others do not (5,6). Thus, fasting insulin secretion rate and total insulin output after an acute stimulus are generally upregulated in insulin-resistant individuals, whereas β-cell glucose sensitivity is largely independent of insulin action. Therefore, β-cell dysfunction originating from β-cell insulin resistance may still be compatible with the increased responses commonly observed in insulin-resistant states.
In this work, we set forth to investigate whether whole-body insulin sensitivity extends to insulin sensitivity in the β-cell and whether it is an important determinant of β-cell function. For this purpose, we used data from the RISC (Relationship between Insulin Sensitivity and Cardiovascular Disease) study (7), in which a large number of nondiabetic subjects, including individuals with impaired glucose regulation (IGR), were recruited. In this study, an isoglycemic-hyperinsulinemic clamp with C-peptide measurements was performed, which allowed evaluation of whole-body insulin sensitivity and the effect of hyperinsulinemia on insulin secretion, assessed from C-peptide. In this cohort, β-cell function was also well characterized by intravenous and oral glucose tolerance tests (OGTTs); we could thus assess whether the β-cell response to hyperinsulinemia was an important determinant of β-cell function.
RESEARCH DESIGN AND METHODS
RISC is a prospective observational cohort study for which rationale and methodology have been described previously (7). In brief, participants were recruited from the local population at 19 centers in 14 countries in Europe, according to the following inclusion criteria: either sex, age range 30–60 years, and clinically healthy. Initial exclusion criteria were as follows: treatment for obesity, hypertension, lipid disorders or diabetes, pregnancy, cardiovascular or chronic lung disease, weight change of ≥5 kg in the last 3 months, cancer (in the last 5 years), and renal failure. Exclusion criteria after screening were as follows: arterial blood pressure ≥140/90 mmHg, fasting plasma glucose ≥7.0 mmol/L, 2-h plasma glucose (on a standard 75-g OGTT) ≥11.0 mmol/L, total serum cholesterol ≥7.8 mmol/L, serum triglycerides ≥4.6 mmol/L, or electrocardiogram abnormalities. Baseline examinations began in June 2002 and were completed in November 2005. The present analysis is based on the baseline data of 1,314 subjects who satisfied all criteria, were nondiabetic on the OGTT by the 1997 American Diabetes Association criteria (8), and had all the parameters used for this analysis. These subjects were classified as having normal glucose tolerance (NGT)—defined as a fasting glucose level <6.11 mmol/L and a 2-h glucose level <7.78 mmol/L—or IGR, the latter category encompassing impaired fasting glycemia (i.e., a fasting plasma glucose level between 6.11 and 7.00 mmol/L) and impaired glucose tolerance (i.e., a fasting glucose <7.00 mmol/L and a 2-h glucose between 7.78 and 11.1 mmol/L).
Local ethics committee approval was obtained by each recruiting center. Volunteers were given detailed written information on the study as well as oral explanation, and they all signed a consent form.
OGTT.
On the first day, in the morning after an overnight fast, a 75-g OGTT was performed with sampling at baseline and 30, 60, 90, and 120 min after glucose ingestion for the measurement of glucose, insulin, and C-peptide concentrations.
Isoglycemic clamp.
On a separate day within 1–3 weeks of the OGTT, an isoglycemic-hyperinsulinemic clamp was performed in all subjects. Exogenous insulin was administered as a primed-continuous infusion at a rate of 240 pmol ⋅ min–1 ⋅ m−2 simultaneously with a variable 20% dextrose infusion adjusted every 5 min to maintain plasma glucose level within 15% of the individual fasting glucose level. The clamp procedure was standardized across centers with the use of a demonstration video and written operating instructions; the raw data from each clamp study were immediately transferred to the coordinating center, where they underwent quality control scrutiny according to preset criteria. Blood was sampled throughout the clamp for the measurement of glucose. One or two fasting blood samples and two samples during the last 40 min of the clamp were taken for measurement of glucose, insulin, and C-peptide concentrations.
Glucose bolus.
In 935 subjects, at the end of the isoglycemic clamp, a glucose bolus (0.3 g per kilogram body weight) was administered intravenously over 1 min; plasma glucose and C-peptide concentrations were measured 2, 4, 6, and 8 min after the injection.
Analytical methods.
Blood collected during the studies was separated into plasma and serum, aliquoted, and stored at –20°C for glucose and –80°C for insulin and C-peptide measurement in the central laboratories. Samples were transported on dry ice at prearranged intervals to the laboratories. Plasma glucose was measured by the glucose oxidase technique. Plasma insulin and C-peptide were measured by a two-sited time-resolved fluoroimmunoassay (AutoDELFIA Insulin Kit; Wallac Oy, Turku, Finland) using monoclonal antibodies, with the following assay characteristics: sensitivity 1–2 and 5 pmol/L, respectively; within-assay coefficient of variation 4–5% for both; and between-assay variation 5 and 3.5%.
