Abstract
Extracellular signaling molecules have crucial roles in development and homeostasis, and their incorrect deployment can lead to developmental defects and disease states. Signaling molecules are released from sending cells, travel to target cells and act over length scales of several orders of magnitude, from morphogen-mediated patterning of small developmental fields to hormonal signaling throughout the organism. We discuss how signals are modified and assembled for transport, which routes they take to reach their targets and how their range is affected by mobility and stability.
Introduction
The exchange of information between cells is essential for the development and homeostasis of all multicellular organisms. Developmental signals govern cell fate decisions, tissue morphogenesis and the migration of cells to specific destinations within the organism. In both developing and adult individuals, signaling molecules coordinate physiological processes such as neurotransmission and immune responses. Disease states, including cancer, can occur if signals or signaling pathways are deployed at the wrong time or place.
Intriguingly, many of the signaling pathways that control these diverse processes are employed repeatedly during development and are evolutionarily conserved. For example, the Hedgehog (Hh) signaling pathway is utilized during development of the fly wing and the mammalian spinal cord. How then are signaling molecules able to achieve specificity? In addition to the developmental history and genotype of a tissue, the spatial and temporal distribution of signaling molecules governs their activity. Some signals mediate communication between direct neighbors (juxtacrine) or over several cell diameters (paracrine), whereas others act at ultra-long (endocrine) ranges. In the case of endocrine signaling, the entire body can be affected by a signal produced in a single localized gland. The temporal distribution of signals is also regulated. Hormones such as insulin are released by the endocrine system only under the appropriate conditions, and developmental signals must be activated and repressed at the correct times in order to generate properly patterned organisms and to prevent disease states later in life.
Many important signaling pathways and their major components have now been catalogued and characterized. However, we still know little about how the signals that activate these pathways become distributed correctly within tissues. Do signaling molecules travel as individuals, or are they packaged as cargo into vehicles? What routes do signaling molecules take to reach their destination? What are the mechanisms that modulate the direction, mobility and stability of signals? In this review, we discuss the extracellular movement of signals at cellular, tissue and organism scales. We begin by discussing the biophysical principles underlying the transport of molecules over short and long distances. We then describe how signaling molecules are modified and packaged at the source for their journey. Finally, we discuss the extracellular routes that signals take to reach target tissues and how the modulation of a signal’s direction, mobility and stability can affect its range.
Biophysics of molecular transport
Many signals are proteins or small molecules that are secreted by localized groups of cells. The range of a signal is the domain over which it exerts its effects. In other words, the signaling range is the distance from the source at which a response is observed. Different signals have vastly different signaling ranges (Chen and Schier 2001; Chen and Schier 2002; Williams et al. 2004; Blilou et al. 2005; Sawamoto et al. 2006; Kicheva et al. 2007; Shilo and Barkai 2007; Yu et al. 2009; Gallet 2011). For example, the ultra-short juxtacrine signal Delta only signals to direct neighbors (Nichols et al. 2007), the medium- to long-range paracrine TGFβ signals Dpp and Nodal act over distances from 40 to 200 μm, respectively, corresponding to approximately 15 cell diameters (Bollenbach et al. 2008; Harvey and Smith 2009), and ultra-long range endocrine signals such as insulin are secreted from localized sources but act throughout the entire body.
Several factors control signaling range, including the concentration of signal at the source, as well as the activity, mobility and stability of the signal. First, the signaling range can be influenced by the amount of the signal that is produced; high rates of signal production result in high levels of extracellular signal. Higher levels of extracellular signal enhance the likelihood that molecules will reach receptors on distant cells. Some signals are thought to be secreted during development with a constant flux from source cells over long time scales (Wartlick et al. 2011), whereas others, such as neurotransmitters, are stored in a readily accessible pool at the source and only released in a short pulse upon stimulation by specific inputs. Second, the strength or signaling ability of a ligand affects its signaling range. For example, a mutation or polymorphism that decreases receptor binding but does not affect the distribution of a ligand will nonetheless shorten its signaling range. Third, the ability of a signal to move through a tissue will affect its signaling range; molecules that move more freely or directionally through tissues will move farther from their source than molecules that are restricted in their movement. Fourth, signal stability helps determine signaling range. For example, very stable signals can move a long distance away from their source before being degraded. Finally, even highly expressed, stable, active and diffusive signals can have short-range effects because of dilution in target tissues.
In the following section on transport biophysics, we describe the differences between stationary and dynamic sources, introduce diffusion, active transport and fluid flow as mechanisms of signal movement and discuss how signal stability influences signaling range.
Stationary and dynamic sources
Signal sources are often localized and stationary, and the signaling molecules that they secrete travel away from the source. Although this review focuses on the extracellular movement of signaling molecules, it is worth pointing out that signals can also be distributed by other means. For example, cell divisions that partition mRNA along a cell lineage (Pfeiffer et al. 2000; Dubrulle and Pourquie 2004; Harfe et al. 2004) or highly dynamic expression patterns (Doitsidou et al. 2002; Boldajipour et al. 2008) can move the source and thus transport signals. Furthermore, signals can act at long distances by traveling inside neurites, nanotubes and other cellular extensions (Huang and Kunes 1996; Rustom et al. 2004; Watkins and Salter 2005; Davis and Sowinski 2008; Sherer and Mothes 2008; Hurtig et al. 2010; Wang et al. 2010). Conversely, signals can be perceived far from cell bodies by neurites, growth cones, filopodia, cytonemes, and other thin extensions (Miller et al. 1995; Ramirez-Weber and Kornberg 1999; Ribeiro et al. 2002; Sato and Kornberg 2002; Wolf et al. 2002; De Joussineau et al. 2003; Hsiung et al. 2005; Lidke et al. 2005; Roy et al. 2011).
Mechanisms of signal movement
The ability of a signal to move through a tissue is the primary determinant of signaling range. Paracrine and endocrine signals must travel over vastly different distances in order to reach their final targets. For example, during fly development tissues are patterned by paracrine signals that move tens of micrometers over hours to days (Wartlick et al. 2011), whereas in the large vascular system of humans the endocrine signal insulin is transported over meters within minutes from the pancreas to the target tissue. Three principal mechanisms are used for the transport of molecules: diffusion, directional active transport, and fluid flow. Each transport mechanism has features that make it uniquely suited for the movement of different signals in different tissue contexts. In the following, we discuss which mechanisms are optimized for paracrine and endocrine signals, respectively.
There has been much debate about whether movement of paracrine signals involves directional active transport from sending to receiving cells, or whether a diffusive process would be sufficiently reliable to guarantee timely and robust transport (Wolpert 2009). One limitation with signal movement by diffusive transport is that it takes a very long time for diffusing molecules to travel long distances away from their source (Figure 1). Let us consider a typical protein with a diameter of 5 nm (corresponding to a molecular weight of 40-50 kDa). The Stokes-Einstein equation describes the diffusivity D of a molecule with a radius R moving through a fluid with a viscosity η at a temperature T as D = kBT/6πηR (kB is the Boltzmann constant) (Berg 1993; Phillips et al. 2009). A protein with a diameter of 5 nm moving through water at room temperature would therefore be expected to have a diffusion coefficient of approximately 100 μm2/s. The time scale of diffusion increases with the square of the distance. More precisely, the average time t it takes molecules with a diffusion coefficient D to diffuse a distance L in one dimension is approximately t = L2/2D (Berg 1993; Phillips et al. 2009). Stable proteins with a diffusion coefficient of 100 μm2/s could therefore easily traverse a one-dimensional field of 1 mm within approximately 2 hours. However, it would take close to 6 days on average to traverse 1 cm and more than 150 years to travel a distance of 1 meter. This illustrates that diffusion is useful for signal movement over short but not long distances. In 1970, Francis Crick reasoned that developing embryonic tissues are sufficiently small for diffusing molecules to reach target cells in a timely fashion, and therefore signal dispersal by diffusion could be a plausible mechanism for patterning tissues during development (Crick 1970).
