Abstract
OBJECTIVE
The glucoincretin hormone glucagon-like peptide 1 (GLP-1) enhances glucose-stimulated insulin secretion and stimulates pancreatic β-cell mass expansion. We have previously shown that the forkhead transcription factor FoxO1 is a prominent transcriptional effector of GLP-1 signaling in the β-cell. FoxO1 activity is subject to a complex regulation by Akt-dependent phosphorylation and SirT1-mediated deacetylation. In this study, we aimed at investigating the potential role of SirT1 in GLP-1 action.
RESEARCH DESIGN AND METHODS
FoxO1 acetylation levels and binding to SirT1 were studied by Western immunoblot analysis in INS832/13 cells. SirT1 activity was evaluated using an in vitro deacetylation assay and correlated with the NAD+-to-NADH ratio. The implication of SirT1 in GLP-1–induced proliferation was investigated by BrdU incorporation assay. Furthermore, we determined β-cell replication and mass in wild-type and transgenic mice with SirT1 gain of function after daily administration of exendin-4 for 1 week.
RESULTS
Our data show that GLP-1 increases FoxO1 acetylation, decreases the binding of SirT1 to FoxO1, and stunts SirT1 activity in β-INS832/13 cells. GLP-1 decreases both the NAD+-to-NADH ratio and SirT1 expression in INS cells and isolated islets, thereby providing possible mechanisms by which GLP-1 could modulate SirT1 activity. Finally, the action of GLP-1 on β-cell mass expansion is abolished in both transgenic mice and cultured β-cells with increased dosage of SirT1.
CONCLUSIONS
Our study shows for the first time that the glucoincretin hormone GLP-1 modulates SirT1 activity and FoxO1 acetylation in β-cells. We also identify SirT1 as a negative regulator of β-cell proliferation.
The glucoincretin hormone glucagon-like peptide 1-[7–36]amide (GLP-1) (1–3) is a potent therapeutic agent in the treatment of diabetes (4). GLP-1 improves insulin secretion in subjects with impaired glucose tolerance and type 2 diabetes (5). It also stimulates insulin gene expression and insulin biosynthesis (6), in part via increased expression and activity of the β-cell–specific transcription factor Pdx1 (7,8). Moreover, GLP-1 has been shown to promote β-cell mass expansion in both experimental animal models (8,9) and cultured β-cells (7,10–14). However, the molecular mechanism by which GLP-1 exerts its action is not fully elucidated.
We have previously shown that GLP-1 transactivates the epidermal growth factor receptor (12) to subsequently activate phosphatidylinositol-3 kinase and Akt signaling (7,11). Activation of epidermal growth factor receptor/phosphatidylinositol-3 kinase/Akt signaling by GLP-1 stimulates β-cell proliferation (7,11) and survival (13,14). Of interest, this signaling pathway has been suggested to play a role in the glucoincretin effect of GLP-1 as well (15). We have also demonstrated that the forkhead transcription factor FoxO1, an important regulator of β-cell mass (16–18), is a prominent transcriptional effector of GLP-1 action in β-cells (10). Thus, GLP-1 inhibits FoxO1 via Akt-mediated phosphorylation and nuclear exclusion. Inhibition of FoxO1 by GLP-1 increases both Pdx1 and Foxa2 expression and triggers β-cell mass expansion (10).
FoxO1 activity is regulated in a complex fashion by various posttranslational modifications, including reversible Ser-Thr phosphorylation and Lys acetylation (19). Acetylation at Lys-242, -245, and -262 of FoxO1 attenuates its ability to bind cognate DNA sequence and increases its susceptibility to phosphorylation by Akt (20). Conversely, deacetylation of FoxO1 by the NAD+-dependent protein deacetylase SirT1 increases its transcriptional activity (21–23).
We therefore sought to test the possible implication of SirT1 in GLP-1 action. The current study shows that GLP-1 stunts SirT1-mediated FoxO1 deacetylation, thereby relieving a molecular brake on β-cell mass expansion. Our work describes a novel mechanism for GLP-1 action. It also identifies SirT1 as a negative regulator of β-cell proliferation.
RESEARCH DESIGN AND METHODS
Reagents.
Human GLP-1 fragment 7–36 amide, exendin-4, nicotinamide, and resveratrol were obtained from Sigma (St. Louis, MO). RPMI-1640 medium, FCS, and other culture media were purchased from Invitrogen (Burlington, ON, Canada). Anti-FKHR antibody was purchased from Cell Signaling (Beverly, MA). Antiacetyl-lysine and anti-SirT1 antibodies were obtained from Millipore (Bedford, MA). Anti–guinea-pig insulin was purchased from Sigma.
