Abstract
OBJECTIVE
Since the pioneering work of Claude Bernard, the scientific community has considered the liver to be the major source of endogenous glucose production in all postabsorptive situations. Nevertheless, the kidneys and intestine can also produce glucose in blood, particularly during fasting and under protein feeding. The aim of this study was to better define the importance of the three gluconeogenic organs in glucose homeostasis.
RESEARCH DESIGN AND METHODS
We investigated blood glucose regulation during fasting in a mouse model of inducible liver-specific deletion of the glucose-6-phosphatase gene (L-G6pc−/− mice), encoding a mandatory enzyme for glucose production. Furthermore, we characterized molecular mechanisms underlying expression changes of gluconeogenic genes (G6pc, Pck1, and glutaminase) in both the kidneys and intestine.
RESULTS
We show that the absence of hepatic glucose release had no major effect on the control of fasting plasma glucose concentration. Instead, compensatory induction of gluconeogenesis occurred in the kidneys and intestine, driven by glucagon, glucocorticoids, and acidosis. Moreover, the extrahepatic action of glucagon took place in wild-type mice.
CONCLUSIONS
Our study provides a definitive quantitative estimate of the capacity of extrahepatic gluconeogenesis to sustain fasting endogenous glucose production under the control of glucagon, regardless of the contribution of the liver. Thus, the current dogma relating to the respective role of the liver and of extrahepatic gluconeogenic organs in glucose homeostasis requires re-examination.
Since the pioneering work of Claude Bernard in the 19th century, the scientific community has long considered the liver to be the major source of endogenous glucose production (EGP) in any postabsorptive situation (1). Nevertheless, EGP can also be performed by the kidneys and the intestine, particularly during fasting (2–6) or under protein feeding (7,8). In these two situations, quantifications of kidney and intestinal glucose release have suggested that the liver might contribute far less glucose than expected (<50%). These data questioned the predominance of the liver in EGP. The liver, kidney, and intestine are the only organs capable of producing glucose in blood because they are the only organs known to express the catalytic subunit (G6PC) of the glucose-6-phosphatase (G6Pase) system (9). Indeed, G6Pase is the crucial enzyme of EGP. It catalyzes the hydrolysis of glucose-6 phosphate, an obligate step, common to glycogenolysis and gluconeogenesis, occurring immediately before the release of glucose in blood. Moreover, in humans, mutations in the G6PC gene result in glycogen storage disease type 1a (GSD1a), an autosomal recessive metabolic disorder. Patients are unable to maintain blood glucose concentration and suffer from severe hypoglycemia episodes if not treated by continuous feeding (10–12). A total knock-out model for the G6pc gene was developed, but these mice are unable to survive weaning despite glucose injections every 8 h (13).
Recently, we developed a novel mouse model of GSD1a in which G6pc deletion exclusively targeted the liver (L-G6pc−/− mice) (14). L-G6pc−/− mice are viable and show all hepatic hallmarks of GSD1a, i.e., accumulation of glucose-6 phosphate, glycogen, and triglycerides (14). This results in marked hepatomegaly and steatosis. It is noteworthy that L-G6pc−/− mice exhibit normoglycemia in the fed state (14). Thus, this animal model can be used to address the question of the predominance of the liver and of the capacity of extrahepatic gluconeogenesis in the control of glucose homeostasis in the absence of food glucose. We hypothesized that L-G6pc−/− would regulate their blood glucose during long-term fasting as a result of the induction of extrahepatic glucose production. We also identified novel mechanisms involved in the induction of gluconeogenesis in the kidneys and intestine, in the absence of liver glucose production. These novel findings were also extended to the wild-type (WT) physiology.
RESEARCH DESIGN AND METHODS
Generation of liver-specific G6pc-null mice.
The lack of hepatic G6Pase was obtained by a specific deletion of G6pc exon 3 in the liver using a CRE-lox strategy in mice, which was recently described by Mutel et al. (14). Male adult (6–8 weeks old) B6.G6pclox/lox.SAcreERT2/w and C57Bl/6J mice (Charles Rivers Laboratories) were injected intraperitoneally once daily with 100 µL of tamoxifen (10 mg/mL) on 5 consecutive days, to obtain L-G6pc−/− and L-G6pc+/+ (WT) mice, respectively (14). Mice were housed in the animal house of Lyon 1 University, in controlled temperature (22°C) conditions, with a 12-h light–12-h-dark cycle. Mice had free access to water and standard chow (unless fasted). Mice were studied 5 weeks after tamoxifen treatment. The specific hepatic deletion of G6pc exon 3 in the L-G6pc−/− mice was always verified by PCR on purified liver genomic DNA after euthanasia, as described by Mutel et al. (14). All procedures were performed in accordance with the guidelines established by the European Convention for the Protection of Laboratory Animals. The animal care committee of the University approved all experiments.
Metabolic studies.
Blood was withdrawn from the retro-orbital vein for plasma metabolite determinations. Glucose, triglyceride, and total cholesterol concentrations were determined with Biomérieux colorimetric kits. Free fatty acid and glycerol concentrations were determined with a Diasys colorimetric kit. Insulin, glucagon, and corticosterone concentrations were determined with mouse ELISA kits from Chrystal Chem, Biovendor, and Neogen, respectively. Catecholamines and amino acids levels were determined by high-performance liquid chromatography (Centre Hospitalier Universitaire, Lyon). β-Hydroxybutyrate was measured using Optium β−ketone test strips with Optium Xceed sensors (Abbott Diabetes Care, U.K.). Blood glucose was determined with an Accu-Chek Go glucometer (Roche Diagnostics) during fasting experiments. Hepatic glycogen determinations were carried out as described by Keppler and Decker (15).
In vivo analyses.
EGP was determined in conscious, unrestrained, catheterized mice. A catheter was inserted into the right jugular vein under anesthesia, and mice were allowed to recover for 4–6 days. After 6 h or 24 h of fasting, mice were infused with a bolus (92.5 kBq) of [3-3H] glucose. We then administered [3-3H] glucose at a rate of 6.3 kBq/min. Blood glucose was monitored every 15 min. In preliminary experiments, we checked that a steady state of plasma [3-3H] glucose-specific activity reached from 30 min of infusion (data not shown). Plasma glucose concentration did not vary during the infusion time. At a steady state of glycemia and [3-3H] glucose-specific activity (between 60 to 90 min), blood was collected to determine glucose specific activity and glucose concentration (16). EGP was calculated as Ra using the simplified equation of Steele, by dividing the [3-3H] glucose infusion rate by the [3-3H] glucose-specific activity (16).
l-Alanine and l-glutamine tolerance tests were performed after 6 h or 24 h of fasting. Mice were injected intraperitoneally with l-alanine or l-glutamine (2 g/kg BW). Blood glucose levels were determined from the tail vein at 0, 15, 30, 45, 60, and 120 min after injection.