Calculations.
Insulin sensitivity was expressed as the M value (9), calculated during the final 40 min of the 2-h clamp. Insulin sensitivity was normalized to fat-free mass (FFM)—as measured by the Tanita bioimpedance balance (Tanita UK, International Division)—in units of μmol ⋅ min–1 ⋅ kgFFM–1. Glucose, insulin, and C-peptide concentrations during the clamp were calculated as the mean values in the final 40 min of the test, when a steady state had been achieved for each variable.
The acute insulin secretory response to the intravenous glucose bolus (glucose-induced secretory response [GISR]) was expressed as the mean incremental C-peptide concentration during the first 8 min after the glucose bolus (GISRC-pep) as well as the ratio of incremental insulin secretion (calculated by C-peptide deconvolution [10]) to the plasma glucose increment during the same time interval (GISRsecr).
β-Cell function was assessed from the OGTT using a model that describes the relationship between insulin secretion and glucose concentration, which has been illustrated in detail previously (11,12). In brief, the model expresses the dependence of insulin secretion (in pmol ⋅ min–1 ⋅ m−2) on absolute glucose concentration at any time point during the OGTT through a dose-response function relating the two variables. The characteristic parameter of the dose response is the mean slope over the observed glucose concentration range, denoted as β-cell glucose sensitivity (β-GS) (13). Model parameters were estimated from glucose and C-peptide concentrations by regularized least-squares, as previously described (11,12). Regularization involves the choice of smoothing factors that were selected to obtain glucose and C-peptide model residuals with SDs close to the expected measurement error (∼1% for glucose and ∼4% for C-peptide). Insulin secretion rates were calculated from the model every 5 min.
Statistical analysis.
Data are reported as means ± SD or medians (interquartile range) for variables with a skewed distribution (by Shapiro-Wilk W test). Because both insulin sensitivity and steady-state plasma insulin concentration have a skewed distribution, their impact on insulin secretory parameters was first analyzed by stratifying the primary data (in quartiles) and using two-way ANOVA to simultaneously assess sex differences. Next, general linear models were used to test the simultaneous dependence of each insulin secretory parameter on multiple explanatory variables (i.e., systematically controlling for center, sex, age, BMI, family history of diabetes, and plasma glucose concentration); the effect size was expressed as the regression coefficient (calculated for 1 SD of the explanatory variable). Logistic regression was performed by standard methods and reported as odds ratio and 95% CI, calculated for 1 SD of the explanatory variable.
Statistical analyses were run using JMP 7.0 (SAS Institute, 2007); a P ≤ 0.05 was considered significant.
RESULTS
In the whole cohort of subjects with NGT, insulin sensitivity (M value, from the isoglycemic-hyperinsulinemic glucose clamp) had a median value of 53 μmol ⋅ min–1 ⋅ kgFFM–1 and a 95% tolerance interval of 21–92 μmol ⋅ min–1 ⋅ kgFFM–1, a 4.5-fold range. Likewise, the mean plasma glucose concentration during the steady-state period of the clamp had a median of 5.03 mmol/L and a 95% tolerance interval of 4.08–6.06 mmol/L, a 1.5-fold range; mean steady-state plasma insulin concentration had a median of 401 pmol/L and a 95% tolerance interval of 216–614 pmol/L, a threefold range.
The main clinical and metabolic data of the entire cohort by sex-specific quartile of insulin sensitivity value are shown in Table 1. Although matched on sex, age, and fasting glucose concentrations, more insulin-resistant NGT subjects were characterized by higher BMI, fasting hyperinsulinemia, higher fasting insulin secretion rate, and GISR (from postclamp intravenous glucose bolus injection) but similar β-GS (from OGTT modeling analysis). Fasting C-peptide concentrations were progressively higher across insulin sensitivity quartiles.
TABLE 1.