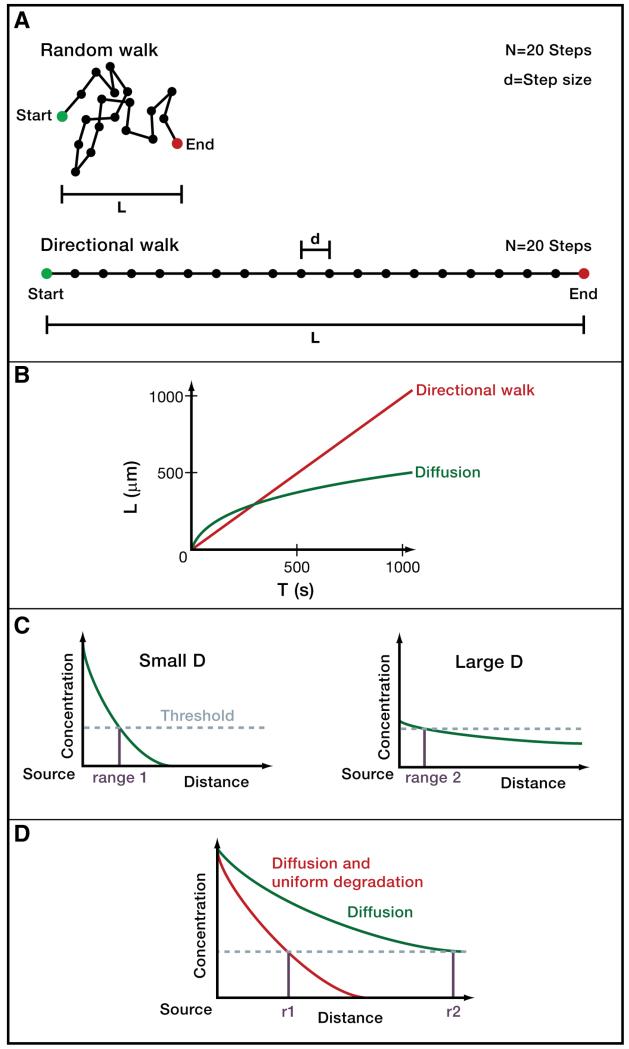
Figure 1. Biophysics of signal movement.
A) Directional movement and random walks. The distance L that a molecule moving in a constant direction covers with N steps of step size d is L= Nd. However, diffusing molecules do not move in a constant direction but rather undergo random walks, in which the direction of motion changes randomly after each step due to collision with surrounding molecules. A diffusing molecule will therefore on average cover a distance (Berg 1993; Phillips et al. 2009). For example, with 20 steps of size 1 a molecule moving in a constant direction could travel a distance L=20, whereas a randomly walking molecule would only be displaced from its starting position by about L=5 on average. Conversely, to travel a distance of L=20, a randomly walking molecule would need to make 400 steps of size 1 on average.
B) Timescales of diffusive and directional movement. The displacement L in micrometers as a function of time t (in seconds) for a molecule moving in a constant direction with a velocity v of 1 μm/s is described by L=tv (red). The average displacement L as a function of time t for an ensemble of molecules diffusing with a diffusion coefficient D of 100 μm2/s (e.g. a small protein diffusing in water) is described by (green). Diffusing molecules can move rapidly away from their starting positions over short distances, but take a long time to move long distances.
C) Concentration thresholds, signaling range and diffusivity in paracrine signaling. Typically, cells must be exposed to a concentration of paracrine signal above a certain threshold (indicated by dashed horizontal line) in order to respond to signaling. Signals with large diffusion coefficients (right graph) travel farther from their sources than signals with small diffusion coefficients (left graph). However, less diffusive signals can counter-intuitively have longer signaling ranges (r1) than more diffusive signals (r2), since these relatively immobile signals “pile up” near the source at levels above the threshold required for a cellular response.
D) Mobility and stability affect signaling range. Molecules that are cleared uniformly and rapidly in the target field accumulate to lower concentrations.
Directional active transport mechanisms and fluid flow commonly exhibit constant velocities that allow transport of endocrine signals over long distances. The time it takes molecules to travel a distance L by directional transport is linear and not quadratic as in the case of diffusion (Phillips et al. 2009). For example, flow velocities of 300-500 μm/s in human blood vessels (Phillips et al. 2009) and in the vasculature of olive trees (Lopez-Bernal et al. 2010) have been measured. A molecule moving by fluid flow in the blood system would be able to travel about 1 mm within 3 seconds, 1 cm within 30 seconds, and 1 meter within 40 minutes. This very fast transport mechanism is employed mostly by endocrine signals - diffusion alone would take years to transport these molecules to their distant target tissues. Thus, diffusive transport is sufficient to move paracrine signaling molecules over short distances, whereas some long-range paracrine and most endocrine signals moving over longer distances require directional active transport or fluid flow to shorten the transport time by several orders of magnitude.
Stability
The stability of a signaling molecule is another important determinant of signaling range. Highly stable signals can spread over large distances, whereas unstable signals can act only locally (Figure 1D). Specific clearance mechanisms such as signal uptake by cells and signal degradation by extracellular enzymes ensure that cells within tissues are exposed to the appropriate levels of signaling molecules (Scholpp and Brand 2004; Boldajipour et al. 2008; Hagemann et al. 2009; Yan et al. 2009; Naumann et al. 2010). Localized degradation can help to generate more intricate signal distributions than those achieved by simple active transport or diffusive mechanisms alone (White et al. 2007). Feedback regulation of signal stability also plays an important role in some paracrine signaling processes. Theoretical studies have demonstrated that “self-enhanced clearance” can provide a simple but powerful mechanism that fine-tunes the distribution of a signaling molecule and renders patterning processes robust to many kinds of perturbations, such as changes in the rate of signal production (Eldar et al. 2003; Barkai and Shilo 2009; Lander et al. 2009).
In summary, the clearance kinetics – i.e. localized or uniform, linear or nonlinear – and the transport mechanism – i.e. diffusion or directional transport - together affect the dynamics of signal distribution and therefore ultimately the signaling range.
Preparing for the journey
Before signals begin their extracellular journey, they often undergo post-translational modifications that can affect their production, activity, mobility and stability. These modifications can dramatically affect signal transport and in some cases have been suggested to necessitate the packaging into vehicles to allow mobility. In the following, we illustrate these concepts with two examples. We discuss how the pro-domains of Transforming Growth Factor β (TGFβ) family ligands and lipid modifications of Hedgehog (Hh) ligands decrease their mobility and describe how these molecules can be mobilized to act at a longer range by association with carrier proteins or by packaging into membranous particles.