Cell culture.
INS832/13 cells (24) were grown in RPMI-1640 medium supplemented with 10 mmol/L HEPES, 10% heat-inactivated FCS, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 50 μmol/L β-mercaptoethanol, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified 5% CO2 atmosphere. Cells at 70% confluence were washed with phosphate-buffered saline and preincubated in serum-free RPMI-1640 medium supplemented with 3 mmol/L glucose and 0.1% BSA (Sigma) for at least 4 h before treatment. This condition mimics calorie restriction and was shown to activate SirT1.
Islet isolation.
Rat islets were isolated from male Wistar rats (250 g) by collagenase digestion. Islets were subsequently purified over a Histopaque gradient and handpicked under a microscope.
Human islets were isolated from organ donors at the Department of Surgery, Montreal General Hospital, McGill University Health Center, Montreal, Quebec, Canada (three separate donors were received). Human ethics approval was obtained through the McGill University Health Center ethics committee. Donors were between ages 42 and 65, and none had a history of diabetes or metabolic disorder. Islets were isolated by digestion with Liberase CI (Boehringer Mannheim, Indianapolis, IN) followed by purification over a Ficoll gradient using a CORE 2991 Cell Processor (COBE BCT, Denver, CO). After isolation, islets were rested overnight in complete CMRL medium.
Western blot.
Proteins were extracted and quantified by BCA assay (Roche, Indianapolis, IN). FoxO1 protein complexes were immunoprecipitated from 1 mg of total protein extracts and subjected to electrophoresis on 8 or 10% polyacrylamide gels.
Chromatin immunoprecipitation.
Cells were fixed to isolate intact chromatin and sheered in 200-base pair fragments, and SirT1 DNA/protein complexes were immunoprecipitated using an anti-SirT1 antibody (Millipore). Bound DNA was amplified by PCR using sequence-specific primers designed for amplification of the forkhead binding site region of the Foxa2 promoter (16).
SirT1 activity.
Cells were treated for 15 min, and SirT1 activation was measured using a commercially available fluorometric assay (Biomol, Plymouth Meeting, PA). In brief, the kit uses a SirT1 substrate consisting of amino acids 379–382 of human p53 (Arg-His-Lys-Lys[Ac]). The assay's fluorescence signal is generated in proportion to the amount of deacetylation of Lys-382, a known in vivo target of SirT1. Cell extracts were incubated for 10 min at 37°C with a 25 μmol/L concentration of the fluorogenic SirT1 substrate. Reactions were stopped by the addition of 1 mmol/L nicotinamide, and the fluorescence resonance energy transfer signal was determined.
NAD+-to-NADH ratio and cellular ATP concentrations.
NAD+, NADH, and ATP levels were measured in cell extracts using commercial kits (NAD/NADH quantification kit and ATP fluorometric kit from Biovision, Mountain View, CA).
Nicotinamide phosphoribosyltransferase activity.
Nicotinamide phosphoribosyltransferase (Nampt) activity was measured by the conversion of 14C-labeled nicotinamide to [14C]nicotinamide mononucleotide as described previously (25). In brief, cell lysates (10 μg total protein) were incubated with ATP, 5-phosphoribosyl-1-pyrophosphate, and [14C]nicotinamide for 30 min; filtered through glass fiber filters; and washed with acetone. Filters were air-dried and transferred into scintillation vials for quantification.
Transfection and cell proliferation.
Cells were transfected with either control green fluorescent protein (GFP), wild-type SirT1, or a catalytically inactive SirT1 mutant (26) using Lipofectamine 2000 (Invitrogen) as described previously (16). Transfection efficiency was 70%. The following day, proliferation of transfected cells was evaluated using BrdU incorporation assay (Roche). In brief, cells were treated for 24 h in the absence or presence of 1 nmol/L GLP-1, and BrdU was added to the culture medium for the last 60 min of the incubation period.
Animal studies.
Mice were maintained on a mixed background (129/sv and C57BL/6J). Wild-type and SirBACO transgenic mice (27) aged 6–12 months were treated with daily intraperitoneal injections of exendin-4 (10 nmol/kg) or saline for 7 days (n = 5 each). Mice were killed, and pancreas sections were processed for insulin and Ki67 immunohistochemistry to assess β-cell proliferation. Cross-sectional islet area was measured using Image Pro Plus software (Media Cybernetics, Silver Spring, MD). Results are presented as relative β-cell areas (i.e., percent of insulin + areas relative to total pancreas areas). For each animal, at least four sections spaced 80 μm apart were studied.