Anthropometric and metabolic parameters in subjects with NGT by quartile of insulin sensitivity (M) and in subjects with IGR
| NGT (M quartile) |
IGR | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| n | 287 | 289 | 288 | 287 | 163 |
| Insulin sensitivity (μmol · min–1 · kgFFM–1)° | 80 (19) | 61 (13) | 48 (11) | 34 (13) | 38 (26)++ |
| Women (%) | 55 | 55 | 55 | 55 | 52 |
| Age (years) | 43 ± 8 | 44 ± 9 | 44 ± 8 | 44 ± 8 | 46 ± 8+ |
| Family history of diabetes (%)° | 19 | 22 | 26 | 34 | 43+ |
| BMI (kg/m2)° | 23.9 ± 3.0 | 24.7 ± 3.4 | 25.1 ± 3.8 | 27.2 ± 4.5 | 27.6 ± 4.4+ |
| Fasting glucose (mmol/L) | 5.00 ± 0.46 | 5.04 ± 0.51 | 4.98 ± 0.49 | 5.00 ± 0.56 | 5.36 ± 0.65++ |
| Fasting insulin (pmol/L)° | 21 (12) | 25 (16) | 28 (16) | 37 (29) | 38 (32)++ |
| Fasting C-peptide (pmol/L)° | 403 (203) | 468 (223) | 497 (269) | 627 (339) | 675 (403)++ |
| Steady-state plasma glucose (mmol/L) | 5.14 ± 0.66 | 5.10 ± 0.57 | 4.98 ± 0.53 | 5.04 ± 0.51 | 5.28 ± 0.53++ |
| Steady-state plasma insulin (pmol/L)§ | 393 (109) | 412 (118) | 401 (115) | 405 (135) | 415 (130)++ |
| Steady-state plasma C-peptide (pmol/L)° | 499 (521) | 425 (355) | 411 (313) | 514 (392) | 530 (425) |
| Fasting insulin secretion rate (pmol · min–1 · m−2)° | 57 (23) | 63 (30) | 70 (33) | 87 (43) | 91 (57)++ |
| β-GS (pmol · min–1 · m–2 · mmol−1 · L) | 118 (80) | 124 (86) | 120 (86) | 113 (73) | 68 (48)++ |
| IISR (%) | 25 (105) | −8 (60) | −16 (50) | −22 (43) | −23 (39)++ |
| GISRC-pep (pmol/L)° | 515 (437) | 712 (532) | 822 (709) | 861 (566) | 671 (538)++ |
| GISRsecr (pmol · min–1 · m–2 · mmol−1 · L)* | 425 (346) | 471 (322) | 509 (417) | 483 (383) | 378 (286)++ |
Data are means ± SD or medians (interquartile range), unless indicated otherwise. GISRC-pep and GISRsecr are calculated in 935 subjects (n = 815 for NGT and n = 120 for IGR).
*P = 0.03.
§P < 0.01.
°P < 0.0001 for NGT quartiles by Kruskal-Wallis test.
+P < 0.01 or less for the difference between IGR and NGT by Mann-Whitney U test;
++P = 0.01 or less for the difference between IGR and NGT, adjusted for center, sex, familial diabetes, age, and BMI.
Insulin-induced secretory response.
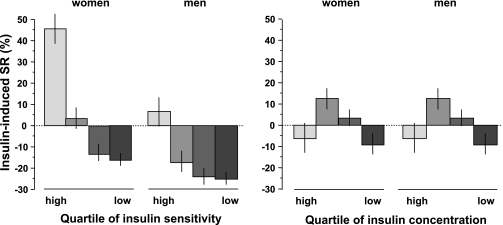
We first examined the impact of insulin sensitivity (M) and antecedent insulinization (i.e., the steady-state plasma insulin concentrations during the clamp) on the β-cell secretory response under isoglycemic conditions. This insulin-induced secretory response (IISR) was calculated as the percent change from baseline of steady-state plasma C-peptide concentrations. IISR was found to be positively associated with insulin sensitivity, ranging from a median 25% increase in the most insulin-sensitive subject group (men and women together) to a median 22% inhibition in the most insulin-resistant group (Table 1); the pattern was significantly accentuated in women (P < 0.0001 for both main effects by two-way ANOVA, P < 0.001 for the sex-by-M interaction) (Fig. 1).
FIG. 1.
Insulin-induced secretory response (SR), as the percent changes in plasma C-peptide concentrations during isoglycemic hyperinsulinemia, by sex-specific quartile of insulin sensitivity (as the M value on the clamp, left panel) and by quartile of steady-state plasma insulin levels (right panel) shown separately for men and women (n = 1,151) with NGT. Plots are median ± SEM.
