Abstract
OBJECTIVE
Norepinephrine is locally released into the ventromedial hypothalamus (VMH), a key brain glucose-sensing region in the response to hypoglycemia. As a result, this neurotransmitter may play a role in modulating counterregulatory responses. This study examines whether norepinephrine acts to promote glucose counterregulation via specific VMH β-adrenergic receptors (BAR).
RESEARCH DESIGN AND METHODS
Awake male Sprague-Dawley rats received, via implanted guide cannulae, bilateral VMH microinjections of 1) artificial extracellular fluid, 2) B2AR agonist, or 3) B2AR antagonist. Subsequently, a hyperinsulinemic-hypoglycemic clamp study was performed. The same protocol was also used to assess the effect of VMH delivery of a selective B1AR or B3AR antagonist.
RESULTS
Despite similar insulin and glucose concentrations during the clamp, activation of B2AR in the VMH significantly lowered by 32% (P < 0.01), whereas VMH B2AR blockade raised by 27% exogenous glucose requirements during hypoglycemia (P < 0.05) compared with the control study. These changes were associated with alternations in counterregulatory hormone release. Epinephrine responses throughout hypoglycemia were significantly increased by 50% when the B2AR agonist was delivered to the VMH (P < 0.01) and suppressed by 32% with the B2AR antagonist (P < 0.05). The glucagon response was also increased by B2AR activation by 63% (P < 0.01). Neither blockade of VMH B1AR nor B3AR suppressed counterregulatory responses to hypoglycemia. Indeed, the B1AR antagonist increased rather than decreased epinephrine release (P < 0.05).
CONCLUSIONS
Local catecholamine release into the VMH enhances counterregulatory responses to hypoglycemia via stimulation of B2AR. These observations suggest that B2AR agonists might have therapeutic benefit in diabetic patients with defective glucose counterregulation.
Lowering glucose toward normal in patients with type 1 diabetes is well documented to reduce long-term complications (1). In clinical practice, however, intensified insulin treatment is often limited by the increased risk of severe hypoglycemia (2). In people without diabetes, a fall in blood glucose is rapidly detected by glucose-sensing neurons in the brain and periphery, and a series of compensatory responses occur to prevent or limit hypoglycemia and to restore euglycemia. Specifically, the activation of counterregulatory hormone release (e.g., glucagon and epinephrine) and the sympathetic nervous system (norepinephrine) promote endogenous glucose production, reduce tissue utilization of glucose, and generate typical warning symptoms. These protective responses against hypoglycemia are disrupted in most patients with type 1 diabetes receiving intensive insulin therapy (3,4). As a result, they display impaired neurohumoral responses to hypoglycemia and, in some cases, the loss of symptomatic awareness of hypoglycemia. The molecular mechanism(s) underlying this phenomenon are not fully understood.
Detection of hypoglycemia by glucose-sensing cells/neurons peripherally (5) and centrally (6) is critical in the defense against hypoglycemia and the prevention of brain injury. One brain region in particular, the ventromedial hypothalamus (VMH), appears to play a key role in hypoglycemia sensing (7). It contains glucose-excited and glucose-inhibited neurons that detect changes in ambient glucose levels and then alter their firing rate accordingly (8). The VMH contains glutamatergic and γ-aminobutyric acid (GABA)ergic innervations, both of which have been shown to have an effect on counterregulatory responses to hypoglycemia (9,10). In addition, monoamine neurotransmitters, such as norepinephrine, are also released into the VMH and appear to regulate VMH function during hypoglycemia. It has been reported that norepinephrine has been reported to stimulate glutamatergic neurotransmission in mechanically dissociated rat VMH neurons and that this effect is mediated via activation of β2-adrenergic receptors (B2AR) (11). The loss of function of glutamatergic neurons in the VMH region, however, suppresses glucose counterregulation in mice (10). In addition, a rise in norepinephrine concentrations in VMH interstitial fluid has been reported during acute hypoglycemia (12), and this rise is prevented when glucose is perfused into the VMH and systemic hypoglycemia is maintained (13). Although local norepinephrine release into the VMH appears to be altered during hypoglycemia, the neuronal projection circuits coordinating this response are not fully understood. It has been suggested that the brainstem and other hypothalamic regions are important components of a glucose-sensing network coordinating defenses against hypoglycemic stress (14,15).