Calculations and statistics.
Data are presented as means ± SEM. Statistical analyses were performed with SPSS (SPSS Inc., Chicago, IL) using ANOVA.
RESULTS
GLP-1 increases FoxO1 acetylation via inhibition of the sirtuin deacetylase SirT1.
We have previously shown that inhibition of the forkhead transcription factor FoxO1 mediates the pleiotropic action of the glucoincretin hormone GLP-1 (10). FoxO1 is subject to various posttranslational modifications, including Akt-dependent phosphorylation and SirT1-mediated deacetylation. Here, we sought to test the hypothesis that GLP-1 inhibits SirT1 to enhance FoxO1 acetylation and to stimulate β-cell mass expansion.
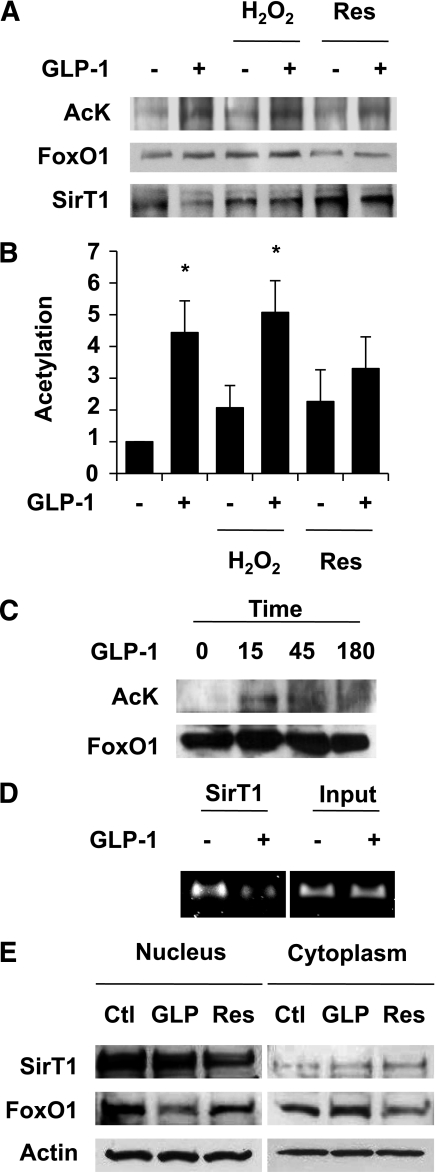
We first evaluated FoxO1 acetylation and binding to SirT1 by Western blot in INS832/13 cells. We resorted to the use of a pancreatic β-cell line because of the high amount of biological material required to perform the assays. INS cells were incubated in serum-free medium, a condition known to activate SirT1, in the presence or absence of GLP-1 and the SirT1 activators H2O2 or resveratrol. Figure 1A and B shows that GLP-1 induced FoxO1 acetylation, an action prevented by resveratrol but not by H2O2. Coimmunoprecipitation experiments revealed that GLP-1 inhibited the physical association between FoxO1 and SirT1 (Fig. 1A). However, this action was inhibited by both H2O2 and resveratrol. The action of GLP-1 on FoxO1 acetylation was transient, with a maximal effect observed between 15 and 45 min. FoxO1 acetylation returned to basal value after ∼180 min (Fig. 1C). Taken together, these results suggest that GLP-1 inhibits SirT1-mediated FoxO1 deacetylation.
FIG. 1.
GLP-1 inhibits SirT1-mediated FoxO1 deacetylation in the β-cell. A: FoxO1 acetylation and binding to SirT1 were evaluated by Western blot after immunoprecipitation of FoxO1 protein complexes. INS832/13 cells were incubated in serum-free medium in the presence or absence of GLP-1 (10 nmol/L), with or without the SirT1 activators resveratrol (10 μmol/L) and H2O2 (25 μmol/L) for 45 min. B: Densitometry analysis of five different experiments as shown in A. *P < 0.05. C: FoxO1 acetylation was evaluated as described in A after the indicated periods of time. D: SirT1 binds the Foxa2 promoter. SirT1 was immunoprecipitated from cross-linked chromatin extracted from INS832/13 cells treated with or without GLP-1 for 45 min. Eluted DNA was PCR-amplified using oligonucleotides flanking the forkhead binding site in the rat Foxa2 promoter. E: Western blots showing the subcellular localization of SirT1 and FoxO1 after 45-min incubation with either 10 nmol/L GLP-1 (GLP) or 10 μmol/L resveratrol. Actin is shown as control. Representative images of at least three experiments are shown. Res, resveratrol; AcK, acetyl-lysine, Ctl, control.