For glucagon challenge, WT mice were injected intraperitoneally with 0.9% NaCl or glucagon (Glucagen, Novo Nordisk) at a dose of 200 ng/g of weight. Mice were killed by cervical dislocation after 30 min. Kidneys and intestine were rapidly removed and fixed in formaldehyde for chromatin immunoprecipitation (ChIP) experiments, as described previously (17).
For GcgR antagonist experiments, L-G6pc−/− mice were dosed by gavage with 50 mg/kg of GcgR antagonist L168,049 (Glucagon receptor antagonist II, Calbiochem) in 5% PEG-400/5% Tween-80/90% H2O or vehicle, as described previously (18). Immediately after gavage, mice were fasted and killed 6 h later.
Gene expression analysis.
Mice were killed by cervical dislocation. The liver and kidneys were rapidly removed, with tongs chilled previously in liquid N2, and frozen. The intestine (proximal jejunum) was rinsed and immediately frozen in liquid N2. G6Pase and PEPCK-c activities were assayed at maximal velocity, as described previously (19). Immunoblotting was carried out using purified anti-rat G6PC (20), anti-PEPCK (Santa Cruz Biotechnology), and antiglutaminase (kindly provided by Dr. N. Curthoys) antibodies. Membranes were reprobed with anti-β-actin monoclonal antibody for standardization. Total RNAs were isolated from tissues with TRIzol reagent. Reverse transcription and real-time PCR were performed using sequence-specific primers (Supplementary Table 1), with ribosomal protein mL19 transcript (Rpl19) as a reference.
ChIP assays.
Nuclear isolation and ChIP assays were performed as described (17). Chromatin obtained was then sheared with the Enzymatic shearing kit (Active Motif), yielding chromatin fragments of 500–200 bp in size. Each immunoprecipitation was performed with about 10 µg of chromatin using the ChIP-It Express kit (Active Motif). Chromatin complexes were immunoprecipitated for 16–18 h at 4°C while rotating with either phospho-CREB (pS133) antibody (Epitomics), glucocorticoid receptor antibody (sc-1004; Santa Cruz Biotechnology), or GFP antibody (Santa Cruz) as a negative control. After DNA purification, quantitative PCR amplification was performed using primers specific for the cAMP or glucocorticoid response units of Pck1 and G6pc promoters (Supplementary Table 1).
Statistical analyses.
Results are reported as means ± SEM. The various groups were compared by Mann-Whitney tests. Differences were considered statistically significant at P < 0.05.
RESULTS AND DISCUSSION
Control of blood glucose in L-G6pc−/− mice during fasting.
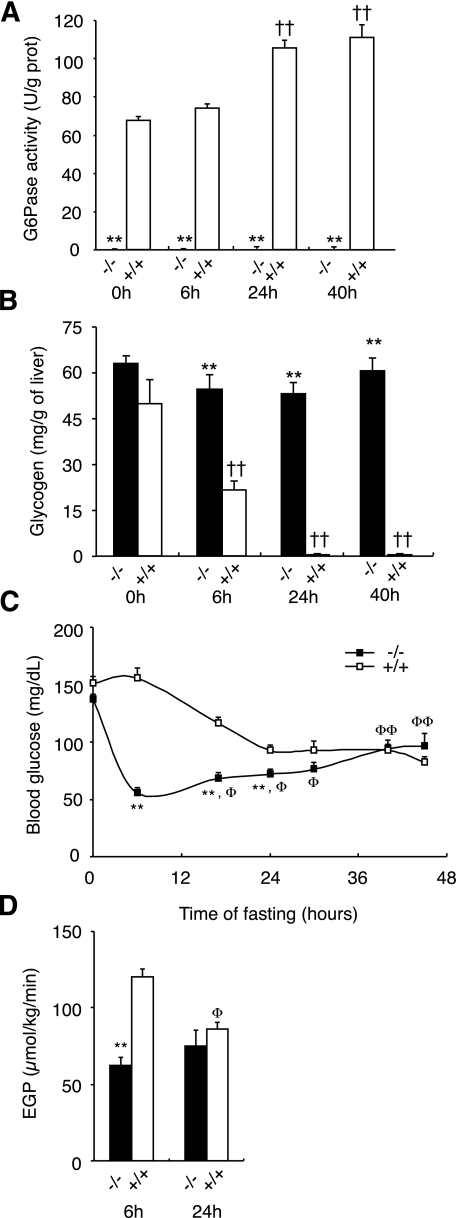
G6Pase activity was disrupted specifically in the liver by temporal and tissue-specific deletion of the G6pc gene based on a CRE-lox strategy (14). In brief, transgenic B6.G6pclox/lox mice were crossed with transgenic B6.SAcreERT2/w mice (21) to generate B6.G6pclox/lox.SAcreERT2/w mice, expressing inducible CREERT2 specifically in the liver. The treatment of adult B6.G6pclox/lox.SAcreERT2/w mice with tamoxifen induced the excision of G6pc exon 3, specifically in the liver (14), leading to a complete lack of hepatic G6Pase activity (Fig. 1A). This finding is consistent with these mice being unable to mobilize their glycogen stocks during fasting (Fig. 1C), leading to a hepatomegaly (Table 1). In WT mice, indeed, glycogen stock was about 40% of the fed level at 6 h of fasting and was completely exhausted after 12 h of fasting (0.6 mg/g of liver, n = 4). Although mice with the total knock-out of G6pc die after weaning, these L-G6pc−/− mice are viable. Moreover, all L-G6pc−/− mice survived prolonged fasting (45 h), although hepatic G6Pase activity was still undetectable throughout the fasting period (Fig. 1A). Because hepatic glucose production is thought to be critical for glucose homeostasis during fasting periods, plasma glucose concentration and EGP were determined in L-G6pc−/− and WT mice during prolonged fasting. L-G6pc−/− mice displayed the same blood glucose level as control mice in the fed state (Fig. 1C). Consistent with the role of hepatic glycogenolysis as an important pathway of glucose production in the early postabsorptive state, EGP levels were found to be lower in 6 h–fasted L-G6pc−/− animals than in control animals (Fig. 1D). This was correlated with a lower blood glucose in 6 h–fasted L-G6pc−/− mice (Fig. 1C).
FIG. 1.
Hepatic glucose production is not crucial for the control of fasting glycemia. G6Pase activities (A) and glycogen content (B) were assayed in the liver of L-G6pc−/− (black bar) and WT (white bar) mice in the fed state (0 h) or in fasted states (6 h, 24 h, and 40 h; n = 5 to 6 mice per group). C: Blood glucose concentrations were determined afterward for 0, 6, 16, 24, 30, 40, and 45 h fasting in L-g6pc−/− and WT mice. D: EGP was determined in conscious L-G6pc−/− (black bar) and WT (white bar) mice fasted for 6 h or 24 h (n = 6 mice per group). Mice had free access to water during fasting. Data were obtained 5 weeks after gene deletion and are expressed as means ± SEM. Values significantly different from WT (**P < 0.01). ††, significantly different with respect to the value in fed state in each group (P < 0.01). Φ and ΦΦ, significantly different with respect to the value at 6 h of fasting in each group (P < 0.05 and P < 0.01, respectively).