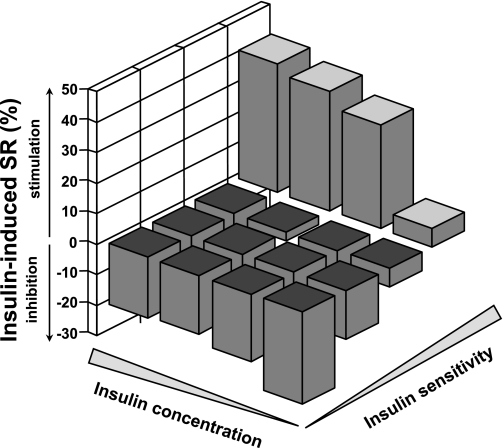
The continuous relationship between IISR and insulin sensitivity was well described by a linear fit (standardized regression coefficient = 0.39, adjusted for center, sex, age, and BMI; P < 0.0001). The relationship between IISR and steady-state plasma insulin concentrations was also positive but less pronounced than with insulin sensitivity and more evident in women (standardized regression coefficient = 0.10, adjusted for center, sex, age, and BMI; P < 0.0001). In bivariate analysis, IISR was a function of both insulin sensitivity (P < 0.001) and steady-state insulin concentrations (P = 0.045), with a significant positive interaction (P < 0.01 for the top quartiles) (Fig. 2).
FIG. 2.
Insulin-induced secretory response (SR), as the percent changes in plasma C-peptide concentrations during isoglycemic hyperinsulinemia, by sex-specific quartile of insulin sensitivity and by quartile of steady-state plasma insulin levels in 1,151 subjects with NGT. The height of each bar represents the median for the corresponding cell.
A full regression analysis controlling for relevant modifiers of both insulin action and secretion—namely, sex, age, family history of diabetes, and BMI—revealed that IISR increased by 23% for each 21 μmol ⋅ min–1 ⋅ kgFFM–1 of insulin sensitivity increment and, independently, by 5% for each 114 pmol/L increment in steady-state plasma insulin concentrations, while maintaining a strong dependence on plasma glucose levels (17% increase per each 0.58 mmol/L increase in plasma glucose) (Table 2). Of note, IISR was predicted to decrease by 8% for each 8 years of age, independently of the other factors.
TABLE 2.
Multivariate analysis of IISR (as percent change in C-peptide from baseline) and GISRC-pep
| Variable (category or 1 SD) | Regression coefficient | SE | Partial correlation coefficient | P |
|---|---|---|---|---|
| IISR (%)* | ||||
| Sex (F) | 5 | 2 | 0.086 | 0.005 |
| Familial diabetes (yes vs. no) | −2 | 2 | −0.040 | 0.185 |
| Age (8 years) | –8 | 1 | –0.156 | <0.0001 |
| BMI (3.9 kg/m2) | 1 | 2 | 0.015 | 0.612 |
| Steady-state plasma glucose (0.58 mmol/L) | 17 | 2 | 0.282 | <0.0001 |
| Steady-state plasma insulin (114 pmol/L) | 5 | 2 | 0.080 | 0.008 |
| Insulin sensitivity (21 μmol ⋅ min–1 ⋅ kgFFM–1) | 23 | 2 | 0.359 | <0.0001 |
| GISRC-pep (pmol/L)† | ||||
| Sex (F) | –39 | 16 | –0.087 | 0.014 |
| Familial diabetes (yes vs. no) | –44 | 17 | –0.093 | 0.009 |
| Age (8 years) | –44 | 15 | –0.109 | 0.004 |
| BMI (3.9 kg/m2) | 16 | 16 | 0.034 | 0.335 |
| Glucose increment (1.99 mmol/L) | 109 | 18 | 0.224 | <0.0001 |
| Insulin sensitivity (21 μmol ⋅ min–1 ⋅ kgFFM–1) | –68 | 17 | –0.136 | 0.0002 |
| Steady-state plasma insulin (114 pmol/L) | 64 | 16 | 0.140 | <0.0001 |
For continuous variables, the regression coefficient (mean ± SE) is calculated for 1 SD of the variable.
*Total explained variance = 40%.
†Total explained variance = 34%.
Glucose-induced secretory response.
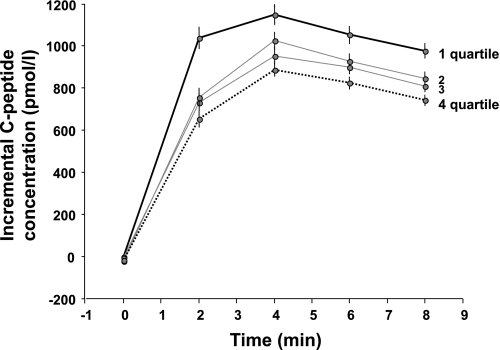
We next examined whether insulin sensitivity and pre-exposure to hyperinsulinemia had any effect on the secretory response to acute hyperglycemia applied by an intravenous glucose bolus (GISR). GISR was positively associated with the degree of antecedent hyperinsulinemia, whether expressed as GISRC-pep (P < 0.0001 between extreme quartiles; Fig. 3) or GISRsecr (P = 0.02 between extreme quartiles; data not shown).
