Abstract
Objective
To describe the patient profiles of the Leber hereditary optic neuropathy (LHON) Gene Therapy Clinical Trial, year 1. This study aims to identify and characterize affected patients and carriers with the G11778A mutation in mitochondrial DNA for planned gene therapy that will use “allotopic expression” by delivering a normal nuclear-encoded ND4 gene into the nuclei of retinal ganglion cells via an adeno-associated virus vector injected into the vitreous.
Methods
Patients with LHON with visual loss as well as asymptomatic maternally related family members were molecularly screened for ND1, ND4, and ND6 mutations in mitochondrial DNA commonly associated with LHON. All patients and maternal relatives also underwent complete neuro-ophthalmic examination, automated visual field testing, pattern electroretinogram (PERG), and OCT3.
Results
Twenty-five subjects with LHON and 21 carriers positive for the G11778A mitochondrial DNA mutation were recruited. Three additional mutations in the ND4 gene, G11719A, G11947A, or G11914A, were detected. Mean retinal nerve fiber layer (RNFL) thickness was 78.3 μm up to 32 months after visual loss. It was 63.5 μm for all affected patients and 100.7 μm for carriers (P<.01). Mean PERG amplitude was lower in affected patients (40% of normal) than in carriers (94% of normal) (P<.01). Four carriers with PERG amplitudes less than 75% of normal had Early Treatment Diabetic Retinopathy Study acuity more than 20/25, mean defect more than −2 dB, and average RNFL thickness more than 80 μm.
Conclusions
Potential candidates for future gene therapy may include affected patients, as late as 32 months after loss of vision, with mildly reduced RNFL thickness or carriers with low PERG amplitudes and normal RNFL thickness, if the PERG amplitude is a predictor of conversion to LHON in these carriers.
Our understanding of human diseases caused by mutated mitochondrial DNA (mtDNA) has recently evolved dramatically. This is a group of untreatable disorders affecting the eye, nervous system, and heart, some clinically characterized more than a century ago,1 but they are now known to be a spectrum of molecularly defined diseases. In approximately 95% of the cases worldwide, Leber hereditary optic neuropathy (LHON) is caused by 3 pathogenic point mutations in mtDNA coding for the respiratory chain subunits of the nicotinamide adenine dinucleotide ubiquinone-oxidoreductase (complex I) genes: 3460G>A in ND1, 11778G>A in ND4, and 14484T>C in ND6.2 Of these 3 mutations, the 11778G>A resulting in an arginine to histidine substitution at amino acid 340 is responsible for half of the LHON cases. We have chosen to start the journey to gene therapy with one of the most severe, the G11778A mutation in mtDNA responsible for LHON, a disease renowned for causing blindness in later childhood and early adulthood.3 It shows little or no propensity for recovery and there is no effective therapy for this or any disease caused by mutated mtDNA.4
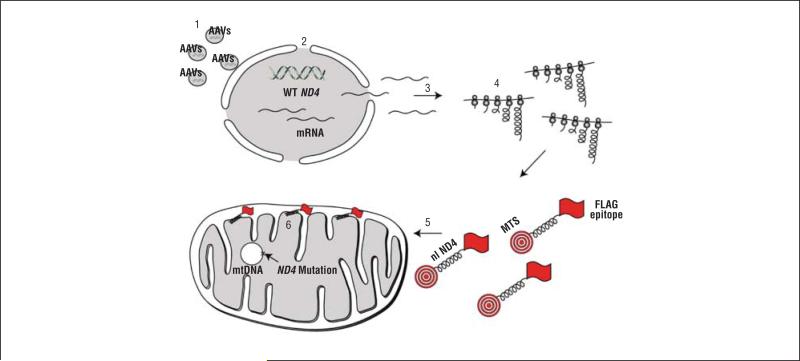
One of the major limitations in this aspect is that there are few practical methods currently available to deliver genes to the mitochondria.5,6 Since no technology existed to introduce DNA directly into mitochondria, we overcame this deficiency in oxidative phosphorylation by constructing a “nuclear version” of the mitochondrial gene. We then directed the cytoplasmically synthesized protein to the mitochondria by using a targeting sequence appended to the reading frame.7-9 An illustration of this technique, coined allotopic expression, is shown in Figure 1. Allotopic expression of the normal ND4 gene normalized the defective adenosine triphosphate synthesis of LHON cells and it improved their survival.10
Figure 1.