TABLE 1.
Body and liver weights and plasmatic parameters of 6 h–fasted L-g6pc−/− mice
| L-g6pc−/− | L-g6pc+/+ | |
|---|---|---|
| Mice per group | 6 | 6 |
| Body wt (g) | 27.8 ± 0.8 | 26.5 ± 0.8 |
| Liver wt (g) | 1.8 ± 0.04** | 1.1 ± 0.1 |
| Glucose (mmol/L) | 3.4 ± 0.2** | 7.2 ± 0.3 |
| Insulin (ng/mL) | 0.35 ± 0.02** | 0.67 ± 0.07 |
| Glucagon (pg/mL) | 552 ± 50** | 302 ± 28 |
| Corticosterone (ng/mL) | 191 ± 19** | 71.0 ± 9.7 |
| Norepinephrine (ng/mL) | 0.43 ± 0.06 | 0.43 ± 0.03 |
| Epinephrine (ng/mL) | 0.36 ± 0.10 | 0.36 ± 0.04 |
| Total cholesterol(g/L) | 1.33 ± 0.09** | 0.89 ± 0.04 |
| Triglycerides (g/L) | 1.42 ± 0.1** | 0.78 ± 0.03 |
| Free fatty acids (mmol/L) | 1.07 ± 0.09** | 0.69 ± 0.06 |
| Glycerol (mg/dL) | 4.3 ± 0.2 | 4.2 ± 0.3 |
| Lactate (mmol/L) | 5.5 ± 0.5** | 3.5 ± 0.3 |
| Alanine (µmol/L) | 336 ± 34** | 224 ± 18 |
| Glutamine (µmol/L) | 538 ± 16* | 618 ± 26 |
Values are expressed as means ± SEM. Data were determined 5 weeks after gene invalidation from L-g6pc−/− and WT L-g6pc+/+ mice upon 6 h of fasting.
Values significantly different from WT (*P < 0.05 and **P < 0.01) are indicated (Mann-Whitney test).
Concomitant with this drop in blood glucose observed at 6 h fasting, ketogenesis was rapidly induced in L-G6pc−/− animals. At 6 h fasting, the amounts of ketone bodies (β-hydroxybutyrate) in L-G6pc−/− mice were fivefold greater compared with the control mice that were able to produce high levels of glucose by inducing glycogenolysis (Supplementary Fig. 1A). These results were accounted for by fast induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (Hmgcs2) expression in 6 h–fasted L-G6pc−/− mice (Supplementary Fig. 1B). It is important to recall here that ketone bodies provide an alternative form of cellular energy fuel in postabsorptive situations characterized by low glucose availability. This is true notably for the brain, which may derive up to 70% of its energy from ketone bodies (6), and for the kidney and intestine, the two alternative gluconeogenic organs, which derive 50% of their energy from ketone bodies in postabsorptive state (22,23). It therefore seems unlikely that L-G6pc−/− mice lacked energy fuels for these essential organs.
It is noteworthy that differences in EGP between L-G6pc−/− and control animals were no longer observed after longer fasting periods, and that L-G6pc−/− mice controlled blood glucose effectively during long fasting periods (Fig. 1D). We even observed a significant increase in blood glucose between 6 h and 16 h fasting, such that the blood glucose concentration of L-G6pc−/− mice eventually reached that of control mice after 30 h fasting (Fig. 1C). By contrast, blood glucose concentration decreased steadily during fasting in control mice, eventually reaching a plateau at 100 mg/dL after 24 h of fasting (Fig. 1C).
Induction of extrahepatic gluconeogenesis in L-G6pc−/− mice.
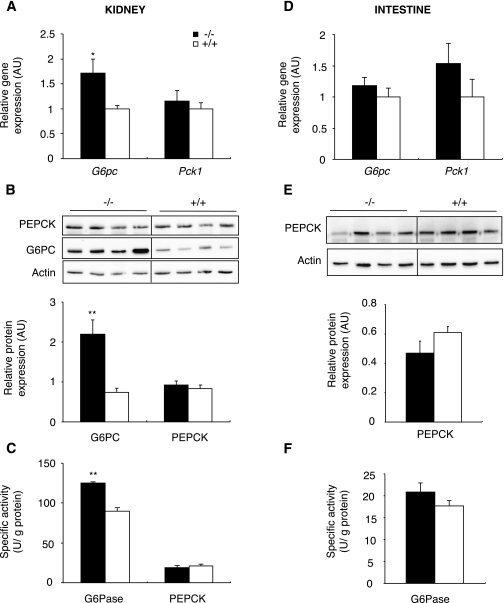
It was hypothesized that L-G6pc−/− mice maintained their glycemia through rapid induction of extrahepatic gluconeogenesis, compensating for lack of glucose production by the liver. It is noteworthy that blood glucose was the same in L-G6pc−/− and control mice in the fed state, although glucose injection was necessary in the total G6pc knockout mice to maintain blood glucose (24). To explain this difference, we assessed the expression of gluconeogenic genes in the kidneys and intestine of fed animals. Renal G6pc mRNA levels of fed L-G6pc−/− mice were about 1.7-fold higher than those in control mice (Fig. 2A). This was associated with an increase in renal G6PC protein (Fig. 2B). Finally, specific G6Pase activity in the kidney was 40% higher in L-G6pc−/− than in control mice (Fig. 2C). On the contrary, renal Pck1 expression and PEPCK activity were not modified (Fig. 2A–C). In the fed state, no modification of G6PC and PEPCK-c expression was observed in the intestine of L-G6pc−/− compared with control mice (Fig. 2D–F). Thus, only G6Pase was upregulated in the kidneys of L-G6pc−/− mice in the fed state, reflecting constitutive induction of the gluconeogenic pathway in the kidneys of L-G6pc−/− compared with WT mice.
FIG. 2.
Expression of main gluconeogenic enzymes in the kidneys (A–C) and the intestine (D–F) of fed L-G6pc−/− mice. A and D: Level of G6pc and Pck1 mRNA expressed as a ratio relative to the Rpl19 mRNA level. B and E: Western blot and quantification analysis for G6PC and PEPCK-C proteins. Actin is shown as a loading control. C and F: Specific G6Pase and PEPCK-c activity of homozygous L-G6pc−/− (black bar) and WT (white bar) mice. Data were obtained 5 weeks after gene deletion in fed mice (n = 6 mice per group) and are expressed as the mean ± SEM. Values significantly different from WT (*P < 0.05, **P < 0.01) are indicated. AU, arbitrary units.