FIG. 3.
Glucose-induced secretory response (as the incremental C-peptide concentrations in response to an intravenous glucose bolus) by quartile of antecedent steady-state plasma insulin concentration in 815 men and women with NGT who underwent the glucose bolus test. Median (interquartile range) plasma insulin concentrations for the quartiles (1–4) are 522 (85), 436 (35), 378 (30), and 309 (51) pmol/L. Plots are mean ± SEM.
On the intravenous glucose test, plasma glucose concentrations rose by a median of 7 mmol/L over the 8 min after bolus injection, with a wide inter-individual variability (95% tolerance interval of 3.48–9.61 mmol/L). Because plasma glucose is the primary stimulus for endogenous insulin secretion, it was necessary to also adjust multivariate analyses for the mean glucose increment during the 8 min of hyperglycemia. The full model showed that GISRC-pep increased by 63 pmol/L for each 118 pmol/L increment in antecedent plasma insulin concentrations; both insulin sensitivity and age were negatively related to GISRC-pep (Table 2). When replacing GISRC-pep with GISRsecr in this model, prior hyperinsulinemia was still positively associated with GISRsecr, with a predicted increase of 31 pmol ⋅ min–1 ⋅ m–2 ⋅ mmol−1 ⋅ L for each 118 pmol/L increment in steady-state plasma insulin concentrations (P = 0.003).
β-Cell glucose sensitivity.
Finally, we analyzed β-GS as determined from modeling of the OGTT. This parameter was not related to insulin sensitivity either in univariate analysis (Table 1) or after controlling for standard covariates (center, sex, age, BMI, and familial diabetes). However, in a fully adjusted model, β-GS was significantly related to both IISR (r= 0.16 [95% CI 0.097–0.22], P < 0.0001) and GISRC-pep (0.24 [0.18–0.30], P < 0.0001).
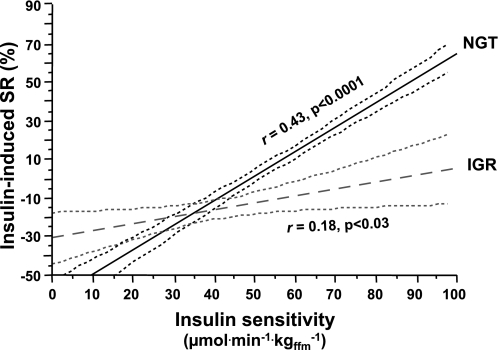
To examine the relative role of the three measures of β-cell function (IISR, GISR, and β-GS) in glucose tolerance, we analyzed the data of the subjects with IGR (comprising subjects with impaired fasting glucose or glucose intolerance). As expected, subjects in this category were older, heavier, and more insulin resistant than the NGT group (Table 1). With regard to β-cell function, IISR (−23 [39] vs. −9 [2]%, median [interquartile range], P < 0.03), GISRC-pep (674 [549] vs. 712 [611] pmol/L, P < 0.0001), and β-GS (69 [47] vs. 118 [83] pmol ⋅ min–1 ⋅ m–2 ⋅ mmol−1 ⋅ L, P < 0.0001) were all impaired in IGR compared with NGT (P adjusted for center, sex, age, BMI, familial diabetes, insulin sensitivity, and plasma glucose and insulin levels). In particular, in IGR subjects, IISR was not significantly related to antecedent insulinemia, and its relationship to insulin sensitivity was weak and with a flatter slope than in NGT (Fig. 4). By multiple logistic regression adjusting for sex, age, BMI, and familial diabetes, IGR was predicted by β-GS (odds ratio 4.84 [95% CI 2.89–8.09]) and insulin sensitivity (3.06 [2.19–4.27]) but not by IISR (1.11 [0.77–1.61]) or GISRC-pep (1.15 [0.89–1.48]).
FIG. 4.
Relationship between insulin-induced secretory response response (SR), as the percent changes in plasma C-peptide concentrations during isoglycemic hyperinsulinemia, and insulin sensitivity (as the M value on the clamp) in subjects with NGT and subjects with IGR. Dotted lines are 95% CIs for the linear fits. The two lines differ from one another at the P = 0.0004 level.