Illustration of allotopic strategy. Adeno-associated viruses (AAVs) deliver a nuclear-encoded version of ND4 to the nucleus of the cell (step 1). Messenger RNA (mRNA) is transcribed in the nucleus (step 2). The mRNA diffuses into the cytoplasm (step 3), where it directs the synthesis of a mitochondrial-targeting sequence (MTS) attached to the nuclear ND4 protein (nl ND4) that also has a FLAG epitope for immunodetection. The entire fusion protein is translated on cytoplasmic ribosomes (step 4). The MTS directs the MTS/ND4/FLAG fusion protein into the mitochondria where the MTS is cleaved off (step 5). Within the inner mitochondrial matrix the mature protein (ND4/FLAG), without the MTS, is assembled into complex I (step 6). mtDNA indicates mitochondrial DNA; WT, wild type.
Next, we created a bona fide animal model for LHON. Using site-directed mutagenesis of the nuclear version of ND4, we replaced the codon for arginine with that for histidine at amino acid 340. Injection of this construct into the mouse visual system disrupted mitochondrial cytoarchitecture, elevated reactive oxygen species, induced swelling of the optic nerve head, and induced apoptosis, with a progressive demise of ganglion cells in the retina and their axons composing the optic nerve.11 In contrast, ocular expression of the wild-type human ND4 subunit appeared safe, suggesting that it may be useful for treatment of patients with LHON.12 Here, by using the scientific and clinical knowledge acquired to date, we begin the journey toward genetic therapy for human optic neuropathy and mitochondrial disease. Our goal is to test the relevance and the effectiveness of this therapy in preventing and restoring visual loss in recruited patients with LHON and maternally related family members.
METHODS
CLINICAL
Patients with LHON and maternally related family members were recruited at the Bascom Palmer Eye Institute by neuro-ophthalmologists (J.G. and B.L.L.). Informed consent, approved by the University of Miami institutional review board, was obtained from patients with LHON and their maternally related family members. Best-corrected visual acuity was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Neuro-ophthalmic examination also included assessments of the pupils and anterior segment and ophthalmoscopy of the optic nerve head and retina. Visual field test results were obtained with the Humphrey 30-2 white-on-white standard automated perimetry.
MOLECULAR ANALYSIS
Genomic DNA was obtained from the patient's white blood cells obtained from venous blood. A polymerase chain reaction–based test using the amplification refractory mutation system was used to detect the presence or absence of 3 nucleotide substitutions known to cause LHON (3460G>A, 11778G>A, or 14484T>C). Two polymerase chain reaction cocktails, 1 with primers corresponding to the normal sequence and 1 with primers corresponding to the mutant sequences, were used to amplify patient DNA. Together, these amplifications revealed the normal and mutant genotypes. An additional primer pair, corresponding to a region of chromosome 7, was included in the mutant polymerase chain reaction cocktails as an internal amplification control. All positive amplification refractory mutation system results were confirmed with bidirectional DNA sequencing. Twenty affected patients and 15 carriers had molecular analysis that was performed by the Carver Laboratory at the University of Iowa.13 This cohort of subjects was tested for heteroplasmy and secondary mutations in the ND4 gene. Five affected patients and 6 carriers had molecular analysis that was performed by Athena Laboratories. Heteroplasmy and secondary mutations were not reported to us by Athena.
OPTICAL COHERENCE TOMOGRAPHY
Peripapillary retinal nerve fiber layer (RNFL) thickness was evaluated using the fast RNFL program of the Stratus OCT (Carl Zeiss Meditec, Dublin, California) and analyzed using software version 3.0. The RNFL thickness was determined at 256 points around a circular scan (diameter, 3.4 mm) around the center of the optic disc that was repeated 3 consecutive times. For each eye, RNFL scans were repeated 4 times and exported on an electronic worksheet, and an average scan was computed. The RNFL thickness was evaluated from the average scan.