Posttranslational modifications affect signaling range
TGFβ superfamily members have multiple roles in development (e.g. patterning of the germ layers, dorsal-ventral patterning and establishing left-right asymmetry), homeostasis (e.g. regulation of proliferation, immune response and blood vessel maintenance) and disease (e.g. cancer, heart disease and Marfan syndrome). These ligands are produced as pro-proteins consisting of a pro-domain and a mature domain and are processed via cleavage by specific convertases (Figure 2). In many cases the pro-domain stays attached to the mature ligand after processing and regulates signal activity, stability and mobility (De Crescenzo et al. 2001; Le Good et al. 2005; Blanchet et al. 2008; Tian et al. 2008; Sengle et al. 2011). Pro-domains can target ligands to the extracellular matrix to restrict their mobility and to create a ligand pool that can be rapidly mobilized. Tethering of complexes to the extracellular matrix can be achieved via interactions with latent TGFβ binding proteins (LTBPs) (Nunes et al. 1997), fibrillin microfibrils (Neptune et al. 2003; Sengle et al. 2008; Nistala et al. 2010) and heparin sulfate proteoglycans (HSPGs, discussed in more detail below). The tethered latent complex can then be mobilized and activated by extracellular stress signals that terminally remove the pro-domain (Lyons et al. 1988; Annes et al. 2003; Wolfman et al. 2003). Proteolytic cleavage of LTBPs, competition with binding to microfibrils as well as mechanical forces have been implicated in mobilizing the latent ECM-bound TGFβ complex (Ge and Greenspan 2006; Chaudhry et al. 2007; ten Dijke and Arthur 2007; Maeda et al. 2011).
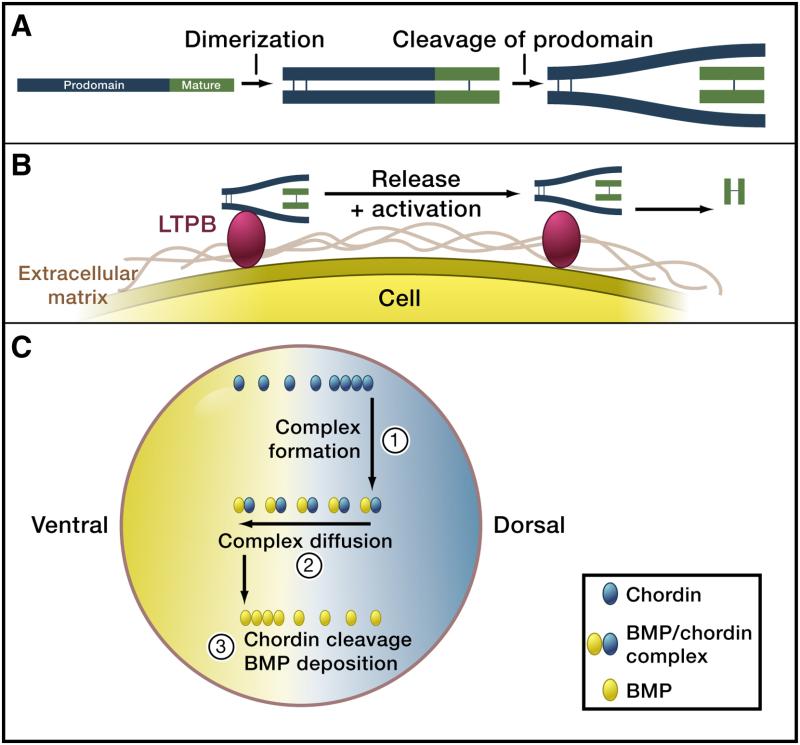
Figure 2. TGFβ signal trafficking.
A) Pro-protein cleavage. TGFβ superfamily ligands are produced as pro-proteins, dimerize and require cleavage of the pro-domain by convertases (e.g. Furin). For many TGFβ ligands the prodomain (blue) stays attached to the mature domain (green) after cleavage. Modified from (ten Dijke and Arthur 2007).
B) Tethering to the extracellular matrix and release. Pro-domain – mature domain complexes can be tethered to the extracellular matrix (brown), e.g. via the interaction of the pro-domain with latent TGFβ binding proteins (LTBP, red). After cleavage of LTBP and the pro-domain (e.g. by matrix metalloproteinases such as BMP1 and MMP2), the mature domain is released and can now signal to distant cells. Modified from (ten Dijke and Arthur 2007).
C) Heteromerization with carrier proteins and shuttling. BMP molecules (green) are thought to be relatively immobile unless bound to Chordin (red). During early embryogenesis in frogs, BMPs are uniformly distributed. Production of Chordin on the dorsal side of the embryo leads to complex formation between BMPs and Chordin. The BMP/Chordin complex is mobile and diffuses. Repeated rounds of Chordin cleavage by a uniformly distributed protease, subsequent release of free BMP and remobilization by Chordin binding is thought to eventually result in the accumulation of free BMP on the ventral side. Additional downstream feedback signaling networks can result in robust pattern formation. Figure modified from (Lewis 2008).
Many paracrine ligands are lipid-modified and inserted into the plasma membrane to restrict their mobility or to decrease their secretion or signaling activity (Willert et al. 2003; Cong et al. 2004; Takada et al. 2006; Komekado et al. 2007; Kurayoshi et al. 2007; Franch-Marro et al. 2008; Steinhauer and Treisman 2009). For example, Hh proteins are cholesterylated at their C-termini (Porter et al. 1996), which increases their membrane affinity and restricts their dispersal (Gallet 2011) (Figure 3A). Mutant Shh proteins lacking the cholesterol modification have an extended distribution and an increased signaling range (Li et al. 2006), leading to dramatic patterning defects (Huang et al. 2007; Huang et al. 2007).
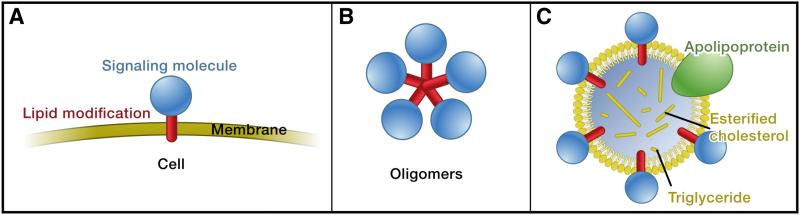
Figure 3. Trafficking of hydrophobic signal molecules.
Signaling molecules (blue) are often modified by lipid attachments (red), and they can be inserted into membranes (A). In order to act on cells at a distance from the producing cell, these signaling molecules have to move through a hydrophilic environment. Formation of oligomers (B) and lipoprotein particles (C) are thought to mask hydrophobic residues or modifications and have been implicated in the transport of hydrophobic signals such as Hh and Wg. Figure modified from (Eaton 2008).
Specific proteins are dedicated to handle lipid-modified Hh. Dispatched is thought to be required for the release of Hh from cell surfaces and its subsequent long-range signaling activities. Mutants for dispatched retain cholesterol-modified Hh on the cell surface and show a reduced Hh signaling range (Burke et al. 1999). Similarly, the membrane microdomain protein Reggie-1 is important for the secretion and spreading of Hh (Katanaev et al. 2008). Although the precise mechanisms of Dispatched and Reggie activity on Hh ligands remain unclear, these molecules illustrate the importance of dedicated pathways to handle modified signaling proteins.