The current study was undertaken to assess the mechanisms by which the local release of catecholamines might act within the VMH to influence the magnitude of the counterregulatory response to hypoglycemia. Our data suggest that norepinephrine modulates VMH neurons during hypoglycemia at least in part through B2AR.
RESEARCH DESIGN AND METHODS
Male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 275–350 g were individually housed in the Yale Animal Resource Center in temperature-controlled (22–23°C) and humidity-controlled rooms. The animals were fed rat chow (Agway Prolab 3000, Syracuse, NY), given water ad libitum, and acclimatized to a 12-h light/dark cycle. The Yale University Institutional Animal Care and Use Committee approved the experimental protocols.
Surgery.
Seven to 10 days before each study, all animals were anesthetized with an injection (1 mL/kg i.p.) of a mixture of xylazine (20 mg/mL AnaSed; Lloyd Laboratories, Shenandoah, IA) and ketamine (100 mg/mL Ketaset; Aveco, Fort Dodge, IA), in a ratio of 1:2 (vol/vol), before undergoing vascular surgery for the implantation of vascular catheters in a carotid artery and jugular vein. Microinjection guide cannulae were then bilaterally inserted into the VMH, targeting the ventromedial nucleus (coordinates from bregma: anteroposterior −2.6 mm, mediolateral ±0.8 mm, and dorsoventral −8.0).
Microinjection.
On the morning of the study, after an overnight fast and 60 min after opening the catheters, 22-gauge microinjection needles, designed to extend 1 mm beyond the tip of the guide cannula (Plastics One, Roanoke, VA), were inserted through the guide cannula bilaterally into each VMH. The rat was then microinjected over 2 min (0.5 µL/min) using a CMA-102 infusion pump (CMA Microdialysis, North Chelmsford, MA).
The animals were microinjected immediately before the initiation of a hyperinsulinemic-hypoglycemic clamp with 1 µL of one the following pharmacologic agents: artificial extracellular fluid as a control (n = 12); 1 μmol/L concentration of the B2AR antagonist ICI-118,551 (n = 12); or 1 μmol/L concentration of the B2AR agonist formoterol (n = 10). The doses of B2AR agonists and antagonists selected were based on preliminary dose-ranging studies using a 1 nmol/L to 200 μmol/L concentration of the agonist and antagonist. In another set of studies, we microinjected 1 μmol/L CGP 20712 dihydrochloride, a potent, selective B1AR (n = 7), or 1 μmol/L SR59230A hydrochloride, a potent, selective B3AR antagonist (n = 6).
Hyperinsulinemic-hypoglycemic clamp.
A primed-continuous intravascular infusion of 20 mU ⋅ kg−1 ⋅ min−1 regular insulin was started, and a variable infusion of 20% dextrose was adjusted in response to 10-min measurements of plasma glucose to reach a target value of 50 mg/dL within 30 min. Thereafter, plasma glucose was monitored at 10-min intervals using a glucose analyzer (Analox Instruments, Lunenburg, MA) to adjust the dextrose infusion rate to maintain a stable 50 mg/dL glucose level until the end of the study at 90 min. Blood was drawn at baseline and at 30, 60, and 90 min for measurement of plasma glucagon, epinephrine, norepinephrine, and insulin.
Probe placement.
At the end of experiments, the rats were killed with an overdose of sodium pentobarbital (Sleepaway; Fort Dodge Animal Health, Fort Dodge, IA). Brains were removed, frozen, and stored at −80°C until analysis. Accuracy of microinjection needle placements was verified histologically.
Analytic procedures.
Catecholamine analysis was performed by high-performance liquid chromatography using electrochemical detection (ESA, Acton, MA). Plasma insulin and glucagon were measured by radioimmunoassay (Linco, St. Charles, MO).
Data analysis.
All data are expressed as means ± SEM. Baseline comparisons were performed using ANOVA. A mixed-model analysis with an unstructured covariance matrix was used to accommodate correlated responses from repeated measures. Post hoc linear contrasts were used to localize effects. Analyses were performed using SAS 9.2 software (SAS Institute, Inc., Cary, NC), with a threshold P < 0.05 considered as statistically significant.