ELECTROPHYSIOLOGY
The pattern electroretinogram (PERG) was simultaneously recorded from both eyes according to a new paradigm optimized for glaucoma detection (PERGLA)14 that is incorporated in a commercially available system (Lace Elettronica, Rome, Italy). The method is considered highly reproducible,14,15 and normative data are available.16 Details of the PERGLA protocol have been previously described.14 In brief, retinal signals were recorded by means of skin electrodes taped on the lower eyelids. Subjects were fitted with the appropriate add and were instructed to fixate on a target at the center of a pattern stimulus placed at a viewing distance of 30 cm. Subjects did not receive dilating drops and were allowed to blink freely. The pattern stimulus consisted of horizontal gratings (1.7 cycles/degree, 25° diameter circular field, 98% contrast, 40 candelas [cd]/m2 mean luminance) reversing in counterphase at 8.14 Hz (16.28 reversals/s). The pattern stimulus was generated on a cathode ray tube display (65-Hz refresh rate, not interlaced) placed at the bottom of a Ganzfeld bowl having a background luminance of 3 cd/m2. Electrical signals were bandpass filtered (1-30 Hz), amplified (100 000-fold), and averaged in synchrony with the reversal period. During signal acquisition, sweeps contaminated by eye blinks or gross eye movements were automatically rejected over a threshold voltage of 25 μV. Two successive responses of 300 artifact-free sweeps each were recorded, separated by a brief pause. The first 30 sweeps of each response were rejected to allow steady-state conditions. The software allowed visual inspection of the 2 consecutive responses superimposed to check for consistency and then computed the final PERG waveform (600 artifact-free sweeps). Since the PERG was recorded in response to relatively fast alternating gratings, the response waveforms were typically sinusoidal-like, with a temporal period corresponding to the reversal rate. The PERG waveforms were automatically analyzed in the frequency domain by discrete Fourier transform to isolate the frequency component at the contrast-reversal rate (16.28 Hz) and compute its amplitude in microvolts.
STATISTICAL ANALYSIS
Values were expressed as mean (SD). Data were analyzed via the t test for paired or unpaired data. A P value less than .05 was considered significant and those less than .01 were considered to be highly significant. ETDRS visual acuity was converted to logMAR units for analysis. Because of the strong age dependency of PERG, these measurements were expressed as percentage of age-specific normal.17 Except where otherwise noted, measurements of interval-level variables, such as ETDRS acuity, RNFL thickness, and PERG amplitude, collected from the 2 eyes on an individual were averaged for analysis.
RESULTS
MOLECULAR ANALYSIS
Affected patients were homoplasmic, with 1 exception who was heteroplasmic, for the G11778A mutation in mtDNA that changes the highly conserved amino acid 340 from arginine to histidine in the ND4 subunit of complex I. Maternally related family members were also homoplasmic for the G11778A mutation in mtDNA, except for 2 carriers who were heteroplasmic. These 2 carriers had at least 70% to 80% of the mutated G11778A mtDNA.
Twelve subjects with the G11778A mutation had additional mutations in the ND4 gene (Table). Seven subjects with the G11778A mutation had 2 additional mutations in the ND4 mtDNA (A11947G and G11719A, n=5 subjects; G11719A and G11914A, n=2 subjects). Five subjects with the G11778A mutation had only 1 additional mutation (G11719A). Eleven subjects were homoplasmic for the G to A transition at nucleotide 11719 (G11719A). This mutation resulted in a substitution of glutamate for glycine at amino acid 320 in the ND4 protein. Thus, the single affected patient who was heteroplasmic for the primary G11778A mtDNA mutation (>50% G11778A mtDNA) had 2 amino acid substitutions in the ND4 protein. An alignment of the amino acid changes caused by the G11719A and G11778A mutations in mtDNA is shown relative to the normal mitochondrial-encoded ND4 amino acid sequence in Figure 2. Subjects with the G to A transition at nucleotide 11914 (G11914A, n=2 subjects) or the A to G transition at nucleotide 11947 (A11947G, n=5 subjects) were homoplasmic. These mutations resulted in no amino acid changes of the specified amino acid (threonine) in the ND4 protein.