Signal assemblies and vehicles
Signals are often assembled into higher-order complexes that modulate and regulate their dispersal. For example, hormones such as cortisol have long been known to utilize carrier proteins for long-range movement through the blood stream. The Stokes-Einstein equation introduced above states that larger molecules move more slowly but paradoxically, in the context of live animals, larger assemblies are often more mobile than the free molecules. In vivo, large assemblies can act as vehicles that transport signals that would otherwise be immobilized on cell surfaces. We illustrate this concept with three examples. We first introduce how carrier proteins can change the mobility of TGFβ family signals. Then we describe strategies to move hydrophobic proteins such as Hh through aqueous environments and lastly we discuss how some signals are packaged for long-range transport in membranous particles.
Association with carrier proteins
The association of many TGFβ superfamily signals with carrier proteins enhances ligand mobility and thereby increases signaling ranges (Figure 2C). For example, Bone Morphogenetic Proteins (BMPs) use carrier proteins to regulate their dispersal during patterning of the dorsal-ventral axis (Eldar et al. 2002; Shimmi et al. 2005; van der Zee et al. 2006; Ben-Zvi et al. 2008; Umulis et al. 2009). In Xenopus, BMPs have very low mobility, possibly due to high-affinity interactions with extracellular matrix (ECM) molecules (Ohkawara et al. 2002). The secreted BMP antagonist Chordin/Sog forms a complex with BMPs and inhibits their activity. Mathematical modeling suggests that BMP-Chordin complexes are highly diffusive compared to BMPs that are not complexed with Chordin (Ben-Zvi et al. 2008). BMPs are initially uniformly distributed in the embryo, whereas Chordin is locally produced on the dorsal side. Repeated rounds of BMP mobilization by Chordin, subsequent enhanced diffusion of the heteromeric complex, and finally cleavage of Chordin in the BMP-Chordin complex by a uniformly distributed protease are thought to eventually result in the clearance of BMP on the dorsal side and effective transport by “shuttling” to the ventral side.
Moving hydrophobic signals through aqueous environments
As discussed above, several signaling molecules require hydrophobic modifications for their normal activity. Although such hydrophobic molecules should be trapped by plasma membranes, they often move over long distances through predominantly aqueous extracellular environments. This conundrum is partially resolved by the observation that hydrophobic signals can form oligomers and can be packaged into lipoprotein complexes that hide hydrophobic residues or modifications (Figure 3).
Hydrophobic domains may be hidden in the center of oligomers, whereas hydrophilic domains are exposed to the aqueous extracellular milieu (Figure 3B). For example, Hh molecules form large multimeric complexes (Zeng et al. 2001; Chen et al. 2004; Feng et al. 2004; Goetz et al. 2006; Vyas et al. 2008). Mutation of a conserved lysine in the Hh protein abolishes oligomerization (Vyas et al. 2008), and the mutant signal is thereby restricted to act at a shorter range than the wild-type signal. Recent studies in cell culture suggest that the formation of Shh oligomers depends on lipid modifications, but that the hydrophobic modifications are cleaved off before the oligomers are released from the Shh producing cells (Dierker et al. 2009; Ohlig et al. 2011).
Another strategy to overcome the problem of moving a hydrophobic molecule through an aqueous environment is the use of carriers that bind signals and bury their hydrophobic residues or modifications within the complex. Some hydrophobic signaling molecules are packaged into lipoprotein particles that can diffuse through tissues. Lipoprotein particles are secreted vesicles and composed of a phospholipid monolayer containing the lipoprotein apolipoprotein (Figure 3C) (Eaton 2008). The hydrophobic proteins Wingless (Wg) and Hh are thought to be packaged into lipoprotein particles for long-range signaling (Greco et al. 2001; Panáková et al. 2005; Neumann et al. 2009) and fail to disperse from their sources when lipoprotein levels are decreased (Panáková et al. 2005). Other lipid-based mechanisms have also recently been discovered to control the distribution of signals (Pizette et al. 2009), but how these mechanisms, oligomerization and packaging into lipoprotein particles are connected remains unclear.
Other membranous vehicles
Comparable in size to typical lipoprotein particles, exosomes are membrane-encapsulated signal vesicles proposed to operate as vehicles for the packaging and transport of signaling molecules (Liegeois et al. 2006; Lakkaraju and Rodriguez-Boulan 2008; Korkut et al. 2009; Ristorcelli et al. 2009; Sheldon et al. 2010; Higginbotham et al. 2011). Their role in vivo is controversial, but is well established that exosomes originate from endosomal multivesicular compartments whose outer membrane fuses with the plasma membrane to release the vesicles contained therein.
One model proposes that the Notch ligand Delta requires dispersal by exosomes. Delta is tethered to the membrane via its single-pass transmembrane domain. Interestingly, Delta must be taken up by the sending cell in order to signal to the receiving cell (Itoh et al. 2003; Le Borgne and Schweisguth 2003). It has been proposed that endocytosed Delta in the sending cell is packaged into multivesicular bodies that are then recycled and released as exosomes to activate Notch signaling in receiving cells (Le Borgne and Schweisguth 2003). Although Delta does not need to be trafficked through known recycling pathways to be able to signal (Windler and Bilder 2010), the Notch ligand Delta-like 4 can be found in exosomes outside of cells (Sheldon et al. 2010). Purified exosomes can transfer the Delta-like 4 signal to other cells suggesting the possibility that exosomes could mediate Notch signal transfer independent of classic cell-cell contact (Sheldon et al. 2010), potentially at a longer range.
Other signals that act at a long range have been proposed to be packaged into microparticles. Microparticles are large membrane-enclosed vehicles that originate by budding from the plasma membrane and range from 100-1000 nm in diameter (Mause and Weber 2010). Microparticles can move in the vascular system and may serve as long-range and globally distributable signal vehicles. For example, the cell death signal Caspase I has recently been shown to be delivered to smooth muscle cells by microparticles originating from activated immune cells (Sarkar et al. 2009). Interestingly, large protein quantities and even mRNAs can be transported in exosomes and microparticles, and these vehicles may thus be able to modulate the target cells more profoundly than an extracellular signal alone (Whale et al. 2006; Valadi et al. 2007). These and other studies suggest that membranous vesicles are an attractive model for the transport of hydrophobic signals, but in the absence of methods to directly interfere with the generation of these assemblies in vivo, their relevance for signaling remains unclear.
On the road
What routes do signals take to reach their destination? The foregoing biophysical considerations showed that paracrine signals can diffuse through the extracellular matrix to act over short distances. In contrast, endocrine signals require specialized routes such as the vascular systems of plants and animals for their long-range transport. In the following, we discuss how transport routes and transport mechanisms are connected. We begin with long-range transport mechanisms and then go on to discuss transport over medium, short and ultra-short ranges. Finally, we discuss how altering the stability of signaling molecules can change their range.