Chromatin immunoprecipitation assay was performed to assess whether SirT1 is recruited to the promoter region of FoxO1 target genes. Figure 1D shows that SirT1 was physically associated to a region of the Foxa2 promoter encompassing the forkhead binding site (10). Moreover, SirT1 binding to the Foxa2 promoter was reduced upon GLP-1 treatment. This result indicates that SirT1 binding to the Foxa2 promoter mirrors that of FoxO1, as we have previously demonstrated (10). Altogether, these findings indicate that SirT1 interacts with FoxO1 and is recruited to the promoter of FoxO1 target genes in a GLP-1–inhibitable manner. These data suggest a role for SirT1 in the transcriptional response to the glucoincretin hormone in β-cells. We next performed subcellular fractionation to address whether GLP-1 provokes SirT1 nuclear exclusion, as it does for FoxO1 (Fig. 1E). Our results show that SirT1 is predominantly nuclear in INS cells and that its subcellular localization was not altered by GLP-1 treatment. Conversely, GLP-1 induced cytoplasmic translocation of FoxO1, as we have published previously (10).
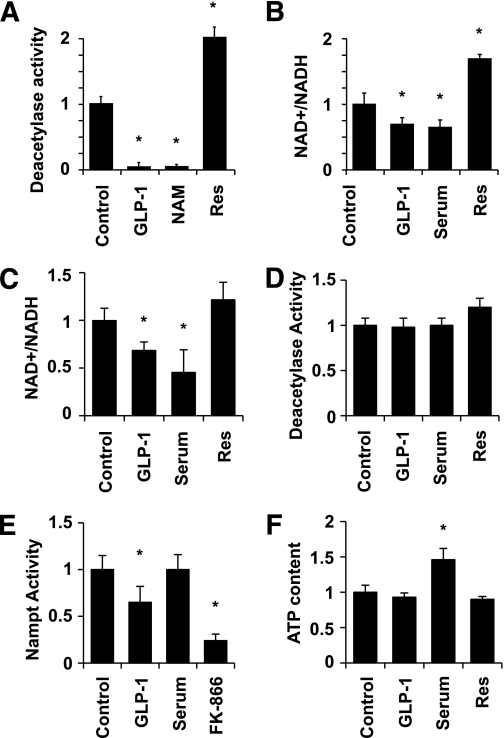
We next sought to directly test whether GLP-1 affects SirT1 activity using an in vitro deacetylase assay. Figure 2A shows that GLP-1 blunted SirT1 activity to the same extent as the SirT1 inhibitor nicotinamide. Conversely, SirT1 activity was increased in resveratrol-treated cells. Because SirT1 activity is dependent on NAD+, we measured changes in the NAD+-to-NADH ratio in response to GLP-1 treatment in INS cells and isolated human islets. Figure 2B shows that GLP-1 decreased the NAD+-to-NADH ratio by 30% in INS cells, an action mimicked by the addition of 10% serum. Conversely, resveratrol increased the NAD+-to-NADH ratio by 60%. Both GLP-1 and serum also elicited a reduction in the NAD+-to-NADH ratio in human islets (Fig. 2C), whereas the effect of resveratrol (∼25%) did not reach statistical significance. The reduction in NAD+ availability provides a possible mechanism by which GLP-1 could acutely regulate SirT1 activity. To directly test this possibility, we supplemented the assay buffer with 450 μmol/L NAD+ (Fig. 2D). Supplementation of NAD+ suppressed GLP-1 as well as serum inhibition of SirT1 activity. In an effort to unravel the mechanism by which GLP-1 could alter the NAD+-to-NADH ratio, we investigated its action on the activity of Nampt, the rate-limiting enzyme for the biosynthesis of NAD, and on the cellular ATP content. GLP-1 decreases the activity of Nampt (Fig. 2E), thereby revealing a novel mechanism by which GLP-1 could decrease the NAD+-to-NADH ratio. FK-866 (10 nmol/L), a specific Nampt pharmacological inhibitor, was used as a positive control. Conversely, GLP-1 did not significantly change the cellular ATP content (Fig. 2F), consistent with a recent publication by Peyot et al. (28). It is interesting that the addition of serum did not alter Nampt activity but significantly increased the cellular ATP concentration. This suggests that serum and GLP-1 could inhibit SirT1 via distinct mechanisms.