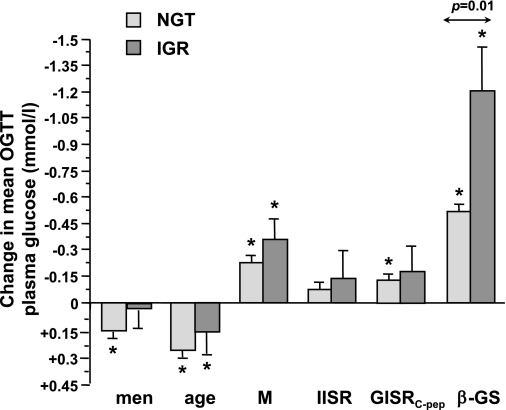
Finally, using the mean plasma glucose concentration during the OGTT as the outcome variable, the contribution of relevant factors was calculated separately for the NGT and IGR group. As plotted in Fig. 5, the contribution of sex (male), age, insulin sensitivity, IISR, and GISRC-pep was similar in absolute value between IGR and NGT subjects, whereas the contribution of β-GS was more than twice as high in IGR as in NGT (all changes calculated for 1 SD of the predictor). Percentagewise, a 1-SD decrement in β-GS accounted for 38% of glucose increments in NGT and 59% in IGR.
FIG. 5.
Contribution of sex, age, insulin sensitivity (M), IISR, GISRC-pep, and β-GS to the mean glucose concentration during the OGTT. Bars are regression coefficients (mean ± SEM) calculated for 1 SD of the continuous variable in separate multiple regression models for NGT and IGR, each adjusted for center, family history of diabetes, and BMI. Asterisks denote mean values that are significantly different from zero. The P value is for the difference between NGT and IGR.
DISCUSSION
In our study cohort, plasma glucose and insulin concentrations and insulin sensitivity values on the isoglycemic clamp each spanned a wide range, thereby making it possible to explore their role in β-cell response from interindividual variations. The large number of observations also allowed adequate control for confounding by sex, age, BMI, and familial diabetes.
We show that, in subjects with NGT, the β-cell response to insulin under isoglycemic conditions (IISR) is positively related to both degree of hyperinsulinemia and insulin sensitivity independently of one another (Fig. 1 and 2). The statistical model in Table 2 predicts that for the same strength of applied hyperinsulinemia, fasting insulin secretion would increase (from an average of 60 to 89 pmol ⋅ min–1 ⋅ m−2) in the subjects in the most insulin-sensitive quartile and decrease (from 91 to 81 pmol ⋅ min–1 ⋅ m−2) in the most insulin-resistant quartile. This result confirms the findings recently reported in insulin-resistant subjects with type 2 diabetes (14). Incidentally, it should be noted that in the NGT group as a whole, clamp C-peptide levels were suppressed to 91% of the basal value on average; this is in agreement with the historical view that the overall effects of hyperinsulinemia on its own secretion is inhibitory (15). In addition, IISR was predicted to increase by 5% for each 114 pmol/L difference in plasma insulin levels, a smaller effect compared with that of insulin sensitivity. Thus, while maintaining its tight dependence on plasma glucose concentrations, even within the “euglycemic” range, β-cell insulin secretion responds to changes in insulin levels, as dictated by its own insulin sensitivity, i.e., a classic feed-forward mechanism.
Whether the effect of insulin on its own secretion is direct or mediated by changes in other hormones or substrates cannot be decided from the current results with certainty. However, in our dataset, fasting plasma glucagon or proinsulin concentrations had no impact on IISR (data not shown). Furthermore, free fatty acids during the clamp were significantly lower in insulin-sensitive subjects than in insulin-resistant subjects (data not shown) and were unrelated to IISR. Also, previous studies (16) have shown that insulinotropic amino acids decrease with hyperinsulinemia to a lesser extent in insulin-resistant subjects than in insulin-sensitive subjects; thus, their stimulatory effect on insulin secretion would be stronger in insulin-resistant individuals than in insulin-sensitive individuals, i.e., the opposite trend than was observed in our subjects. Therefore, pending a definitive demonstration, the current findings are compatible with direct in vivo effects of insulin exposure on β-cell secretory activity.
The coexistence of stimulating and suppressive effects remains unexplained, as already pointed out (2). A slight C-peptide suppression could be due to increased C-peptide clearance (+7%) induced by hyperinsulinemia, as suggested in a recent study (3). However, there is no evidence that such a small effect may differ between insulin-sensitive and insulin-resistant individuals. The fact that IISR responds to circulating insulin concentrations appears to be at odds with the notion of an autocrine mechanism, by which insulin receptors on β-cells would be engaged by very high pericellular insulin levels. An intriguing possibility is that once stimulated, insulin-sensitive β-cells release more ATP, which then amplifies insulin secretion via ATP-gated P2X3 receptors (17). Other pathways may interfere with the generation of cellular messengers directly stimulating exocytosis (e.g., calcium), which fundamentally depend on glucose metabolism (18). Additional mechanisms based on divergent intracellular pathways may be at play and remain to be identified.