Highways of fluid convection
Highly specialized transport routes allow for the movement of signaling molecules over long distances. In the case of plants, long-distance transport is achieved through the vascular systems of xylem and phloem. Trees can transport water taken up in the roots via the xylem over several tens of meters. The driving force behind this flow is transpiration of water in the leaves. Due to cohesive forces between molecules, the evaporation of water from leaves pulls other water molecules upwards throughout the xylem. Similarly, sugars, plant hormones and other signaling molecules move through the phloem (Corbesier et al. 2007; Robert and Friml 2009; Molnar et al. 2010) due to gradients of osmotic potential from the source to the receiving tissue. Flow velocities in the phloem of plants range from ~10-1000 μm/s (Canny 1973; Windt et al. 2006).
The protein Flowering Locus T (FT) is a prominent example of a long-range signal that is transported via the phloem and mediates communication from leaves to the shoot apex. Flowers arise from the shoot apex; however, the changes in day length that occur as seasons change are sensed by leaves (Knott 1934). Leaves transiently produce FT, and the protein travels along the phloem to the shoot apex, where it communicates information about day length (Zeevaart 2006; Corbesier et al. 2007; Tamaki et al. 2007). Thus, long-range leaf-to-shoot apex communication through the phloem ensures that flowering occurs in the correct growing season.
In animals, the vascular system provides a similar “highway” for the global distribution of signaling molecules. Hormones such as insulin are directly secreted into the bloodstream where they can reach almost all parts of the body leading to a rapid and even distribution. Typical measured flow velocities of blood in capillaries are on the order of 500 μm/s (Phillips et al. 2009), similar to the velocities in the plant phloem discussed above (although blood flow in larger vessels such as the aorta can be up to two orders of magnitude higher (Bahlmann et al. 2001)). At this velocity, insulin secreted from the pancreas can reach a muscle that is several centimeters away within seconds to minutes. But once insulin reaches the skeletal muscle, it moves with much slower kinetics via diffusion (Lauritzen et al. 2006). Mathematical modeling suggests that the low mobility of insulin in muscle is due to interactions with receptors and the increased path lengths that molecules are required to travel due to the presence of highly branched muscle fibers (i.e. increased “tortuosity”) (Shorten et al. 2007). Thus, the mobility of the same ligand can differ dramatically based on its environment.
Fluid flow driven by motile cilia also distributes signaling molecules in organs such as the brain. Since fluid convection dominates over diffusion in speed over long distances, this transport mode might be advantageous in these relatively large substructures. Neuropeptides, for example, are transported in this manner in the cerebrospinal fluid (CSF) after being secreted from the choroid plexus (Veening and Barendregt 2010). Remarkably, despite the rapid distribution of inflowing neuropeptides by fluid flow, it has been proposed that this transport mode can generate a gradient of the signaling molecule Slit, which is important for directing migrating neurons from the choroid plexus to the olfactory bulb (Sawamoto et al. 2006). Cilia-mediated fluid flow is also observed in the mouse node, although it is unclear if fluid flow is required for the directional transport of signals (Hirokawa et al. 2009) or to generate differential fluid pressure leading to asymmetric physical stimulation of mechanosensory cilia (Basu and Brueckner 2008).
Directional active transport
Thus far we have mainly focused on fluid flow as a mechanism for signal dispersal over long ranges. However, occasionally, signals need to move quickly over long distances through environments that lack fluid-flow systems. For example, an interesting combination of passive diffusion and active transport mechanisms is employed in plants to ensure the proper distribution of the plant hormone Auxin (Robert and Friml 2009). In Arabidopsis, Auxin is initially transported via the vasculature from the shoot to the tip of the root (Figure 4). There, a graded Auxin distribution controls cell identity, cell division and cell expansion. Given that the source of Auxin production is far from the root, how can an inverted gradient that peaks at the tip be generated, and how can this distribution be maintained? Auxin can freely diffuse through extracellular spaces and also enter cells. But once Auxin has entered a cell, its protonation state changes and it can only leave through PINs, channel proteins that actively transport Auxin out of cells (Figure 4A). In cells within the root, PIN is localized to the face of the cell that points toward the root tip (the “base” of the cell). Thus, a molecule of Auxin diffusing in the root may enter a cell at any point along the cell surface; however, the molecule can only leave the cell through its base. This concentrates Auxin to the tip of the root (Blilou et al. 2005). This Auxin reflux capacitor is important to stabilize the maximal Auxin concentration at the root tip and to maintain growth of the meristem.
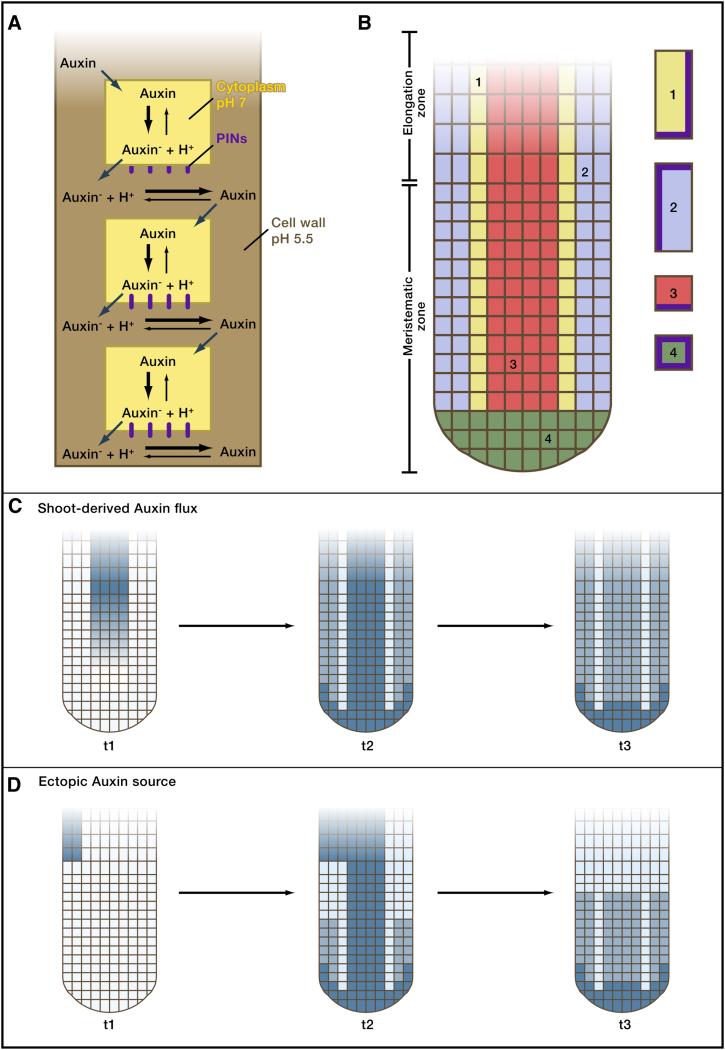
Figure 4. Diffusion and active transport by efflux carrier proteins.
A) Polar auxin transport. Auxin can diffuse in the cell wall (brown) and enter cells. However, once inside the less acidic environment of the cells, Auxin becomes deprotonated (Auxin-) and can no longer leave the cell passively. PINs (red) are specific transport proteins that carry anionic Auxin- out of the cell. PINs are highly localized, often to the base of cells, and thereby lead to a directional transport of Auxin. Figure modified from (Robert and Friml 2009).