Table.
G11778A-Positive Subjects With Secondary mtDNA Mutations (% mtDNA With Mutation)
| Subject No. | Secondary (mtDNA) Mutations, % |
||||
|---|---|---|---|---|---|
| G11778A | G11719A | A11947G | G11914A | Phenotype | |
| 1 | 100 | 100 | 100 | 0 | Affected |
| 2 | 100 | 100 | 100 | 0 | Carrier |
| 3 | 100 | 100 | 100 | 0 | Carrier |
| 4 | 100 | 100 | 0 | 100 | Affected |
| 5 | 100 | 100 | 0 | 100 | Carrier |
| 6 | 70 | 100 | 0 | 0 | Carrier |
| 7 | 100 | 100 | 0 | 0 | Affected |
| 8 | 100 | 100 | 0 | 0 | Affected |
| 9 | 100 | 100 | 0 | 0 | Affected |
| 10 | 100 | 100 | 100 | 0 | Affected |
| 11 | 100 | 100 | 100 | 0 | Carrier |
| 12 | 50 | 100 | 0 | 0 | Affected |
Abbreviation: mtDNA, mitochondrial DNA.
Figure 2.
Alignment of ND4 protein amino acid changes induced by Leber hereditary optic neuropathy mutations. At amino acid 340, arginine (R) is replaced with histidine (H). A secondary mutation at amino acid 320 replaces a glycine (G) with glutamate (E). The top line shows the sequence of the normal ND4 protein.
VISUAL FUNCTION
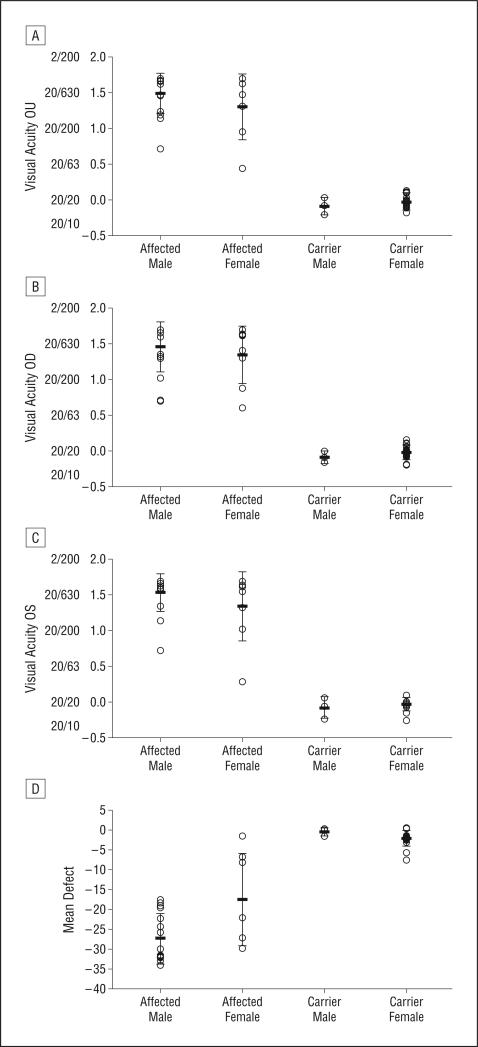
Of the 46 subjects with LHON with the G11778A mutation in mtDNA, there were 25 patients with visual loss, 17 males and 8 females. Median time from visual loss was 33 months. Mean age at the time of recruitment was 31.8 years (range, 9.9-61.5 years). Mean age at the onset of visual loss was 23 years for males and it was 32.8 years for females. All affected patients had bilateral loss of vision. Mean visual acuity was 20/630 in males and 20/400 in females (Figure 3A). Visual acuity results of the right eyes are shown in Figure 3B and those of the left eyes are shown in Figure 3C. There were 21 asymptomatic carriers (3 males and 18 females). Their mean age was 43.2 years (range, 9.3-84.7 years). For asymptomatic carriers, mean ETDRS visual acuity was 20/25 or better (Figure 3).