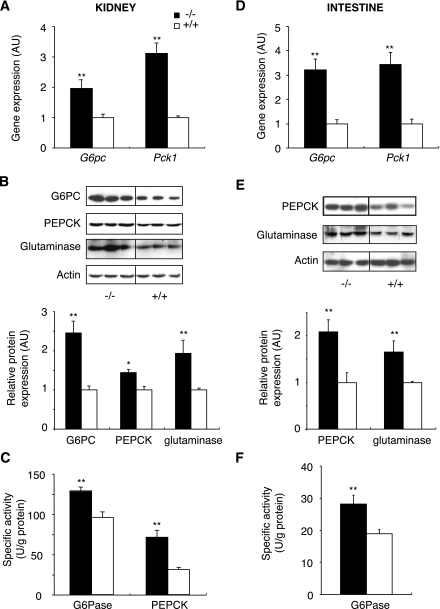
After 6 h fasting, the expression of the genes encoding major regulatory enzymes of extrahepatic gluconeogenesis [G6Pase, PEPCK-c, and glutaminase] was dramatically induced in both the kidneys and intestine of L-G6pc−/− mice. In the kidneys, amounts of G6pc mRNA and protein were about twice those in the control (Fig. 3A and B), and specific G6Pase activity was 36% higher than in control mice (Fig. 3C). Renal PEPCK-c expression was increased concomitantly, resulting in a threefold increase of Pck1 mRNA and protein and specific PEPCK-c activity more than twice that of L-G6pc−/− mice (Fig. 3A–C). There was also an induction of glutaminase in the kidneys of L-G6pc−/− mice (Fig. 3B). A similar pattern of induction of gluconeogenic gene expression was observed for the three enzymes in the intestine of L-G6pc−/− mice (Fig. 3D–F). Thus, the expression of gluconeogenic genes was rapidly induced in both the kidneys and intestine of L-G6pc−/− mice in postabsorptive state.
FIG. 3.
Expression of main gluconeogenic enzymes in the kidneys (A–C) and the intestine (D–F) of 6 h–fasted L-G6pc−/− mice. A and D: Level of G6pc and Pck1 mRNA expressed as a ratio relative to Rpl19 mRNA level. B and E: Western blot analysis and quantification for G6PC, PEPCK-C, and glutaminase proteins. Actin is shown as a loading control. Results are expressed as fold induction versus WT. C and D: Specific G6Pase and PEPCK-c activity of homozygous L-G6pc−/− (black bar) and WT (white bar) mice. Data were obtained 5 weeks after gene deletion, in mice fasted for 6 h (n = 6 mice per group), and are expressed as means ± SEM. Values significantly different from WT (*P < 0.05, **P < 0.01) are indicated.
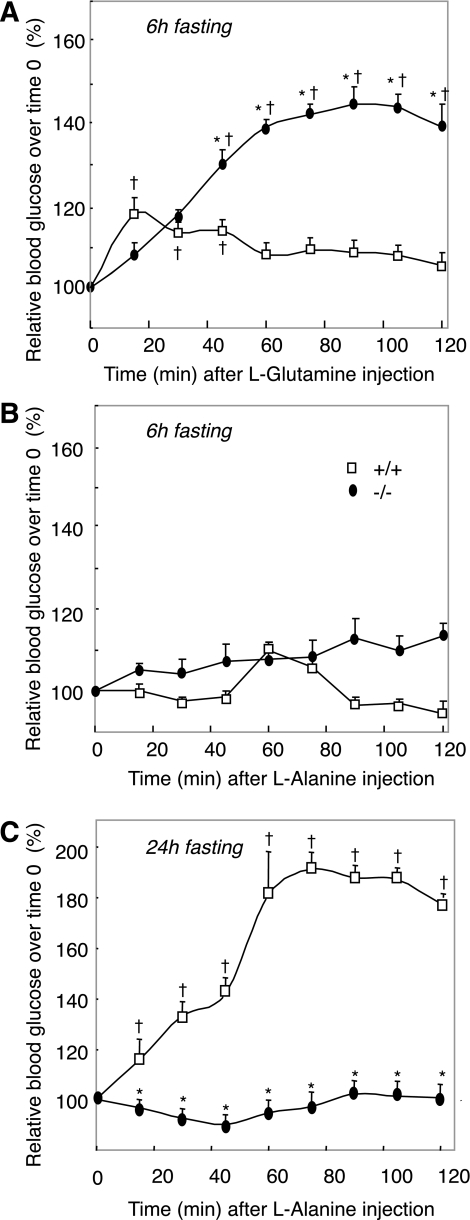
These results strongly suggested that the L-G6pc−/− mouse rapidly upregulated gluconeogenesis in the kidneys and intestine once food glucose was lacking. It is noteworthy that there is clear-cut organ substrate selectivity for the liver, kidney, and intestine gluconeogenesis (9). Whereas gluconeogenesis from glutamine only takes place in the kidneys and intestine, gluconeogenesis from alanine and lactate is the key pathway in the liver (4,9,25). To substantiate the view that postabsorptive or fasting L-G6pc−/− mice were able to regulate plasma glucose from extrahepatic gluconeogenesis in the absence of hepatic gluconeogenesis, we performed tolerance tests from alanine (exclusive hepatic substrate) and glutamine (exclusive extrahepatic substrate). After 6 h of fasting, there was a marked increase in plasma glucose upon glutamine injection in L-G6pc−/− mice, whereas only a marginal increase was observed in WT mice (Fig. 4A). On the contrary, L-G6pc−/− mice were unable to increase their plasma glucose in response to alanine injection (Fig. 4B). Surprisingly, there was no increase in plasma glucose in WT mice injected with alanine; this might be a result of the preponderant role of hepatic glycogenolysis in EGP and the nonactivation of gluconeogenesis at 6 h fasting in normal mice (see Fig. 1). In agreement with this rationale, WT mice dramatically increased plasma glucose in response to alanine injection after 24 h of fasting, a time where glycogen stores were exhausted and liver gluconeogenesis was enhanced (Fig. 1A and B and Fig. 4C). On the contrary, L-G6pc−/− mice were still unable to convert the injection of alanine in increased plasma glucose (Fig. 4C). Also in line with the absence of liver gluconeogenesis in L-G6pc−/− mice, plasma alanine and lactate concentrations were 50–60% higher than in WT mice (Table 1). On the other hand, the plasma concentration of glutamine was slightly lower in L-G6pc−/− mice than in WT mice, which was consistent with an increased utilization of glutamine (Table 1). Taken together, these data strongly suggested that L-G6pc−/− mice regulated plasma glucose from extrahepatic gluconeogenesis exclusively.
FIG. 4.