B) Root architecture and localization of PINs. The Arabidopsis root consists of a meristematic zone, where growth occurs, and an elongation zone. The vasculature is indicated in red, epidermal layers in blue, border cells in yellow and columella tiers in cyan. All cells are surrounded by a cell wall (green). The localization of PIN proteins (brown) in cells within the indicated subregions is shown. Figure modified from (Grieneisen et al. 2007).
C) Establishment and maintenance of an Auxin (blue) concentration maximum in a root with shoot-derived Auxin flux. Three time points of computational simulations are shown. Auxin flows through the phloem from the shoot to the root (t1). The distribution of PINs concentrates Auxin at the root tip (t2) and can maintain the Auxin concentration maximum even when the shoot-derived Auxin flux ceases (t3). Figure modified from (Grieneisen et al. 2007).
D) Accumulation of Auxin (blue) at the root meristem from localized ectopic Auxin production. Three time points of computational simulations are shown. Localized Auxin production from a single cell at timepoint t1 is sufficient to generate an Auxin maximum at the root tip (t3) due to the distribution and subcellular localization of PINs. Figure modified from (Grieneisen et al. 2007).
Interestingly, this transport system is robust to changes in the position of the initial influx – a graded Auxin distribution can even be generated if Auxin concentration is initially uniform, ectopically localized (Figure 4D), or when the flux ceases (Grieneisen et al. 2007). Active transport is therefore not only essential to transport signals over long distances but also ensures a robust spatial localization.
Effective diffusion
Signals that can passively traverse fields of cells by diffusion are expedient, because their transport does not require energy expenditure on the part of the organism. Francis Crick reasoned that signal dispersal by diffusion could be a plausible mechanism for patterning relatively small embryonic tissues (Crick 1970). He hypothesized that to employ such a diffusive mechanism, the diffusing molecule would have to be small enough to rapidly move through cells. Small molecules indeed play an important role in intercellular communication. For example, small molecules such as nitric oxide (NO) or hydrogen peroxide (H2O2) act as rapidly dispersible messengers that diffuse quickly through cells (Niethammer et al. 2009; Schreml et al. 2011).
Most paracrine protein signals cannot diffuse passively through cells and instead move through the extracellular space (Gurdon et al. 1994; Strigini and Cohen 2000; Gritli-Linde et al. 2001; McDowell et al. 2001; Rojo et al. 2002; Lenhard and Laux 2003; Williams et al. 2004), with the exception of a few proteins that diffuse through a cellular environment. These include signals that move in a syncytium (Gregor et al. 2007), some homeodomain transcription factors that move through cell membranes (Prochiantz and Joliot 2003; Brunet et al. 2007; Sugiyama et al. 2008; Wizenmann et al. 2009), and signals that move through special cellular channels such as gap junctions in animals (Esinduy et al. 1995; Mesnil and Yamasaki 2000; Goldberg et al. 2004; Neijssen et al. 2005; Evans et al. 2006; Palacios-Prado and Bukauskas 2009) and plasmodesmata in plants (Sessions et al. 2000; Conti and Bradley 2007; Molnar et al. 2010).
As discussed above, diffusion can be fast over short distances. Therefore, molecules that are too diffusive might not be able to accumulate to sufficiently high concentrations to elicit efficient signaling (Figure 1). Conversely, molecules with very low diffusivity would have extremely short signaling ranges and would not be able to reach distant cells (Lander 2007). Therefore, several mechanisms are employed to fine-tune the temporal and spatial distribution of diffusing molecules.
The extracellular matrix as a signal route
Binding to molecules in the extracellular space affects signal movement. The diffusion of a particle that is interacting with binding partners in this manner is referred to as “effective diffusion” (Crank 1979). Interactions with binding partners can modify ligand dispersal and activity in at least four ways. Binding can 1) alter the mobility/diffusivity of a signal, 2) concentrate ligand at the surface of cells, 3) promote or hinder ligand-receptor interactions, and 4) influence the extracellular stability of a ligand (Figure 5). Below, we illustrate these concepts with several examples.
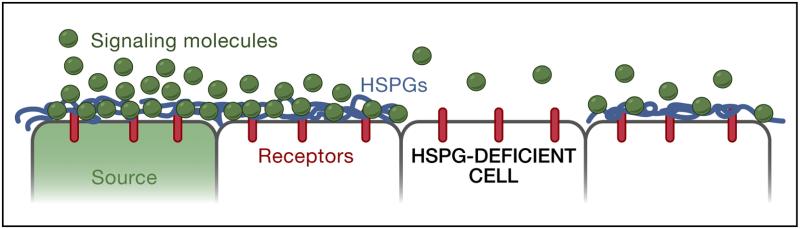
Figure 5. Effective diffusion.
A) Interactions of signaling molecules with HSPGs. HSPGs are often associated with epithelial cell surfaces. Binding to HSPGs can alter the mobility of a signal, concentrate ligand at the surface of cells, promote or hinder ligand-receptor interactions, and influence the extracellular stability of a ligand. For example, in the absence of HSPGs, signaling molecules may not be retained on the cell surface and thereby fail to travel to the next cells.
B) and C) Differential mobility of a signaling molecule in two different tissues. B) The embryonic wing (middle) and haltere discs (right) give rise to the adult wing and haltere, respectively (left). C) Dpp is more mobile in the embryonic wing disc than the haltere disc. The Dpp, Tkv, P-Mad (indicative of signaling range) and HSPG distributions in the wing disc (blue) and haltere disc (red) are shown. It is thought that Dpp movement in the haltere disc is restricted due to Ubx-mediated upregulation of the Dpp receptor Tkv in the medial domain of the disc. Ubx-mediated asymmetric expression of the HSPG core protein Dally is thought to bias Dpp diffusion anteriorly in the haltere disc. Note that Ubx also downregulates dpp transcription leading to decreased Dpp levels in the haltere disc (Crickmore and Mann 2006; de Navas et al. 2006; Crickmore and Mann 2007; Makhijani et al. 2007; Wartlick et al. 2011). Figure modified from (Crickmore and Mann 2008).
Interactions with receptors have been demonstrated to hinder the spread of some signals. (Chen and Struhl 1996; DeWitt et al. 2001). For example, in regions with reduced levels of the Dpp receptor Thickveins (Tkv), Dpp moves farther from a localized source, apparently because its diffusivity is increased (Figure 5C) (Haerry et al. 1998; Crickmore and Mann 2006). In addition, overexpression of tkv shortens Dpp’s signaling range (Haerry et al. 1998; Lecuit and Cohen 1998; Crickmore and Mann 2006). The distribution of other ligands, such as Wg, is not affected in the absence of their receptors (Han et al. 2005), although the distribution can be influenced by overexpression of the signal receptor (Baeg et al. 2004).
Heparan sulfate proteoglycans (HSPGs) are a well-characterized class of ECM components that have been shown to bind to and hinder the spread of some signals. HSPGs are often tethered to cell surfaces and consist of a protein core to which long heparan sulfate sugar chains are attached. Although diffusion has not been directly measured, it is clear that the signaling range or distribution of some signals is modulated in the absence of HSPGs or by overexpression of HSPGs (The et al. 1999; Strigini and Cohen 2000; Baeg et al. 2001; Vincent and Dubois 2002; Belenkaya et al. 2004; Takei et al. 2004; Han et al. 2005; Oki et al. 2007; Yan and Lin 2009; Yan et al. 2009; Marjoram and Wright 2011).