Figure 3.
Plots of visual acuity and visual field mean defects. A, Plots of the mean visual acuity in subjects with Leber hereditary optic neuropathy and maternally related family members. B and C, Mean visual acuity of the right eyes (B) and the left eyes (C). D, Plots of Humphrey visual field mean defects.
Humphrey visual field test results showed central defects consistent with LHON in all affected patients. Mean defect loss was −27.35 for affected males and −18.54 for affected females (Figure 3D). For asymptomatic carriers, mean defect loss was −0.52 for males and −2.13 for females.
OPTICAL COHERENCE TOMOGRAPHY
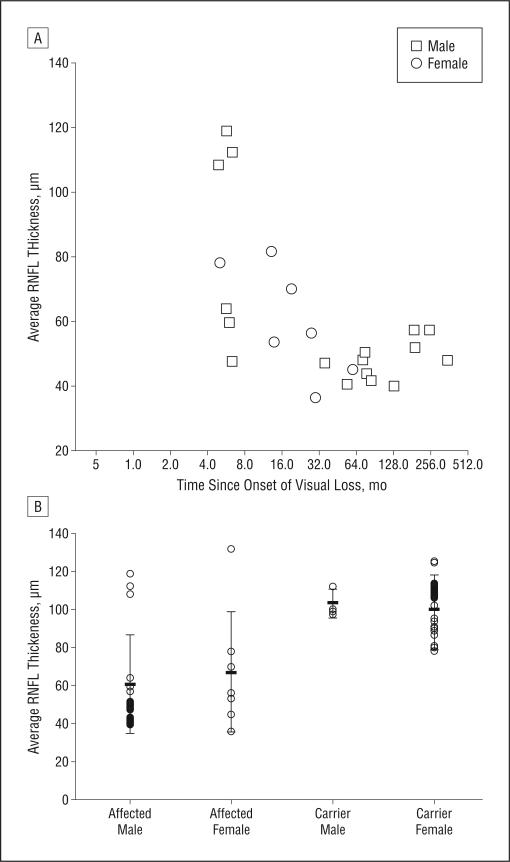
All optical coherence tomography was done during the initial visit. The mean thickness of the RNFL was 78.3 μm up to 32 months after onset of visual loss. After this point, mean RNFL thickness was markedly reduced at 47.5 μm, with the differences being highly significant (P=.003) (Figure 4A). Overall, for all affected patients, the mean RNFL thickness was 63.5 μm. The RNFL thickness was 60.88 μm for males and 69.01 μm for females (Figure 4B). Four patients (16%) with acute visual loss had higher than normal RNFL thickness greater than 100 μm and PERG amplitudes reduced to less than 50% of normal. For asymptomatic carriers, mean RNFL thickness was 100.74 μm. For male carriers, RNFL thickness was 103.67 μm and for female carriers, it was 100.26 μm. Twelve asymptomatic carriers (57%) had RNFL thickness measurements that were greater than 100 μm.
Figure 4.
Plots of optical coherence tomography. A, Scatterplot of the Stratus OCT3 (Carl Zeiss Meditec, Dublin, California) average thickness of the retinal nerve fiber layer (RNFL) relative to the months after initial visual loss. All optical coherence tomography was done during the initial visit. B, A plot of the OCT3 average RNFL thickness of affected subjects and carriers.