Glutamine and alanine tolerance tests in L-G6pc−/− and WT mice . After 6 h (A and B) or 24 h (C) of fasting, L-G6pc−/− (black circles) and WT (open squares) mice were injected with glutamine (A) or alanine (B and C). Blood glucose levels were measured every 15 min for 2 h. Data were obtained 5 weeks after tamoxifen treatment. Percent values relative to time 0 were expressed as means ± SEM (n = 6 mice/group). Values significantly different from WT (*P < 0.01); †significantly different with respect to the value before alanine or glutamine injection (P < 0.01).
Role of glucagon in physiological adaptation of the L-G6pc−/− mouse to fasting.
We then investigated the mechanisms underlying expression changes of gluconeogenic genes in both the kidneys and intestine in the absence of G6pc in the liver.
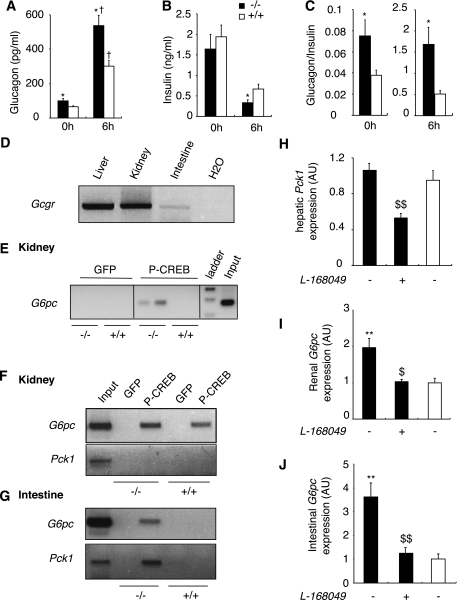
First, we analyzed the hormonal status of L-G6pc−/− mice in both fed and 6 h fasting states. In mammals, sophisticated hormonal control by insulin, glucagon, glucocorticoids, and cathecholamines maintains blood glucose within narrow limits, orchestrating the uptake and production of glucose. In both the fed state and after 6 h fasting, glucagon levels were higher in L-G6pc−/− mice than in control mice (Fig. 5A). On the contrary, L-G6pc−/− mice exhibited a lower insulin level than that of control mice after 6 h fasting (Fig. 5B), but were not hypoinsulinemic in fed state (Fig. 5B). This resulted in a twofold (fed state) to threefold (6 h fasted state) increase in the glucagon-to-insulin ratio in L-G6pc−/− mice in comparison with that of the control mice (Fig. 5C). Moreover, corticosterone levels were markedly increased in L-G6pc−/− mice compared with the control mice after 6 h fasting (Table 1), but not in the fed state (63.0 ± 10.8 ng/mL in L-G6pc−/− mice vs. 65.0 ± 14.0 ng/mL in WT mice). However, the epinephrine and norepinephrine plasma concentrations were similar in both L-G6pc−/− and control mice (Table 1). This is in agreement with the observation that a hypoglycemic state around 60 mg/dL is mild and does not represent a stressed condition from the metabolic viewpoint, especially in the light of the concomitant increased availability of ketone bodies (see above).
FIG. 5.
Glucagon regulates gluconeogenic gene expression in the kidneys and intestine of L-G6pc−/− mice. Plasma glucagon (A), plasma insulin (B), and glucagon-to-insulin ratio (C) of fed or 6 h–fasted L-G6pc−/− (black bars) and control (white bars) mice are shown. D: RT-PCR analysis of the expression of glucagon receptor in the liver, kidney, and intestine of control mice fasted for 6 h. E-G: P-CREB binding to the G6pc or Pck1 promoter was analyzed by ChiP assay from kidneys or intestine of fed mice (E) or 6 h–fasted mice (F and G). H: Level of Pck1 mRNA expressed as a ratio relative to Rpl19 mRNA level in the liver of L-G6pc−/− mice treated or not with GcgR antagonist L-168,049 (black bars) and WT mice (white bars) fasted for 6 h. I and J: Level of G6pc mRNA expressed as a ratio relative to Rpl19 mRNA level in the kidneys (I) and intestine (J) of L-G6pc−/− mice treated or not with GcgR antagonist L-168,049 (black bars) and WT mice (white bars) fasted for 6 h. Data were obtained 5 weeks after gene deletion (n = 6 mice per group) and are expressed as means ± SEM. Values significantly different from WT (*P < 0.05, **P < 0.01) and untreated L-G6pc−/− mice ($P < 0.05, $$P < 0.01) are indicated. †, significantly different fed state (P < 0.01).
In the light of these findings, glucagon could be a major candidate responsible for the induced expression of gluconeogenic genes in the kidneys and intestine of L-G6pc−/− mice. Glucagon receptors (GcgR) are known to be present in the liver, but GcgR are also expressed in the kidneys, notably in the proximal tubules and, to a lesser extent, in the intestine (26–28). We confirmed these data in L-G6pc−/− mice where GcgR mRNA was substantially expressed in the kidneys (10 times less than in the liver, however) and more weakly in the intestine (about 500 times less than in the liver) of both control and L-G6pc−/− mice (Fig. 5D). To date, the specific role of GcgR in these organs is still unexplained. However, two previous articles suggested a possible role of glucagon in the kidney cortex and intestine: 1) glucagon stimulated cAMP production in a kidney cortical cell line (29) and 2) glucagon infusion caused a net release of glucose into portal blood in dog (30). The GcgR is coupled to Gαs proteins, stimulating adenylate cyclase and increasing intracellular cAMP levels (26). This activates protein kinase A (PKA), which phosphorylates cAMP-response element binding protein (CREB). The phosphorylated form of CREB (P-CREB) induces the transcription of G6pc in the kidneys and intestine, via binding to the gene promoter (17). We then examined whether P-CREB could bind to the G6pc and Pck1 promoters by performing a ChIP assay. On the fed state, P-CREB was bound to the G6pc promoter in the kidneys of the fed L-G6pc−/− mice, but not to that of the WT mice (Fig. 5E). P-CREB was bound neither to the G6pc promoter nor to the Pck1 promoter in the intestine of fed L-G6pc−/− or WT mice (not shown). After 6 h fasting, P-CREB was bound to the renal G6pc promoter, but still not to the Pck1 promoter of L-G6pc−/− mice (Fig. 5F). It is noteworthy that in the intestine of 6 h–fasted L-G6pc−/− mice, P-CREB was bound to both G6pc and Pck1 promoters (Fig. 5G). To demonstrate unequivocally that the binding of P-CREB on G6pc promoter in the intestine and kidney of L-G6pc−/− mice was dependent on the increase of plasmatic glucagon, we administered orally a glucagon receptor antagonist to L-G6pc−/− mice (L-168,049, 50 mg/kg body) (18). L-168,049 inhibits the fixation of glucagon on GcgR and glucagon-stimulated cAMP synthesis in CHO cells expressing human GcgR (31). In the liver of L-G6pc−/− mice, Pck1 mRNA expression was decreased by half 6 h after the administration of GcgR antagonist (Fig. 5H), demonstrating the efficiency of the suppression of glucagon signaling. In agreement with the role of glucagon in the induction of extrahepatic gluconeogenesis, the administration of the antagonist prevented the increase of the G6pc expression in both the kidneys and intestine of 6 h–fasted L-G6pc−/− mice (Fig. 5I and J). These results strongly suggested that the increase of glucagon levels could account for the induction of G6pc expression in the kidneys and intestine of L-G6pc−/− mice.