The interaction of two closely related ligands FGF7 and FGF10 with HSPGs provides an interesting example of the effects of signal-HSPG interactions. FGF7 and FGF10 can both guide branching morphogenesis by activating the same receptor (Makarenkova et al. 2009). FGF10 binds more strongly to HSPGs than FGF7 and therefore is thought to be less diffusive. Strikingly, mutation of a single amino acid in FGF10 that is normally involved in binding HSPGs increases its range to that of FGF7 and even causes FGF10 mutants to mimic FGF7 function in branching morphogenesis, possibly due to altered diffusion characteristics. Analogously, a basic domain in the N-terminus of Xenopus BMP4 binds strongly to HSPGs. This interaction restricts the mobility of BMP4 and confines the protein close to its expression domain in the non-neural ectoderm (Ohkawara et al. 2002).
In addition to hindering signal movement, binding to HSPGs can concentrate ligand near cell surfaces and promote receptor-ligand interactions. HSPGs are necessary for the proper distribution and reception of signals such as Wg, Hh, Dpp and Nodal (Hacker et al. 1997; Haerry et al. 1997; Oki et al. 2007; Marjoram and Wright 2011). Concentration of these signals near the cell surface might be especially important in developing epithelial tissues to prevent the release of ligand from the epithelial surface into the lumen. Indeed, cells that cannot synthesize HSPGs fail to retain Dpp and Wg on their surfaces and have attenuated signaling responses (Belenkaya et al. 2004; Takei et al. 2004; Han et al. 2005; Yan et al. 2009).
Several factors that modulate the interactions between ligands and receptors, or ligands and HSPGs, have been identified (Gerlitz and Basler 2002; Giraldez et al. 2002; Kreuger et al. 2004; Glise et al. 2005; Gorfinkiel et al. 2005; Crickmore and Mann 2006; de Navas et al. 2006; Crickmore and Mann 2007; Makhijani et al. 2007; Ayers et al. 2010; Liu et al. 2010; Vuilleumier et al. 2010; Szuperak et al. 2011; You et al. 2011). For example, the heparan sulfate 6-O endosulfatase Sulf1 removes sulfate groups from HSPGs and thereby modulates the HSPGs that concentrate Wg at cell surfaces. This results in decreased Wg signaling possibly due to increased release of Wg from modulated HSPGs (Kleinschmit et al. 2010; You et al. 2011). As Sulf1 is also a transcriptional target of Wg signaling, this provides an elegant way to fine-tune Wg distribution and its signaling activities. If Wg production rates became too high, the signal concentration in the receiving field would increase. But surplus Wg would increase Sulf1 levels, leading to increased removal of sulfate groups from HSPGs and reduced Wg signal retention. This strategy could ensure proper signal distribution by buffering fluctuations in the dynamics of signal dispersal.
Endocytosis
HSPGs, receptors and decoy receptors can also influence ligand stability and distribution by increasing the probability of a ligand to be endocytosed (Scholpp and Brand 2004; Boldajipour et al. 2008; Gallet et al. 2008; Hagemann et al. 2009; Naumann et al. 2010). This internalization results in clearance of ligand from the extracellular space and is thought to be a major regulator of signal stability. For example, overexpression of a receptor of the TGFβ ligand Activin increases the frequency of Activin internalization (Hagemann et al. 2009). The accompanying decrease in signaling range may be caused by the decreased stability of Activin or the sequestration of Activin by its receptor.
Signal stability can also be feedback-regulated. Hh signaling upregulates expression of the Hh receptor Ptc, leading to increased Ptc-mediated endocytosis of Hh (Chen and Struhl 1996). Thus, high levels of Hh signaling promote clearance of Hh from the extracellular space, whereas extracellular Hh is more stable at lower levels of Hh signaling. This “self-enhanced clearance” might be important to fine-tune the signal distribution and to render patterning robust to perturbations (Eldar et al. 2003; Barkai and Shilo 2009; Lander et al. 2009).
Finally, endocytosis can be used as an active transport mechanism to move signals over short distances. Transcytosis—the movement of molecules by cellular uptake and subsequent release—can be either directional or non-directional active transport. Transcytosis directionally transports diverse molecules across tissue barriers such as the placenta or the blood-brain barrier (Tuma and Hubbard 2003; Su et al. 2010) and potentially also redistributes PINs in plants (Kleine-Vehn et al. 2010). Active transport by transcytosis has also been suggested to be necessary for the non-directional, diffusion-like dispersal of some signaling molecules during Drosophila development (Gonzalez et al. 1991; Entchev et al. 2000; González-Gaitán 2003; Kruse et al. 2004; Kicheva et al. 2007; Gallet et al. 2008), but repeated uptake and release of signals has not been demonstrated.
Neuronal signaling routes
In the previous sections we discussed signal movement mechanisms that generally act over time scales of minutes and hours. In contrast, the tasks of the nervous system (e.g. response to sensory stimuli or control of muscle tone) necessitate unique signaling systems that are several orders of magnitude more rapid. The contrast between the nervous system and other tissues nicely illustrates the different strategies utilized for long-range communication.
Signals in the nervous system can act at millisecond time scales i.e at much higher speeds than most developmental and physiological signals. Neurons achieve this speed of information transfer by minimizing the distances over which extracellular signals travel. Information is transmitted intracellularly through changes in membrane potential, and extracellular signaling is generally restricted to synapses, in contrast to the long-range dispersal of developmental signals. Synaptic vesicles store classic neurotransmitters (e.g. dopamine, GABA, acetylcholine), which upon release diffuse across the ~20 nm synaptic cleft in less than one millisecond. Thus, signaling between neurons is extremely rapid, and an input from the periphery can be transmitted via a relay of several neurons to muscles within less than 100 ms and over distances of more than 1 meter.
The regulation of signal secretion is a key step in signal transmission in the nervous system. Classic neurotransmitters are synthesized in the cytoplasm and transported into synaptic vesicles that reside at the presynaptic membrane. Storage of signals allows rapid deployment, a strategy that is uniquely suited to thr rapid communication in the nervous system but is not found for most developmental signals. Exocytosis of neurotransmitters is triggered by increases in calcium levels. Diffusion of released neurotransmitters in the extracellular matrix of the synapse appears to be unhindered, although it has been proposed that the synaptomatrix might play a role in neurotransmitter solubility (Vautrin 2010). After release, signaling is spatially and temporally restricted by the reuptake of neurotransmitters and, in some cases, by enzymatic turnover. Thus, signaling in the nervous system shares with other systems mechanisms such as signal release, degradation and clearance. In contrast to most other signals, however, neurotransmitters are freely diffusible, act at very short time and length scales and can be recycled.
In addition to classic neurotransmitters, neurons can also release hormones and neuropeptides (Scalettar 2006). In contrast to the limited range of classic neurotransmitters in the synaptic cleft, neuropeptides can diffuse over tens of micrometers (Jan and Jan 1982). These molecules are stored in large dense core vesicles, organelles that are also found in neuroendocrine and endocrine cells. Release is stimulus-dependent, but in contrast to the short-term and very local exocytosis of synaptic vesicles at active zones of synapses, dense core vesicles can undergo exocytosis for several minutes and release neuropeptides at axon terminals and the neuronal soma, thus inducing long-term and widespread responses (Nässel 2009).