PERG AMPLITUDE RELATIVE TO VISUAL FUNCTION
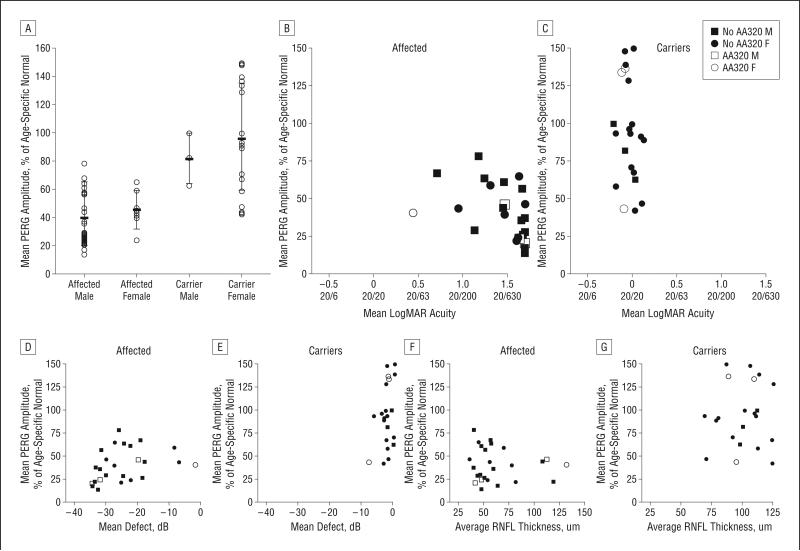
For all affected patients, the mean PERG amplitude was 0.46 μV (40% of normal). For affected males, it was 0.45 μV (39% of normal) and it was 0.49 μV (42% of normal) for females (Figure 5A). For all asymptomatic carriers, the mean PERG amplitude was 0.98 μV (94% of normal). For male carriers, it was 1.04 μV (81% of normal) and for female carriers, it was 0.97 μV (96% of normal).
Figure 5.
Plots of pattern electroretinogram (PERG) amplitude. A, The PERG amplitude relative to normal is shown for affected subjects and carriers. B and C, The PERG amplitude percentage of normal is shown relative to visual acuity with scatterplots in affected subjects (B) and carriers (C). D and E, The PERG amplitude relative to normal is shown relative to the mean defect on Humphrey visual field tests with scatterplots in affected subjects (D) and carriers (E). F and G, The PERG amplitude relative to normal is shown with scatterplots relative to the Stratus OCT3 (Carl Zeiss Meditec, Dublin, California) retinal nerve fiber layer (RNFL) thickness in affected subjects (F) and carriers (G). AA320 F indicates female with primary G11778A mutation plus secondary mutation in ND4 mitochondrial DNA (mtDNA); AA320 M, male with primary G11778A mutation plus secondary mutation in ND4 mtDNA; No AA320 M, male with primary G11778A mtDNA mutation but no secondary mtDNA mutation; No AA320 F, female with primary G11778A mutation but no secondary mtDNA mutation.
For affected patients with LHON, severe reductions in the PERG amplitude were associated with poor visual acuity and severe visual field loss (Figure 5B and D). However, reductions in the PERG amplitude of 25% or more were seen in 7 carriers who had visual acuity of 20/25 or better, only 1 of whom had a mean defect worse than −5 dB (Figure 5C and E) on Humphrey visual field testing.
PERG AMPLITUDE RELATIVE TO OPTICAL COHERENCE TOMOGRAPHY
While most affected patients who had low PERG amplitudes also had RNFL thickness values that were lower than normal, 6 patients had PERG amplitudes that were reduced lower than 50% of normal, but they had an RNFL thickness of 75 μm or more (Figure 5F). Six carriers had PERG amplitudes that were less than 75% of normal, but they had RNFL thickness values that were greater than 80 μm (Figure 5G). Three of them had increased RNFL thickness values that were greater than 100 μm. Four carriers with PERG amplitudes that were less than 75% of normal had ETDRS acuity better than 20/25, mean defect more than −2dB, and average RNFL thickness more than 80 μm.