RESULTS
As summarized in Table 1, the groups of rats did not differ with respect to body weight or concentrations of plasma glucose, insulin, glucagon, epinephrine, and norepinephrine at baseline. During the hyperinsulinemic-hypoglycemic clamp studies, plasma glucose and insulin in each animal group reached levels that were not significantly different (Table 2).
TABLE 1.
Baseline metabolic status of animals in the morning before the study
| Variable | Control (n = 12) | B2AR antagonist ICI-118,551 (n = 12) | B2AR agonist formoterol (n = 10) | B1AR antagonist CGP 20712 (n = 7) | B3AR antagonist SR59230A (n = 6) |
|---|---|---|---|---|---|
| Body weight (g) | 320 ± 7 | 316 ± 7 | 315 ± 6 | 291 ± 3.1 | 289 ± 9 |
| Plasma glucose (mmol/L) | 6.4 ± 0.2 | 6.3 ± 0.1 | 6.5 ± 0.2 | 6.3 ± 0.2 | 6.1 ± 0.2 |
| Insulin (µU/mL) | 7.6 ± 1.8 | 8.2 ± 1.3 | 7.9 ± 1.3 | 10.1 ± 0.7 | 12.4 ± 3.5 |
| Glucagon (ng/L) | 46 ± 3 | 44 ± 3 | 50 ± 5 | 58 ± 6 | 58 ± 5 |
| Epinephrine (pg/mL) | 115 ± 40 | 129 ± 42 | 64 ± 25 | 94 ± 41 | 146 ± 33 |
| Norepinephrine (pg/mL) | 207 ± 34 | 174 ± 26 | 166 ± 33 | 233 ± 63 | 234 ± 54 |
Data are presented as mean ± SEM.
TABLE 2.
Plasma glucose and insulin concentrations during the final 60 min of the hyperinsulinemic-hypoglycemic clamp study for rats in each study group
| Variable | Control (n = 12) | B2AR antagonist ICI-118,551 (n = 12) | B2AR agonist formoterol (n = 10) | B1AR antagonist CGP 20712 (n = 7) | B3AR antagonist SR59230A (n = 6) |
|---|---|---|---|---|---|
| Plasma glucose (mmol/L) | 3.0 ± 0.05 | 3.0 ± 0.03 | 2.8 ± 0.03 | 2.9 ± 0.07 | 3.0 ± 0.06 |
| Insulin (µU/mL) | 1,235 ± 112 | 1,250 ± 164 | 1,302 ± 156 | 1,229 ± 162 | 1,292 ± 160 |
Data are presented as mean ± SEM.
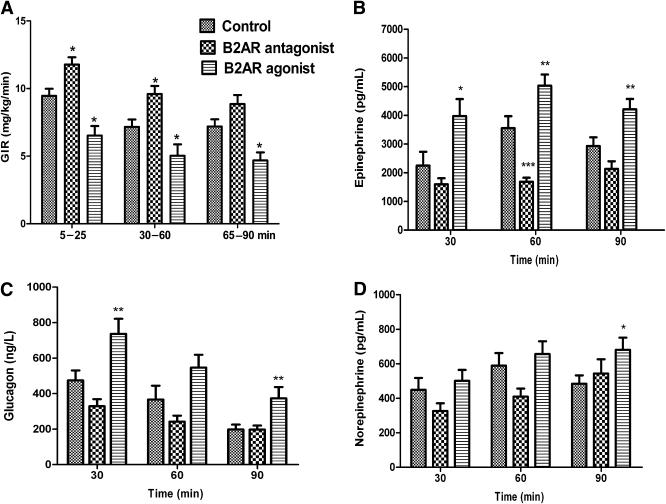
As shown in Fig. 1A, VMH delivery of the B2AR agonist reduced the exogenous glucose infusion rates (GIR) required to maintain target glucose concentration by 32% during the entire hypoglycemic clamp (P < 0.01), whereas B2AR blockade in the VMH raised exogenous glucose requirements by 27% (P < 0.05) compared with the control study.
FIG. 1.
B2AR modulation in the VMH and its effect on GIR and counterregulatory hormones during the hypoglycemic clamp study. GIR (A) and hormonal responses for plasma epinephrine (B), glucagon (C), and norepinephrine (D) for rats receiving microinjection of the artificial extracellular fluid vehicle (control; n = 12), the B2AR antagonist ICI-118,551 (n = 12), or the B2AR agonist formoterol (n = 10) during the hyperinsulinemic-hypoglycemic glucose clamp. Results are presented as mean ± SEM. Post hoc linear contrasts to localized effects; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. controls.