FIG. 2.
GLP-1 inhibits SirT1 deacetylase activity. A: Cells were incubated in the presence or absence of 10 nmol/L GLP-1, 10 mmol/L nicotinamide (NAM), or 10 μmol/L resveratrol (Res). Proteins were extracted after 15 min to perform the in vitro SirT1 deacetylase assay using a fluorogenic SirT1 substrate. B and C: NAD+ and NADH levels were determined in extracts from cells (B) or human islets (C) incubated in the presence or absence of 10 nmol/L GLP-1, 10% serum, or 10 μmol/L resveratrol for 15 min. D: SirT1 activity was measured as described in A with the modification that the assay buffer was supplemented with 25 μmol/L NAD+. E: Nampt activity was measured by the conversion of 14C-labeled nicotinamide to [14C]nicotinamide mononucleotide. The specific Nampt pharmacological inhibitor FK-866 (10 nmol/L) was used as control. F: Total cellular ATP was measured in cells incubated as described above. Means ± SE of three experiments, each comprising duplicates. *P < 0.05.
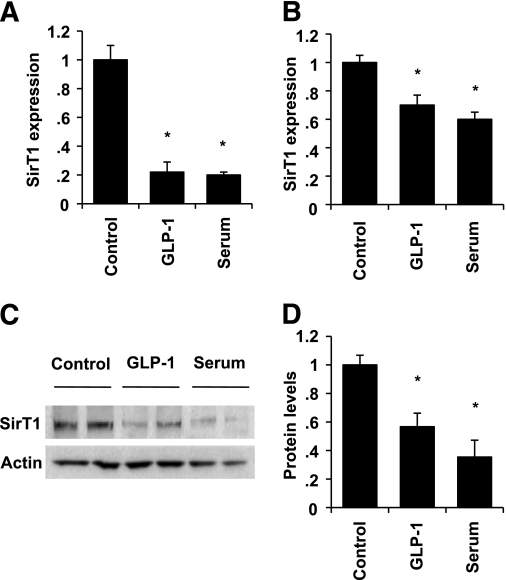
We next measured SirT1 expression by quantitative PCR after overnight incubation in serum-free medium in the absence or presence of GLP-1 (Fig. 3A). Our data show that glucose and serum deprivation, a condition mimicking calorie restriction, increased SirT1 expression by fivefold compared with complete growth medium in INS cells. GLP-1 treatment completely abolished the rise in SirT1 expression. We next sought to confirm these observations in isolated rat islets (Fig. 3B). Both GLP-1 and serum decreased SirT1 expression in rat islets as well, although the detected changes were more modest. Thus, SirT1 expression was repressed by 30% in the presence of GLP-1 and by 40% in response to serum. We assessed SirT1 protein levels by Western blot to test whether alterations in SirT1 expression were translated into changes in protein levels. Figure 3C and D shows that GLP-1 decreased SirT1 protein levels by 70% in INS cells, an effect mimicked by serum.
FIG. 3.
GLP-1 downregulates SirT1 expression. A and B: Cells (A) and isolated rat islets (B) were incubated in serum-free medium in the absence or presence of GLP-1 or left untreated in complete growth medium for 24 h. SirT1 expression levels were measured by quantitative PCR and normalized to actin. C: SirT1 protein levels were evaluated by Western blot in INS cells treated as described in A and B. D: Densitometry analysis of three different experiments as shown in A and B. *P < 0.05.
Overexpression of SirT1 abolishes GLP-1 action on β-cell mass expansion.