The β-cell secretory response during acute hyperglycemia (GISR) also depended on the antecedent degree of insulinization (Fig. 3). This result confirms the recent finding in a group of healthy volunteers, in whom 4 h of high-physiological hyperinsulinemia (∼1,000 pmol/L) led to a 40% higher integrated insulin response compared with basal insulin levels. In fact, our model predicts that a gradient of ∼500 pmol/L in antecedent insulin concentrations (on equal grounds of hyperglycemia) would give rise to a difference in GISRC-pep of ∼300 pmol/L (i.e., +40% of the average GISRC-pep; Fig. 3) and to a difference of 3.7 units of secreted insulin over 8 min. Thus, extent of antecedent insulin exposure is a more powerful stimulus to insulin release under hyperglycemic conditions than under euglycemic conditions. In contrast, the association of GISRC-pep with insulin sensitivity was reciprocal (Table 2). As we have previously emphasized (6), this association is largely attributable to an increase in fasting insulin secretion, which strongly depends on insulin sensitivity. Thus, even after full adjustment for key covariates, we did not find that GISRC-pep was enhanced in subjects with high insulin sensitivity, as was the case for IISR. However, this could be a limitation of correlation analysis, which may be inherently unable to distinguish the indirect influence of insulin resistance (through fasting insulin secretion) from a possible direct enhancing effect of insulin sensitivity.
In the current study, we also show that both IISR and GISR are related to β-cell glucose sensitivity, the key parameter of β-cell function derived from the OGTT (5,6). However, these relationships were weak, suggesting that the three indices—insulin-induced, intravenous glucose–induced, and oral glucose–induced secretory response—reflect distinct modes of β-cell function. In fact, in contrast to IISR, β-cell glucose sensitivity was not different across quartiles of insulin sensitivity (Table 1). The question of the relative role of these secretory responses in the β-cell dysfunction of glucose intolerance was addressed in the IGR group of the study cohort. The key findings are as follows: 1) all three β-cell responses were compromised in IGR individuals, but the adjusted difference in IISR was 5% more suppression and 90 pmol/L less GISRC-pep (or –11%) in IGR than NGT. In contrast, β-GS was markedly reduced in IGR (by ∼40%); 2) in logistic regression analysis, neither IISR nor GISRC-pep was a significant predictor of IGR, whereas a low β-GS carried a fivefold increased probability of a subject being glucose intolerant; 3) in the multiple regression model of Fig. 5, β-cell glucose sensitivity and insulin sensitivity played the most significant role in glucose tolerance, whereas IISR and GISRC-pep provided only a small contribution; and 4) impaired β-GS was the dominant factor in the dysglycemia of IGR, contributing significantly more than in NGT subjects.
Limitations of our study are 1) the multicenter European-only database, 2) the fact that IISR was estimated on the basis of two baseline and two steady-state plasma samples, and 3) the categories of impaired fasting glycemia and impaired glucose tolerance were pooled (as IGR) to not lose statistical power.
In summary, the current study has identified important aspects of β-cell function, such as its control by antecedent insulin exposure and insulin sensitivity, which are likely to imply a direct feedback mechanism. The understanding of the role of insulin signaling in the β-cell is still limited, but there is evidence that stimulation of the insulin receptor activates pathways with different functions (2). In any case, our results suggest that insulin resistance at the β-cell—resulting from defect(s) in intracellular signaling—is not the main secretory abnormality in glucose-intolerant individuals and therefore does not support the contention that insulin resistance, in peripheral tissues and the β-cell, may be the sole cause of hyperglycemia, as previously proposed (3). Conversely, it is possible that a primary defect in the β-cell, expressed in vivo as glucose insensitivity, is the cause rather than the consequence of the diminished role of insulin sensitivity in the secretory response of glucose intolerance.
ACKNOWLEDGMENTS
The RISC study was supported by European Union Grant QLG1-CT-2001-01252.
No potential conflicts of interest relevant to this article were reported.
A.M. and E.F. analyzed the data and wrote the manuscript. A.T. contributed to data analysis and manuscript editing. A.N., C.A., B.B., N.L., and M.W. contributed to data generation and manuscript reviewing.