These changes were accompanied by alternations in peak and in overall epinephrine responses during hypoglycemia. Delivery of B2AR agonist to the VMH increased peak epinephrine responses by 37% (P < 0.01) and epinephrine levels during the entire 90-min period of hypoglycemia by 50% (P < 0.01) compared with the control group. In contrast, VMH delivery of the B2AR antagonist suppressed the peak by 53% (P < 0.001) and epinephrine levels during the entire 90-min period of hypoglycemia by 32% (P < 0.05) compared with the control group (Fig. 1B). Peak and overall glucagon response was also increased by activation of VMH B2AR by 53 and 63%, respectively (P < 0.01 for both vs. control) (Fig. 1C). However, administration of the B2AR antagonist had only a small inhibitory effect on the peak glucagon response, which failed to reach statistical significance (P = 0.051 vs. control at 30 min) (Fig. 1C). Norepinephrine levels were also slightly higher in the B2AR agonist group, but at 90 min only (P < 0.05 vs. control) (Fig. 1D).
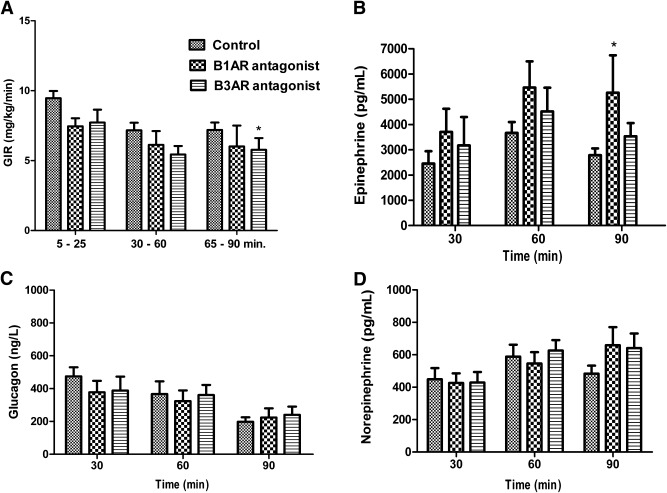
Targeted blockade of VMH B1AR or B3AR failed to suppress counterregulatory hormone responses to hypoglycemia or increase the GIR needed to maintain hypoglycemia (Fig. 2). VMH B1AR blockade had no significant effect on GIR (Fig. 2A) but did produce a modest increase in the overall epinephrine response (P < 0.05 vs. control) (Fig. 2B). Neither glucagon (Fig. 2C) nor norepinephrine (Fig. 2D) responses were significantly affected by B1AR blockade. VMH B3AR blockade produced a small 21% decrease in the GIR required during hypoglycemia (P < 0.05 vs. control). However, B3AR blockade had no significant effect on the release of epinephrine, glucagon, and norepinephrine during hypoglycemia compared with controls (Fig. 2).
FIG. 2.
Effect of VMH B1AR or B3AR blockade on GIR and counterregulatory hormones during the hypoglycemic clamp study. GIR (A) as well as epinephrine (B), glucagon (C), and norepinephrine (D) responses for rats receiving microinjection of the artificial extracellular fluid vehicle (control; n = 12), the B1AR antagonist CGP 20712 (n = 7), and the B3AR antagonist SR59230A (n = 6) during the hyperinsulinemic-hypoglycemic glucose clamp. Results are presented as mean ± SEM. Post hoc linear contrasts to localized effects; *P < 0.05 vs. controls.