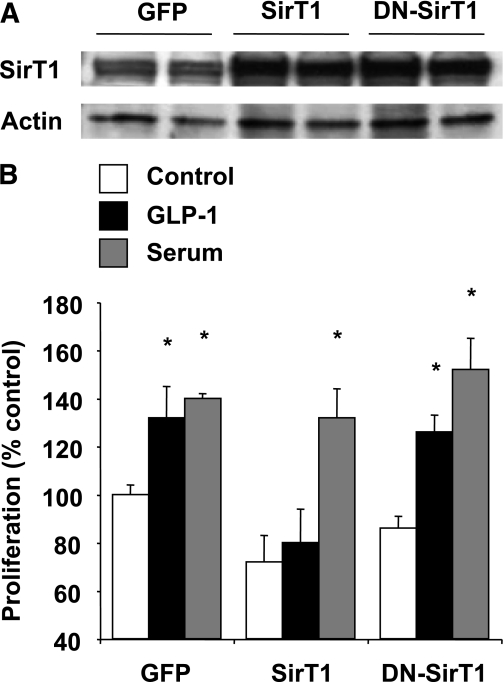
To test whether SirT1 inactivation mediates GLP-1 action on β-cell proliferation, we sought to perform SirT1 gain of function both in vitro and in vivo. INS832/13 cells were either transfected with wild-type SirT1, a catalytically inactive SirT1 mutant, or with control GFP, and β-cell replication was evaluated by BrdU incorporation. Transfection of the cells with SirT1 led to a fivefold increase in protein levels as compared with GFP (Fig. 4A). Overexpression of wild-type SirT1 inhibited GLP-1–induced β-cell proliferation but failed to significantly affect the effect of serum (Fig. 4B). Overexpression of a catalytically inactive SirT1 mutant did not significantly alter β-cell proliferation, indicating that the effects of SirT1 on β-cell proliferation are dependent on its deacetylase activity.
FIG. 4.
Increased dosage of SirT1 suppresses GLP-1–induced β-cell proliferation. A: SirT1 protein levels were evaluated by Western blot after transfection with wild-type (WT) or dominant-negative (DN)-SirT1. GFP was used as control. B: The effect of SirT1 overexpression on β-cell proliferation was evaluated by BrdU incorporation. INS832/13 cells were transfected with WT-SirT1, DN-SirT1, or control GFP for 24 h and then incubated in the presence or absence of GLP-1 (10 nmol/L) or 10% serum for an additional 24-h period. Results represent mean ± SEM of three separate experiments carried out in triplicate. *P < 0.05 compared with the respective control.
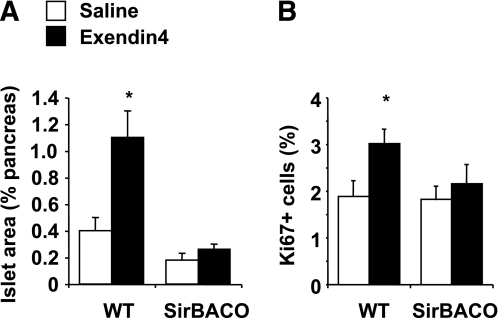
To demonstrate the implication of SirT1 in GLP-1 action in vivo, we determined whether the effects of GLP-1 on β-cell mass were curtailed in transgenic mice with increased dosage of SirT1 (SirBACO mice) as previously described (27). Thus, mice were administered with either saline or the long-acting GLP-1 analog exendin-4 (10 nmol/kg) daily for 7 days. Cross-sectional β-cell areas were estimated by morphometry of pancreatic sections stained for insulin (Fig. 5A), and β-cell proliferation was evaluated by Ki67 staining (Fig. 5B). Exendin-4 administration increased the relative β-cell area by ∼2.5-fold and stimulated β-cell replication by ∼50% in wild-type animals. However, GLP-1 effects on both β-cell area and β-cell replication were abolished in SirBACO mice. Taken together, our results indicate that SirT1 gain of function prevents the mitogenic action of GLP-1 and identify SirT1 as a negative regulator of β-cell mass expansion.
FIG. 5.
The action of exendin-4 on pancreatic β-cell expansion is blunted in SirBACO mice. Wild-type (WT) or SirBACO transgenic mice received daily injections of exendin-4 (10 nmol/kg body wt) or saline for 7 days. Pancreatic β-cell mass and β-cell replication were evaluated by morphometry and immunohistochemistry with anti-insulin and anti-Ki67 antisera. A and B: The relative cross-sectional islet area (A) and the percentage of Ki67-positive β-cells (B) are shown. *P < 0.05 compared with saline controls.