COMMENT
Based on our initial observations herein, there are several options for selection of subjects with LHON with the G11778A mutation for ocular injections with an adeno-associated virus containing a normal ND4 gene. Gene therapy may be highly relevant in affected patients with the least structural injury to the RNFL. Like Seo and coworkers,18 who found a mean RNFL thickness of 82 μm in patients with the G11778A mutation with a disease duration of 4 years, we found that the mean RNFL thickness was 78.3 μm up to 32 months after visual loss. Presently, we have only obtained baseline optical coherence tomography at study entry. The planned follow-up visits every 6 months will be important to determine the rate of RNFL thickness decline and retinal ganglion cell demise. We also found 4 acutely affected patients with low PERG amplitudes who likely had not yet lost many retinal ganglion cells (RNFL thickness >100 μm). With a likely near normal complement of retinal ganglion cells to target with the adeno-associated virus containing a normal ND4 gene, these patients may be the best candidates for entry into a phase 1/2 clinical trial. With restoration of adenosine triphosphate synthesis in cultured LHON cells treated with allo-topic ND4 that was described in our previous work,10 visual function may be restored. Still, oxidative injury and apoptosis may be irreversible11 and treatment may need to be initiated prior to visual loss.
In LHON, visual loss in the second eye occurs within a mean duration of approximately 2 months after visual loss in the first eye. Gene therapy to rescue retinal ganglion cells prior to visual loss of the second eye may be possible in these patients during this window. Unfortunately, our affected patients had bilateral visual loss by the time of entry into our study. For us to target these patients, we will have to get them referred to us much sooner. Newman and coinvestigators4 were able to design such a study. They used topical brimonidine tartrate (Alphagan). Treatment was initiated prior to visual loss in the “asymptomatic” second eye in their patients with LHON. Unfortunately, treatment proved to be ineffective in their small series of 8 patients. Although they did not measure the RNFL thickness in their study, it is unlikely that their patients had significant RNFL loss in the treated study eyes, because we found average RNFL thickness values of 78 μm up to 32 months after visual loss.
The PERG has provided a sensitive measure of ganglion cell dysfunction in LHON19 and other optic nerve disorders.20,21 To our knowledge, the PERG has not been evaluated in LHON carriers until now. We found 6 carriers who had PERG amplitudes at 75% of normal, but RNFL thickness values were greater than 80 μm. These patients had normal visual function and no evidence for RNFL loss. Perhaps these carriers with low PERG amplitudes and normal RNFL thickness may be candidates for ocular gene therapy, that is, if the PERG amplitude is a predictor of conversion to LHON in them. Since only 50% of males and 10% of females with the G11778A mutation develop visual loss, ocular gene therapy for all carriers may be unnecessary. In a large series of 75 carriers, Sadun and coworkers22 found only 2, both male, who progressed to affected status in 4 years. Presently, we have 21 asymptomatic carriers enrolled. At the current rate of enrollment, we should achieve similar numbers with 4 additional years for patient recruitment. We are reexamining all subjects every 6 months to determine whether the PERG amplitude can predict conversion from carrier to affected status. If so, then treatment could be initiated early in those subjects with low PERG amplitudes.
Obviously, those with severe loss of retinal ganglion cells (low RNFL thickness) may be the least desirable subgroup for therapy with a normal human ND4 gene. We found that the RNFL thickness was markedly reduced (47.5 μm) in those patients who presented more than 32 months after their visual loss, thus leaving fewer retinal ganglion cells available to target the adeno-associated virus containing the normal ND4 gene. Still, the Leber congenital amaurosis phase 1 clinical trial showed that partial return of visual function may occur even in blind eyes with long-standing and severe photoreceptor loss.23,24 Perhaps insertion of the normal ND4 gene into the mitochondria of remaining ganglion cells may restore some level of visual function to patients with LHON and blindness with severe RNFL loss.
Rescue of optic neuropathy25 will be proof that allotopic ND4 gene therapy may be effective in patients with LHON. Retinal ganglion cell survival with dysfunction many months after loss of vision may provide a long window of opportunity for intervention and rescue by adeno-associated virus–mediated ocular gene delivery of a normal ND4 subunit. Based on these results, possible candidates for future gene therapy may include affected patients with mildly reduced RNFL thickness or carriers with low PERG amplitudes and normal RNFL thickness, if the PERG amplitude is a predictor of conversion to LHON in these carriers. It is our goal to perform the initial intravitreal injections in patients with LHON within the next 4 years.