DISCUSSION
Previous studies have suggested a potential role for local VMH norepinephrine neurotransmission in modulating glucose counterregulation. This view is supported by data showing that 1) norepinephrine delivery into the VMH increases blood glucose and counterregulatory hormone levels (16,17); 2) glucopenia stimulates norepinephrine turnover in the mediobasal hypothalamus, including the VMH (18,19); and 3) levels of norepinephrine increase in VMH interstitial fluid during hypoglycemia (12,13). The current study was undertaken to assess whether norepinephrine acts to promote glucose counterregulation via specific VMH BARs. The data demonstrate that local VMH delivery of a specific B2AR agonist increases both epinephrine and glucagon responses to acute hypoglycemia, whereas delivery of a B2AR antagonist suppresses epinephrine release and tends to reduce the initial glucagon response. In contrast, blockade of VMH B1AR or B3AR failed to have a significant inhibitory effect on counterregulatory responses. It should be noted that for these studies we used similar concentrations of BAR agonists and antagonists (1 μmol/L) as were used in previous in vivo (12,20) and in vitro (11) studies examining the specific receptors mediating the effects of norepinephrine on VMH neurons. Although these concentrations exceed the nanomolar norepinephrine concentrations present in the VMH interstitial fluid, if one takes into account the dilution of the agent in the VMH and in vivo clearance from the tissue during the study, the active local dose level in the VMH was considerably lower. Nevertheless, it seems unlikely that the changes we observed represent off-target effects. We have observed that if the dose of B2AR antagonist is diminished 10-fold, there is a similar degree of suppression of epinephrine responses to hypoglycemia and that if much higher doses of the compound are given, the effect on epinephrine release is less pronounced (B.S., unpublished data). Taken together, our data suggest that norepinephrine release into the VMH during hypoglycemia acts to promote glucose recovery, at least in part via stimulation of B2AR. These findings are consistent with previous studies showing that norepinephrine activates isolated glutamatergic neurons derived from the rat VMH via B2AR (11).
It is noteworthy that earlier studies suggested that norepinephrine’s central actions to regulate peripheral glucose levels were mediated via α2-receptors. Smythe et al. (18,19,21) reported that yohimbine, an α2-receptor antagonist, markedly inhibited stress induced hyperglycemia as well as the hyperglycemic effects of 2-deoxyglucose administration. Moreover, Beverly et al. (20) reported that during hypoglycemia the norepinephrine rise within the VMH followed a bimodal pattern. The initial peaks disappeared when yohimbine was locally delivered, whereas the second rise was suppressed in the presence of timolol, a nonselective BAR antagonist. Neither of these studies, however, examined the effect of hypothalamic adrenergic receptor modulation specifically on the responses of counterregulatory hormones to hypoglycemia. It is noteworthy in this regard that previous studies suggest that the activation of an appropriate counterregulatory response to hypoglycemia is associated with inhibition of GABA tone (9) and activation of glutamate neurons within the VMH (10). It is intriguing to speculate that norepinephrine might act via different adrenergic receptors to exert complementary effects on VMH glutamate and GABA neurotransmission to promote counterregulatory responses to hypoglycemia.
It is now recognized that hypoglycemia is detected by specialized glucose-sensing neurons located within a variety of brain regions (6,7) as well as periphery (5). Watts and Donovan, in a recent review (14), proposed that brainstem catecholaminergic neurons receive neural input from peripheral glucose sensors and then transmit this information to hypothalamic glucose-sensing neurons, including those in the VMH. Thus, these catecholaminergic projections might serve to integrate the VMH into a wide network glucose-sensing neurons.
We conclude that synaptic release of catecholamines acting via B2AR within the VMH plays an important role in modulating the magnitude of the epinephrine responses and, to lesser extent, glucagon responses to insulin-induced hypoglycemia. These observations are consistent with previous studies suggesting that administration of the B2AR agonist terbutaline may restore hypoglycemia awareness and reduce nocturnal hypoglycemia (22,23). This effect may be particularly useful in humans with homozygosity for glycine at codon 16 (GlyGly) of the B2AR who appear to show reduced B2AR sensitivity to antecedent hypoglycemia (24). Moreover, the current data are consistent with recent data indicating that repeated adrenergic receptor activation might also be involved in the generation of hypoglycemia-associated autonomic failure (25) and raise the possibility that B2AR receptor downregulation might play a role in this phenomenon. In our study we used a long-lasting, specific, commercially available β-adrenergic agonist. Given that hypoglycemia remains the major limiting factor in the use of intensified insulin therapy in the management of diabetes, our study may have potential therapeutic implications for strategies aimed at reducing the risk of severe hypoglycemia in diabetes.
ACKNOWLEDGMENTS
This work was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; 20495), the Juvenile Diabetes Research Foundation (JDRF), and the NIDDK-supported Diabetes Endocrinology Research Center. B.S. is the recipient of a postdoctoral fellowship from the JDRF.