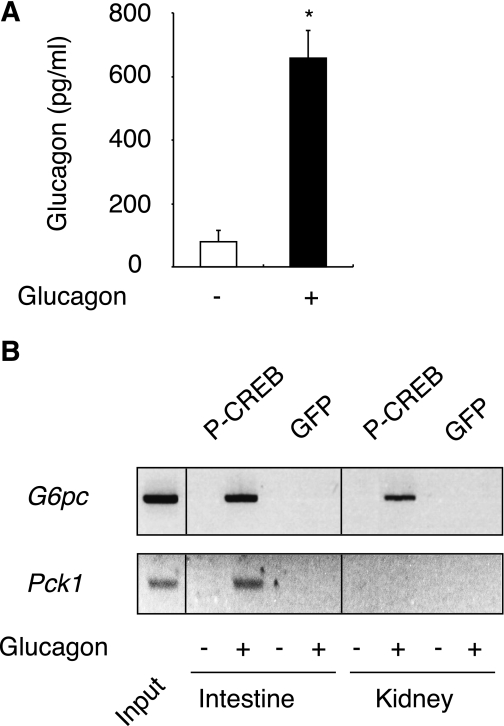
An intriguing observation, however, is that the G6pc gene was induced in the kidneys but not in the intestine in fed L-G6pc−/− mice (Figs. 2 and 5). This might be dependent on differential sensitivity to glucagon in both organs, possibly related to the higher expression level of GcgR in the kidneys (see above). Although this reasoning may appear speculative, this phenomenon might to some extent be related to the fact that the kidney rapidly become predominant in participating in EGP during fasting (8), whereas the participation of the intestine is weaker and is induced later in the same situation (5). In agreement with this rationale, the binding of P-CREB to the renal G6pc promoter occurred from 6 h of fasting in WT mice (Fig. 5F). To increment our findings and confirm that the extrahepatic glucagon effects observed herein were not an adaptation specific to L-G6pc−/− mice but could also take place in WT, we carried out a glucagon challenge in fed WT mice. After 30 min of glucagon administration, the plasma glucagon rose to the level of 6 h–fasted L-G6pc−/− mice (Figs. 5A and 6A). Consistent with the results obtained in L-G6pc−/− mice, P-CREB was firmly bound to the G6pc promoter in both tissues (Fig. 6B). Moreover, in response to glucagon injection, P-CREB bound to the Pck1 promoter only in the intestine (Fig. 6B), as already noted in L-G6pc−/− mice (Fig. 5). In conclusion, these results show definitively for the first time that G6pc gene expression is regulated by glucagon not only in the liver, but also in the kidneys and intestine, under physiological conditions. Moreover, glucagon can also regulate Pck1 in the intestine, but not in the kidneys. It must be noted that the increased concentration of corticosterone might also play a role in the induction of extrahepatic gluconeogenesis. Glucocorticoids activate gluconeogenesis via the binding of glucocorticoid receptors (GRs) to glucocorticoid response element on both G6pc and Pck1 promoters. These regulations have been essentially documented in the liver up to now (for review see [32]). Using ChIP experiments, we showed that GRs were bound to G6pc and Pck1 promoters in both the kidney and intestine of 6 h–fasted L-G6pc−/− and WT mice (Supplementary Fig. 2). The recruitment of GRs to Pck1 promoter was markedly increased in both the kidney (about threefold compared with WT) and intestine (sixfold compared with WT) of L-G6pc−/− mice (Supplementary Fig. 2A and B). There was also a robust recruitment of GRs to the G6pc promoter in the intestine of L-G6pc−/− mice compared with WT mice (about eightfold compared with WT; Supplementary Fig. 2B). These data suggested that glucocorticoids concurred in the induction of G6pc and Pck1 gene expression in the kidney and intestine of L-G6pc−/− mice. This does not exclude that they could act in synergy with P-CREB to stimulate both G6pc and Pck1 gene transcription in the kidney and intestine, as it was observed previously in the liver (33,34).
FIG. 6.
Glucagon induces P-CREB binding to G6pc and Pck1 promoters in WT mice. A: Plasma glucagon of WT mice injected with saline solution (white bar) or glucagon (black bar). Values significantly different from saline solution (*P < 0.05) are indicated. B: P-CREB binding to G6pc or Pck1 promoter was analyzed by ChIP assay from the kidneys and intestine, 30 min after the injection of saline solution or glucagon in WT mice.
Role of metabolic acidosis in the adaptation of renal gluconeogenesis of L-G6pc−/− mice to fasting.
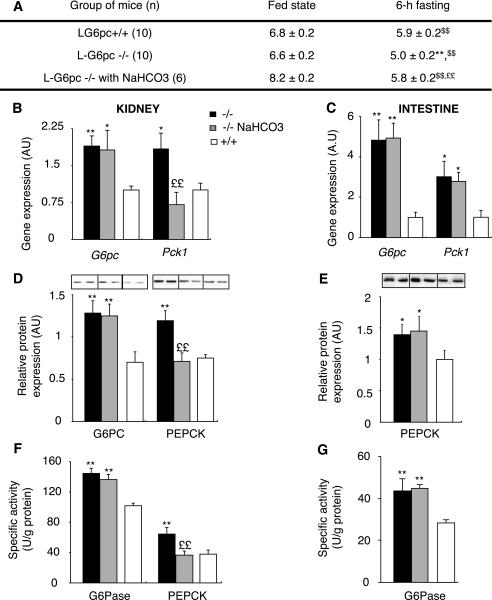
In view of these results, another mechanism should be involved in the induction of PEPCK-c expression in the kidneys of 6 h–fasted L-G6pc−/− mice. Despite a high glucagon-to-insulin ratio, Pck1 was not activated in the kidneys of fed L-G6pc−/− mice. The transcription of Pck1 gene is known to be controlled by metabolic acidosis in the kidneys, whereas it does not respond to changes in pH in the liver (35,36). It is noteworthy that the plasmatic levels of free fatty acids, ketone bodies, and lactate were higher in L-G6pc−/− mice than in control mice, after 6 h fasting (Table 1, Supplementary Fig. 1B). This could lead to a change in the acid-base status. Consistently, urinary pH of 6 h–fasted L-G6pc−/− mice was more acidic compared with that of 6 h–fasted control mice (Fig. 7A). In the fed state, both L-G6pc−/− and WT mice presented the same neutral urinary pH, ranging from 6.5 to 7.0. To test whether acidosis is the mechanism by which the Pck1 gene was induced in L-G6pc−/− mice, we counteracted acidosis by the addition of 0.28M NaHCO3 in the drinking water of L-G6pc−/− mice for 3 days (37). The urinary pH of 6 h–fasted L-G6pc−/− mice increased after treatment with NaHCO3 and was not different from that of control mice (Fig. 7A). This led to a specific normalization of Pck1 expression at the mRNA and protein levels in the kidneys, but not in the intestine, of 6 h–fasted L-G6pc−/− mice treated with NaHCO3 (Fig. 7B–G). On the contrary, G6Pase expression was not affected in the kidneys and in the intestine after NaHCO3 treatment (Fig. 7B–G), confirming that G6pc expression is not regulated by acidosis (35). These data also strongly suggested that Pck1 expression is not regulated by acidosis in the intestine.