This work was submitted to the 47th European Association for the Study of Diabetes (EASD) Annual Meeting, Lisbon, Portugal, 12–16 September 2011, and accepted for poster presentation.
APPENDIX
The RISC centers and principal investigators: Amsterdam, the Netherlands: J. Dekker, EMGO Institute, Vrije Universiteit Amsterdam; Athens, Greece: A. Mitrakou, National and Kapodistrian University of Athens; Belgrade, Serbia: N. Lalic, Clinical Center of Serbia, Institute for Endocrinology, Diabetes and Metabolic Diseases; Dublin, Ireland: J. Nolan, Steno Diabetes Center A/S, Gentofte, Denmark; Frankfurt, Germany: T. Konrad, Clinic of Pediatrics I, Johnann Wolfgang Goethe Universitat am Main; Geneva, Switzerland: A. Golay, Division of Therapeutical Teaching for Chronic Diseases, University Hospital Geneva; Glasgow, Scotland: J.R. Petrie, BHF Glasgow Cardiovascular Research Centre, University of Glasgow; Kuopio, Finland: M. Laakso, Department of Medicine, Kuopio University Hospital; London, England: S.W. Coppack, Academic Medical Unit, The Royal London Hospital, Whitechapel; Lyon, France: M. Laville, Pavillon X, Hopital E Herriot; Madrid, Spain: R. Gabriel, Unidad de Investigacion, Hospital Universitario La Paz; Malmö, Sweden: P. Nilsson, Department of Medicine, University Hospital; Milan, Italy: P.M. Piatti, Unità di Malattie Metaboliche Medicina 1, Istituto Scientifico San Raffaele; Newcastle-upon-Tyne, England: M. Walker, Department of Medicine, University of Newcastle upon Tyne; Odense, Denmark: H. Beck-Nielsen, Odense University Hospital, Department of Endocrinology M; Padova, Italy: A. Mari, National Research Council Institute of Biomedical Engineering; Perugia, Italy: G.B. Bolli, DiMI, University of Perugia; Pisa, Italy: E. Ferrannini, Department of Internal Medicine, University of Pisa; Rome, Italy: G. Mingrone, Istituto di Medicina Interna e Geriatria, Policlinico A Gemelli; Vienna, Austria: C. Anderwald, Clinical Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna; Villejuif, France: B. Balkau, INSERM U 258.
Further information on the RISC study and participating centers can be found at www.egir.org.
Footnotes
A list of the RISC centers and principal investigators is available in the appendix.
REFERENCES
- 1.Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu Rev Physiol 2003;65:313–332 [DOI] [PubMed] [Google Scholar]
- 2.Leibiger IB, Leibiger B, Berggren PO. Insulin signaling in the pancreatic beta-cell. Annu Rev Nutr 2008;28:233–251 [DOI] [PubMed] [Google Scholar]
- 3.Bouche C, Lopez X, Fleischman A, et al. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci U S A 2010;107:4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 6.Mari A, Tura A, Natali A, et al. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia 2010;53:749–756 [DOI] [PubMed] [Google Scholar]
- 7.Hills SA, Balkau B, Coppack SW, et al. The EGIR-RISC STUDY (The European group for the study of insulin resistance: relationship between insulin sensitivity and cardiovascular disease risk): I. Methodology and objectives. Diabetologia 2004;47:566–570 [DOI] [PubMed] [Google Scholar]
- 8.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 10.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 11.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of β-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 2002;283:E1159–E1166 [DOI] [PubMed] [Google Scholar]
- 12.Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 2002;51(Suppl. 1):S221–S226 [DOI] [PubMed] [Google Scholar]
- 13.Mari A, Ferrannini E. Beta-cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab 2008;10(Suppl. 4):77–87 [DOI] [PubMed] [Google Scholar]
- 14.Anderwald C, Tura A, Grassi A, et al. Insulin infusion during normoglycemia modulates insulin secretion according to whole-body insulin sensitivity. Diabetes Care 2011;34:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elahi D, Nagulesparan M, Hershcopf RJ, et al. Feedback inhibition of insulin secretion by insulin: relation to the hyperinsulinemia of obesity. N Engl J Med 1982;306:1196–1202 [DOI] [PubMed] [Google Scholar]
- 16.Tessari P, Cecchet D, Cosma A, et al. Insulin resistance of amino acid and protein metabolism in type 2 diabetes. Clin Nutr 2011;30:267–272 [DOI] [PubMed] [Google Scholar]
- 17.Jacques-Silva MC, Correa-Medina M, Cabrera O, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A 2010;107:6465–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000;49:1751–1760 [DOI] [PubMed] [Google Scholar]