No potential conflicts of interest relevant to this article were reported.
B.S. researched data and wrote the manuscript. W.Z. researched data. O.C. contributed to discussion. A.H. researched data. J.D. performed statistical analysis of data. R.S.S. designed the study and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
The authors are grateful to Aida Groszman, Maria Batsu, Codruta Todeasa, and Ralph J. Jacob, Yale Center for Clinical Investigation Core Laboratory of the Yale Medical School, for excellent technical support and assistance.
REFERENCES
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 3.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 5.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 1997;46:1521–1525 [DOI] [PubMed] [Google Scholar]
- 6.Frizzell RT, Jones EM, Davis SN, et al. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes 1993;42:1253–1261 [DOI] [PubMed] [Google Scholar]
- 7.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 1994;93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin BE, Dunn-Meynell AA, Routh VH. CNS sensing and regulation of peripheral glucose levels. Int Rev Neurobiol 2002;51:219–258 [DOI] [PubMed] [Google Scholar]
- 9.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 2006;55:1080–1087 [DOI] [PubMed] [Google Scholar]
- 10.Tong Q, Ye C, McCrimmon RJ, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 2007;5:383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JG, Choi IS, Park EJ, et al. beta(2)-Adrenoceptor-mediated facilitation of glutamatergic transmission in rat ventromedial hypothalamic neurons. Neuroscience 2007;144:1255–1265 [DOI] [PubMed] [Google Scholar]
- 12.Beverly JL, De Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am J Physiol Regul Integr Comp Physiol 2001;280:R563–R569 [DOI] [PubMed] [Google Scholar]
- 13.de Vries MG, Lawson MA, Beverly JL. Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. Am J Physiol Regul Integr Comp Physiol 2005;289:R977–R981 [DOI] [PubMed] [Google Scholar]
- 14.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 2010;31:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007;22:241–251 [DOI] [PubMed] [Google Scholar]
- 16.Chafetz MD, Parko K, Diaz S, Leibowitz SF. Relationships between medial hypothalamic alpha 2-receptor binding, norepinephrine, and circulating glucose. Brain Res 1986;384:404–408 [DOI] [PubMed] [Google Scholar]
- 17.Steffens AB, Scheurink AJ, Luiten PG, Bohus B. Hypothalamic food intake regulating areas are involved in the homeostasis of blood glucose and plasma FFA levels. Physiol Behav 1988;44:581–589 [DOI] [PubMed] [Google Scholar]
- 18.Smythe GA, Edwards SR. Suppression of central noradrenergic neuronal activity inhibits hyperglycemia. Am J Physiol 1992;263:E823–E827 [DOI] [PubMed] [Google Scholar]
- 19.Smythe GA, Grunstein HS, Bradshaw JE, Nicholson MV, Compton PJ. Relationships between brain noradrenergic activity and blood glucose. Nature 1984;308:65–67 [DOI] [PubMed] [Google Scholar]
- 20.Beverly JL, de Vries MG, Beverly MF, Arseneau LM. Norepinephrine mediates glucoprivic-induced increase in GABA in the ventromedial hypothalamus of rats. Am J Physiol Regul Integr Comp Physiol 2000;279:R990–R996 [DOI] [PubMed] [Google Scholar]
- 21.Smythe GA, Edwards SR. A role for central postsynaptic alpha 2-adrenoceptors in glucoregulation. Brain Res 1991;562:225–229 [DOI] [PubMed] [Google Scholar]
- 22.Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal hypoglycemia in type 1 diabetes: an assessment of preventive bedtime treatments. J Clin Endocrinol Metab 2006;91:2087–2092 [DOI] [PubMed] [Google Scholar]
- 23.Fritsche A, Tschritter O, Häring H, Gerich J, Stumvoll M. The role of beta-adrenergic sensitivity in the pathogenesis of hypoglycaemia unawareness. Diabetes Nutr Metab 2002;15:357–361; discussion 361–362 [PubMed] [Google Scholar]
- 24.Schouwenberg BJ, Smits P, Tack CJ, de Galan BE. The effect of antecedent hypoglycaemia on β2-adrenergic sensitivity in healthy participants with the Arg16Gly polymorphism of the β2-adrenergic receptor. Diabetologia 2011;54:1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramanathan R, Cryer PE. Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes 2011;60:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]