FIG. 7.
Renal gluconeogenesis of L-G6pc−/− mice is regulated by acidosis. A: Follow-up of urinary pH of L-G6pc−/− mice (black bars), L-G6pc−/− mice treated with 0.28 mol/L NaHCO3 in drinking water (gray bars), and WT (L-G6pc+/+ mice, white bars) on the fed or postabsorptive state. Values of pH were determined using strips with ΔpH = 0.2. B and C: Expression levels of mRNA encoding G6pc or Pck1 gene in the kidneys (B) or in the intestine (C) of 6 h–fasted mice. Results are expressed as a ratio relative to Rpl19 expression levels. D–G: Western blot quantification and enzyme activity assays of G6Pase and PEPCK determined in the kidneys (D and F) or in the intestine of 6 h–fasted mice (E and G). Data were obtained 5 weeks after gene deletion and are expressed as mean ± SEM. Values significantly different from WT (*P < 0.05; **P < 0.01), from the fed state ($$P < 0.01), and from L-G6pc−/− without NaHCO3 treatment (££P < 0.01).
Conclusion.
The liver has always been considered the major source of EGP until now. In contradiction with this dogma, we here report that, after a transient drop in plasma glucose as a result of incapacity to mobilize glycogen stores, the absence of hepatic glucose production has no major effect on the control of fasting plasma glucose. Instead, the early induction of gluconeogenesis in the kidneys and intestine occurs, permitting sustentation of EGP and blood glucose right from the start of fasting periods. This perfectly matches what has been observed during the anhepatic phase of liver transplantation in humans (38,39). Our data also emphasize that an essential function of the liver is the rapid tuning of blood glucose during nutritional transitions, via the handling of glycogen stores. However, the first major finding of this study is that the liver is not an irreplaceable source of endogenous glucose in the absence of food glucose. Similarly, glucagon, which is well known to stimulate hepatic glucose release via activation of glycogenolysis and gluconeogenic gene expression, has up to now been considered as only targeting the liver. In opposition to this other dogma, the second major finding here is that glucagon also plays a key role in the transcriptional regulation of renal and intestinal gluconeogenic genes. This may account for the basal induction of renal G6pc in the absence of hepatic glucose production and for the rapid induction of intestinal G6pc and Pck1 once food is lacking. Either fasting or type 2 diabetes is characterized by increased glucagon secretion in humans, which is believed to exert a key role in the augmented EGP of diabetes (40). In these two situations, the renal glucose production is increased (4,41–43). Moreover, the renal glucose production could play a crucial role in the counterregulation of insulin-induced hypoglycemia in humans, a situation of increased glucagon and cortisol secretions (41,42). At least, the important role of the kidney evidenced here might also explain why patients with renal failure are prone to hypoglycemia (44).
In conclusion, our study provides a definitive quantitative estimate of the capacity of extrahepatic gluconeogenesis to sustain fasting EGP, regardless of the contribution of the liver. It also extends the regulatory role of glucagon to the control of gluconeogenesis in the kidneys and intestine. This leads us to conclude that the current dogma relating to the relative role of the liver vis-à-vis extrahepatic gluconeogenic organs in glucose homeostasis should be reconsidered.
ACKNOWLEDGMENTS
This work was supported by research grants from the Agence Nationale de la Recherche (ANR-07-MRAR-011-01) and the Association Francophone des Glycogénoses. A.A.-W. is supported by the Syrian government.
No potential conflicts of interest relevant to this article were reported.
E.M. conducted and designed experiments, performed data analyses, and wrote the manuscript. A.G.-S., A.A.-W., and M.A.-C. contributed to the discussion and interpretation of data. C.Z. assisted in surgical procedures. A.S., I.H., and J.-A.T. assisted in animal breeding, housing, and animal experimentation. G.M. and F.R. supervised the work and wrote the manuscript.
The authors thank Angèle Chamousset and Jean-Michel Vicat for animal care (Animalerie Lyon Est Conventionnelle et SPF, Faculté de Médecine Laennec, IFR62 Lyon-Est, Lyon), Dr. Jean-Marie Cottet-Emard (Centre Hospitalier Universitaire, Lyon) for the determination of catecholamine levels, Dr. Christine Saban (Centre Hospitalier Universitaire, Lyon) for the determination of amino acid levels, and the members of the CECIL Platform (Faculté de Médecine Laennec, IFR62 Lyon-Est, Lyon).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0571/-/DC1.