DISCUSSION
Restoration and maintenance of β-cell mass is a major goal of diabetes treatment (29–31). GLP-1, a potent antidiabetes medication (4), enhances glucose-stimulated insulin secretion and promotes β-cell mass expansion, at least in rodents. However, the molecular mechanism by which GLP-1 exerts its action on β-cell mass is not fully elucidated. We have previously demonstrated that the transcription factor FoxO1, an important regulator of β-cell mass (16–18), is a prominent transcriptional effector of GLP-1 action (10). Thus, GLP-1 provoked nuclear exclusion of FoxO1 to relieve a molecular brake on Pdx1 and Foxa2 expression. Also, the effect of exendin-4 on β-cell mass expansion was abolished in transgenic mice with β-cell–specific FoxO1 gain of function (10). Acetylation/deacetylation of FoxO1 regulates its transcriptional activity (19). On one hand, acetylation of FoxO1 by CBP/p300 reduces its DNA binding activity and increases its susceptibility to phosphorylation by Akt (20). On the other hand, acetylated FoxO1 binds to the promyelocytic leukemia–associated protein Pml and is targeted to specific nuclear subdomains (Pml nuclear bodies) (32). Upon calorie restriction or oxidative stress, FoxO1 is deacetylated by the NAD+-dependent sirtuin deacetylase SirT1, which renders FoxO1 immobile within the nuclear compartment and results in FoxO1 transcriptional activity (26). This prompted us to investigate the role of SirT1 in GLP-1 action.
Our findings show that GLP-1 increases FoxO1 acetylation via inhibition of the NAD+-dependent SirT1 deacetylase. Our study provides two possible mechanisms by which GLP-1 could regulate SirT1. First, we show that GLP-1 acutely decreases the cellular NAD+-to-NADH ratio, possibly via its hitherto uncharacterized action on Nampt. Second, GLP-1 downregulates SirT1 expression, thereby providing a long-term mechanism by which chronic or prolonged exposure to GLP-1 receptor agonists could also inhibit SirT1. The fact that GLP-1 failed to increase the intracellular ATP content seemingly refutes the hypothesis that GLP-1 could inhibit SirT1 via the stimulation of β-cell glucose metabolism. However, because our assay did not take into account compartmentalization of ATP production or breakdown, this possibility cannot be completely ruled out. We also demonstrate that the effects of GLP-1 and its long-acting analog exendin-4 on β-cell mass were blunted in a pancreatic β-cell line with increased dosage or SirT1 as well as in transgenic mice with SirT1 gain of function. Our results are consistent with a model in which GLP-1 inhibits SirT1-mediated FoxO1 deacetylation to relieve the constraint on β-cell proliferation and β-cell mass expansion (Fig. 6). Thus far, the action of GLP-1 agonists on β-cell mass has not been demonstrated in humans in vivo. Moreover, the reported increase in β-cell mass in mice has been shown to be restricted after the initial burst of proliferation in the postnatal period (33,34). However, a recent publication suggests that exendin-4 can stimulate β-cell replication in human islet grafts (35).
FIG. 6.
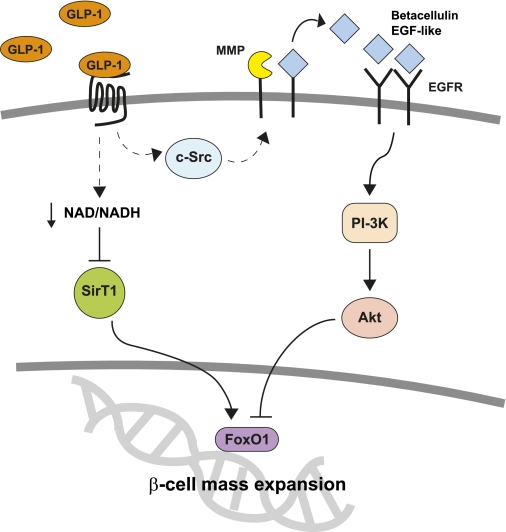
Proposed model. MMP, matrix metalloproteinase; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; PI-3K, phosphatidylinositol-3 kinase. (A high-quality color representation of this figure is available in the online issue.)
Previous studies using genetically engineered mice with SirT1 gain (36) or loss of function (37) indicate that SirT1 could enhance insulin secretion, an effect mediated via reduction of Ucp2 expression. These findings garnered much attention by identifying SirT1, a molecule already scrutinized for its role in longevity, as a potential target for diabetes treatment. However, the action of SirT1 on insulin secretion is difficult to conciliate with its role in calorie restriction (38,39). In addition, these effects were not reproduced in SirBACO mice (27). This discrepancy may be attributed to differences in the level and pattern of SirT1 expression between the various animal models. At first glance, our findings that the glucoincretin hormone GLP-1 inhibits SirT1 may seem conflicting with the action of SirT1 on insulin secretion. However, the short-term effects of GLP-1 on insulin secretion are not transcriptional in nature (40).
In conclusion, we show that GLP-1 suppresses SirT1 activity to stimulate β-cell mass expansion. Our study describes a novel mechanism of action for the glucoincretin hormone and identifies for the first time SirT1 as a negative regulator of pancreatic β-cell mass. Thus, SirT1 could link β-cell replication to the hormonal/nutritional status of the cells.