Acknowledgments
Funding/Support: This work was supported by National Eye Institute grants R24EY018600 (Dr Guy), RO1 EY014957 (Dr Porciatti), and P30-EY014801 (Dr Porciatti) and an unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness and partially supported at the University of Florida by National Eye Institute grant R01 EY-11123 (Dr Hauswirth), Macula Vision Research Foundation, and RPB Inc.
Footnotes
Additional Contributions: Mabel Wilson edited the manuscript.
Financial Disclosure: Dr Hauswirth and the University of Florida have a financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc) that might, in the future, commercialize some aspects of this work.
REFERENCES
- 1.Leber T. Über hereditare und congenital-angelegete Sehnerverleiden. Graefe's Archiv für klinische und experimentelle Opthalmologie. 1871;7:249–271. [Google Scholar]
- 2.Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- 3.Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- 4.Newman NJ, Biousse V, David R, et al. Prophylaxis for second eye involvement in Leber hereditary optic neuropathy: an open-labeled, nonrandomized multi-center trial of topical brimonidine purite. Am J Ophthalmol. 2005;140(3):407–415. doi: 10.1016/j.ajo.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Keeney PM, Quigley CK, Dunham LD, et al. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum Gene Ther. 2009;20(8):897–907. doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachibana M, Sparman M, Sritanaudomchai H, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461(7262):367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glick B, Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. doi: 10.1146/annurev.ge.25.120191.000321. [DOI] [PubMed] [Google Scholar]
- 8.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 9.Manfredi G, Fu J, Ojaimi J, et al. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nat Genet. 2002;30(4):394–399. doi: 10.1038/ng851. [DOI] [PubMed] [Google Scholar]
- 10.Guy J, Qi X, Pallotti F, et al. Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann Neurol. 2002;52(5):534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 11.Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci. 2007;48(1):1–10. doi: 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- 12.Guy J, Qi X, Koilkonda RD, et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009;50(9):4205–4214. doi: 10.1167/iovs.08-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone EM. Genetic testing for inherited eye disease. Arch Ophthalmol. 2007;125(2):205–212. doi: 10.1001/archopht.125.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111(1):161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredette MJ, Anderson DR, Porciatti V, Feuer W. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology. 2008;115(6):957–963. doi: 10.1016/j.ophtha.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK., II Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112(1):10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47(9):3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo JH, Hwang JM, Park SS. Comparison of retinal nerve fibre layers between 11778 and 14484 mutations in Leber's hereditary optic neuropathy. Eye (Lond) 2010;24(1):107–111. doi: 10.1038/eye.2009.36. [DOI] [PubMed] [Google Scholar]
- 19.Kurtenbach A, Leo-Kottler B, Zrenner E. Inner retinal contributions to the multifocal electroretinogram: patients with Leber's hereditary optic neuropathy (LHON): multifocal ERG in patients with LHON. Doc Ophthalmol. 2004;108(3):231–240. doi: 10.1007/s10633-004-8676-8. [DOI] [PubMed] [Google Scholar]
- 20.Porciatti V, Ventura LM. Physiologic significance of steady-state pattern electroretinogram losses in glaucoma: clues from simulation of abnormalities in normal subjects. J Glaucoma. 2009;18(7):535–542. doi: 10.1097/IJG.0b013e318193c2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura LM, Venzara FX, III, Porciatti V. Reversible dysfunction of retinal ganglion cells in non-secreting pituitary tumors. Doc Ophthalmol. 2009;118(2):155–162. doi: 10.1007/s10633-008-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadun AA, Salomao SR, Berezovsky A, et al. Subclinical carriers and conversions in Leber hereditary optic neuropathy: a prospective psychophysical study. Trans Am Ophthalmol Soc. 2006;104:51–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellouze S, Augustin S, Bouaita A, et al. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83(3):373–387. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]