REFERENCES
- 1.Wahren J, Ekberg K. Splanchnic regulation of glucose production. Annu Rev Nutr 2007;27:329–345 [DOI] [PubMed] [Google Scholar]
- 2.Croset M, Rajas F, Zitoun C, Hurot JM, Montano S, Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes 2001;50:740–746 [DOI] [PubMed] [Google Scholar]
- 3.Ekberg K, Landau BR, Wajngot A, et al. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 1999;48:292–298 [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 2001;24:382–391 [DOI] [PubMed] [Google Scholar]
- 5.Mithieux G, Bady I, Gautier A, Croset M, Rajas F, Zitoun C. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 2004;286:E370–E375 [DOI] [PubMed] [Google Scholar]
- 6.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest 1969;48:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mithieux G, Misery P, Magnan C, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab 2005;2:321–329 [DOI] [PubMed] [Google Scholar]
- 8.Pillot B, Soty M, Gautier-Stein A, Zitoun C, Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology 2009;150:616–624 [DOI] [PubMed] [Google Scholar]
- 9.Mithieux G, Rajas F, Gautier-Stein A. A novel role for glucose 6-phosphatase in the small intestine in the control of glucose homeostasis. J Biol Chem 2004;279:44231–44234 [DOI] [PubMed] [Google Scholar]
- 10.Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med 2002;2:121–143 [DOI] [PubMed] [Google Scholar]
- 11.Ozen H. Glycogen storage diseases: new perspectives. World J Gastroenterol 2007;13:2541–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moses SW. Historical highlights and unsolved problems in glycogen storage disease type 1. Eur J Pediatr 2002;161(Suppl. 1):S2–S9 [DOI] [PubMed] [Google Scholar]
- 13.Lei KJ, Chen H, Pan CJ, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet 1996;13:203–209 [DOI] [PubMed] [Google Scholar]
- 14.Mutel E, Abdul-Wahed A, Ramamonjisoa N, et al. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J Hepatol 2011;54:529–537 [DOI] [PubMed] [Google Scholar]
- 15.Keppler D, Decker K. Methods of Enzymatic Analysis. New York, VCH Publishers, 1974 [Google Scholar]
- 16.Guignot L, Mithieux G. Mechanisms by which insulin, associated or not with glucose, may inhibit hepatic glucose production in the rat. Am J Physiol 1999;277:E984–E989 [DOI] [PubMed] [Google Scholar]
- 17.Gautier-Stein A, Zitoun C, Lalli E, Mithieux G, Rajas F. Transcriptional regulation of the glucose-6-phosphatase gene by cAMP/vasoactive intestinal peptide in the intestine. Role of HNF4alpha, CREM, HNF1alpha, and C/EBPalpha. J Biol Chem 2006;281:31268–31278 [DOI] [PubMed] [Google Scholar]
- 18.de Laszlo SE, Hacker C, Li B, et al. Potent, orally absorbed glucagon receptor antagonists. Bioorg Med Chem Lett 1999;9:641–646 [DOI] [PubMed] [Google Scholar]
- 19.Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 1999;117:132–139 [DOI] [PubMed] [Google Scholar]
- 20.Rajas F, Jourdan-Pineau H, Stefanutti A, Mrad EA, Iynedjian PB, Mithieux G. Immunocytochemical localization of glucose 6-phosphatase and cytosolic phosphoenolpyruvate carboxykinase in gluconeogenic tissues reveals unsuspected metabolic zonation. Histochem Cell Biol 2007;127:555–565 [DOI] [PubMed] [Google Scholar]
- 21.Schuler M, Dierich A, Chambon P, Metzger D. Efficient temporally controlled targeted somatic mutagenesis in hepatocytes of the mouse. Genesis 2004;39:167–172 [DOI] [PubMed] [Google Scholar]
- 22.Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes Metab 2010;36:257–262 [DOI] [PubMed] [Google Scholar]
- 23.Windmueller HG, Spaeth AE. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem 1978;253:69–76 [PubMed] [Google Scholar]
- 24.Zingone A, Hiraiwa H, Pan CJ, et al. Correction of glycogen storage disease type 1a in a mouse model by gene therapy. J Biol Chem 2000;275:828–832 [DOI] [PubMed] [Google Scholar]
- 25.Mithieux G. The new functions of the gut in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care 2005;8:445–449 [DOI] [PubMed] [Google Scholar]
- 26.Authier F, Desbuquois B. Glucagon receptors. Cell Mol Life Sci 2008;65:1880–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burcelin R, Li J, Charron MJ. Cloning and sequence analysis of the murine glucagon receptor-encoding gene. Gene 1995;164:305–310 [DOI] [PubMed] [Google Scholar]
- 28.Marks J, Debnam ES, Dashwood MR, Srai SK, Unwin RJ. Detection of glucagon receptor mRNA in the rat proximal tubule: potential role for glucagon in the control of renal glucose transport. Clin Sci (Lond) 2003;104:253–258 [DOI] [PubMed] [Google Scholar]
- 29.Prié D, Friedlander G, Coureau C, Vandewalle A, Cassingéna R, Ronco PM. Role of adenosine on glucagon-induced cAMP in a human cortical collecting duct cell line. Kidney Int 1995;47:1310–1318 [DOI] [PubMed] [Google Scholar]
- 30.Weber FL, Jr, Veach GL, Friedman DW. Effects of insulin and glucagon on the uptake of amino acids from arterial blood by canine ileum. Dig Dis Sci 1981;26:113–118 [DOI] [PubMed] [Google Scholar]
- 31.Cascieri MA, Koch GE, Ber E, et al. Characterization of a novel, non-peptidyl antagonist of the human glucagon receptor. J Biol Chem 1999;274:8694–8697 [DOI] [PubMed] [Google Scholar]
- 32.Yabaluri N, Bashyam MD. Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci 2010;35:473–484 [DOI] [PubMed] [Google Scholar]
- 33.Waltner-Law M, Duong DT, Daniels MC, et al. Elements of the glucocorticoid and retinoic acid response units are involved in cAMP-mediated expression of the PEPCK gene. J Biol Chem 2003;278:10427–10435 [DOI] [PubMed] [Google Scholar]
- 34.Vander Kooi BT, Onuma H, Oeser JK, et al. The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol Endocrinol 2005;19:3001–3022 [DOI] [PubMed] [Google Scholar]
- 35.Curthoys NP, Gstraunthaler G. Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol 2001;281:F381–F390 [DOI] [PubMed] [Google Scholar]
- 36.Curthoys NP, Taylor L, Hoffert JD, Knepper MA. Proteomic analysis of the adaptive response of rat renal proximal tubules to metabolic acidosis. Am J Physiol Renal Physiol 2007;292:F140–F147 [DOI] [PubMed] [Google Scholar]
- 37.López-Flores I, Peragón J, Valderrama R, et al. Downregulation in the expression of the serine dehydratase in the rat liver during chronic metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 2006;291:R1295–R1302 [DOI] [PubMed] [Google Scholar]
- 38.Battezzati A, Caumo A, Martino F, et al. Nonhepatic glucose production in humans. Am J Physiol Endocrinol Metab 2004;286:E129–E135 [DOI] [PubMed] [Google Scholar]
- 39.Joseph SE, Heaton N, Potter D, Pernet A, Umpleby MA, Amiel SA. Renal glucose production compensates for the liver during the anhepatic phase of liver transplantation. Diabetes 2000;49:450–456 [DOI] [PubMed] [Google Scholar]
- 40.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 1985;28:574–578 [DOI] [PubMed] [Google Scholar]
- 41.Cersosimo E, Garlick P, Ferretti J. Renal glucose production during insulin-induced hypoglycemia in humans. Diabetes 1999;48:261–266 [DOI] [PubMed] [Google Scholar]
- 42.Meyer C, Gerich JE. Role of the kidney in hyperglycemia in type 2 diabetes. Curr Diab Rep 2002;2:237–241 [DOI] [PubMed] [Google Scholar]
- 43.Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest 1998;102:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am 1989;18:103–121 [PubMed] [Google Scholar]