ACKNOWLEDGMENTS
J.B. is a Canadian Diabetes Association scholar and a Fonds de la Recherche en Santé du Québec Junior Investigator. This work was supported by a research grant to J.B. from the National Sciences and Engineering Research Council.
No potential conflicts of interest relevant to this article were reported.
P.-O.B.-D. and L.V. conducted experiments and performed data analyses. N.K. and W.G. supplied SirBACO mice and reviewed the manuscript. J.B. supervised the project, conducted experiments, and wrote the manuscript.
The authors acknowledge Drs. Domenico Accili (Columbia University), Yves Deshaies (Université Laval), and Frédéric Picard (Université Laval) for numerous useful discussions.
REFERENCES
- 1.Fehmann HC, Göke R, Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev 1995;16:390–410 [DOI] [PubMed] [Google Scholar]
- 2.Holz GG, 4th, Kühtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 1993;361:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorens B, Waeber G. Glucagon-like peptide-I and the control of insulin secretion in the normal state and in NIDDM. Diabetes 1993;42:1219–1225 [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 [DOI] [PubMed] [Google Scholar]
- 5.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992;326:1316–1322 [DOI] [PubMed] [Google Scholar]
- 6.Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology 1992;130:159–166 [DOI] [PubMed] [Google Scholar]
- 7.Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 1999;42:856–864 [DOI] [PubMed] [Google Scholar]
- 8.Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 2000;49:741–748 [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 2002;45:1263–1273 [DOI] [PubMed] [Google Scholar]
- 10.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic beta-cell mass. Diabetes 2006;55:1190–1196 [DOI] [PubMed] [Google Scholar]
- 11.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 2001;50:2237–2243 [DOI] [PubMed] [Google Scholar]
- 12.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003;52:124–132 [DOI] [PubMed] [Google Scholar]
- 13.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 2004;47:806–815 [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia 2004;47:478–487 [DOI] [PubMed] [Google Scholar]
- 15.MacDonald PE, Wang X, Xia F, et al. Antagonism of rat beta-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 2003;278:52446–52453 [DOI] [PubMed] [Google Scholar]
- 16.Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem 2007;282:287–293 [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 2005;280:22297–22307 [DOI] [PubMed] [Google Scholar]
- 18.Nakae J, Biggs WH, 3rd, Kitamura T, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 2002;32:245–253 [DOI] [PubMed] [Google Scholar]
- 19.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004;117:421–426 [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A 2005;102:11278–11283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J Biol Chem 2004;279:28873–28879 [DOI] [PubMed] [Google Scholar]
- 22.Daitoku H, Hatta M, Matsuzaki H, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A 2004;101:10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004;303:2011–2015 [DOI] [PubMed] [Google Scholar]
- 24.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000;49:424–430 [DOI] [PubMed] [Google Scholar]
- 25.Garten A, Petzold S, Barnikol-Oettler A, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun 2010;391:376–381 [DOI] [PubMed] [Google Scholar]
- 26.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 2005;280:20589–20595 [DOI] [PubMed] [Google Scholar]
- 27.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008;8:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyot ML, Gray JP, Lamontagne J, et al. Glucagon-like peptide-1 induced signaling and insulin secretion do not drive fuel and energy metabolism in primary rodent pancreatic beta-cells. PLoS ONE 2009;4:e6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology 2000;141:1926–1929 [DOI] [PubMed] [Google Scholar]
- 30.Bonner-Weir S, Sharma A. Pancreatic stem cells. J Pathol 2002;197:519–526 [DOI] [PubMed] [Google Scholar]
- 31.Edlund H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat Rev Genet 2002;3:524–532 [DOI] [PubMed] [Google Scholar]
- 32.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab 2005;2:153–163 [DOI] [PubMed] [Google Scholar]
- 33.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009;58:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 2009;58:1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian L, Gao J, Weng G, et al. Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transpl Int 2011;24:856–864 [DOI] [PubMed] [Google Scholar]
- 36.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2005;2:105–117 [DOI] [PubMed] [Google Scholar]
- 37.Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 2006;4:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell 2005;120:473–482 [DOI] [PubMed] [Google Scholar]
- 39.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004;305:390–392 [DOI] [PubMed] [Google Scholar]
- 40.Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7-36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes 1998;47:57–65 [DOI] [PubMed] [Google Scholar]