Similar to many other signaling molecules, neuropeptides (and endocrine signals) undergo complex biosynthesis steps preceding their release. Pro-neuropeptides are translocated into the lumen of the endoplasmic reticulum, transported through the Golgi complex and sorted into large dense core vesicles. Post-translational processing includes the cleavage of pro-peptides by convertases and carboxypeptidases, C-terminal amidation, and N-terminal cyclization of glutamine. The generation of multiple, modified peptides from a common precursor is thought to contribute to protection from extracellular peptidases. After release, neuropeptides diffuse to nearby target neurons. Although it is clear that the extracellular range of neuropeptides is limited by dilution and inactivation by membrane-bound peptidases (Stephenson and Kenny 1987), the extracellular diffusion of neuropeptides has garnered relatively little attention.
Prospects
Research in the last decade has significantly increased our knowledge of the mechanisms underlying the dispersal of many signaling molecules important for development and homeostasis. The modification and packaging of signals have been recognized as important determinants of range, interactions with extracellular factors have been shown to modulate signal movement and activity, and different routes of signal transfer have been described.
Many questions remain. First, the modifications of morphogens and the stoichiometry of morphogen vehicles are poorly described. For example, how heterogeneous are signal modifications and assemblies? What is the exact composition of lipoprotein particles? How does vehicle composition influence dispersal and activity? Second, the in vivo biophysical properties of signals are poorly understood. For example, what is the concentration and flux of signals from the source? What are the diffusion coefficients and half-lives of signals? What are the signal concentrations that elicit specific responses in vivo? How can highly related signals have different ranges of activity (Chen and Schier 2001; Tanaka et al. 2007)? Third, the localization of many signaling molecules within tissues remains uncharacterized. For example, do most signals form gradients? Are there different extracellular compartments that partition signals into specific domains? How complex are the trafficking routes of signaling molecules? Finally, it is unclear how the many extracellular factors modulate signal movement. Do they affect signal diffusion, clearance, trafficking, release, localization or activity? The recent developments in imaging technologies (Helmstaedter et al. 2008; Lichtman et al. 2008; Huang et al. 2010) promise answers to these questions in the near future.
Acknowledgements
We thank Katherine W. Rogers as well as Christian Mosimann, Ben Jordan, Andrea Pauli and Julien Dubrulle for helpful discussions, the European Molecular Biology Organization and the Human Frontier Science Program (HFSP) for postdoctoral fellowships and NIH and HFSP for funding of our research on signal movement. We apologize to our colleagues whose work could not be cited due to reference limits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Ayers KL, Gallet A, Staccini-Lavenant L, Therond PP. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev Cell. 2010;18:605–620. doi: 10.1016/j.devcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. doi: 10.1016/j.ydbio.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Bahlmann F, Wellek S, Reinhardt I, Krummenauer F, Merz E, Welter C. Reference values of fetal aortic flow velocity waveforms and associated intra-observer reliability in normal pregnancies. Ultrasound Obstet Gynecol. 2001;17:42–49. doi: 10.1046/j.1469-0705.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- Barkai N, Shilo BZ. Robust generation and decoding of morphogen gradients. Cold Spring Harb Perspect Biol. 2009;1:a001990. doi: 10.1101/cshperspect.a001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B, Brueckner M. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–174. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- Berg HC. Random walks in biology. Princeton University Press; Princeton: 1993. [Google Scholar]
- Blanchet MH, Le Good JA, Mesnard D, Oorschot V, Baflast S, Minchiotti G, Klumperman J, Constam DB. Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation. EMBO J. 2008;27:2580–2591. doi: 10.1038/emboj.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Bollenbach T, Pantazis P, Kicheva A, Bökel C, González-Gaitán M, Jülicher F. Precision of the Dpp gradient. Development. 2008;135:1137–1146. doi: 10.1242/dev.012062. [DOI] [PubMed] [Google Scholar]
- Brunet I, Di Nardo AA, Sonnier L, Beurdeley M, Prochiantz A. The topological role of homeoproteins in the developing central nervous system. Trends Neurosci. 2007;30:260–267. doi: 10.1016/j.tins.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–815. doi: 10.1016/s0092-8674(00)81677-3. [DOI] [PubMed] [Google Scholar]
- Canny MJ. Phloem translocation. Cambridge University Press; London: 1973. [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFβ1. Journal of Cell Biology. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18:641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Schier AF. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–610. doi: 10.1038/35079121. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol. 2002;12:2124–2128. doi: 10.1016/s0960-9822(02)01362-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Conti L, Bradley D. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell. 2007;19:767–778. doi: 10.1105/tpc.106.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Crank J. The mathematics of diffusion. Clarendon Press; Oxford: 1979. [Google Scholar]
- Crick F. Diffusion in embryogenesis. Nature. 1970;225:420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- Crickmore MA, Mann RS. Hox control of organ size by regulation of morphogen production and mobility. Science. 2006;313:63–68. doi: 10.1126/science.1128650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore MA, Mann RS. Hox control of morphogen mobility and organ development through regulation of glypican expression. Development. 2007;134:327–334. doi: 10.1242/dev.02737. [DOI] [PubMed] [Google Scholar]
- Crickmore MA, Mann RS. The control of size in animals: insights from selector genes. Bioessays. 2008;30:843–853. doi: 10.1002/bies.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- De Crescenzo G, Grothe S, Zwaagstra J, Tsang M, O’Connor-McCourt MD. Real-time monitoring of the interactions of transforming growth factor-β (TGF-β) isoforms with latency-associated protein and the ectodomains of the TGF-β type II and III receptors reveals different kinetic models and stoichiometries of binding. J Biol Chem. 2001;276:29632–29643. doi: 10.1074/jbc.M009765200. [DOI] [PubMed] [Google Scholar]
- De Joussineau C, Soule J, Martin M, Anguille C, Montcourrier P, Alexandre D. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature. 2003;426:555–559. doi: 10.1038/nature02157. [DOI] [PubMed] [Google Scholar]
- de Navas LF, Garaulet DL, Sanchez-Herrero E. The ultrabithorax Hox gene of Drosophila controls haltere size by regulating the Dpp pathway. Development. 2006;133:4495–4506. doi: 10.1242/dev.02609. [DOI] [PubMed] [Google Scholar]
- DeWitt AE, Dong JY, Wiley HS, Lauffenburger DA. Quantitative analysis of the EGF receptor autocrine system reveals cryptic regulation of cell response by ligand capture. J Cell Sci. 2001;114:2301–2313. doi: 10.1242/jcs.114.12.2301. [DOI] [PubMed] [Google Scholar]
- Dierker T, Dreier R, Petersen A, Bordych C, Grobe K. Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem. 2009;284:8013–8022. doi: 10.1074/jbc.M806838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature. 2004;427:419–422. doi: 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- Eaton S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat Rev Mol Cell Biol. 2008;9:437–445. doi: 10.1038/nrm2414. [DOI] [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. doi: 10.1038/nature01061. [DOI] [PubMed] [Google Scholar]
- Eldar A, Rosin D, Shilo BZ, Barkai N. Self-enhanced ligand degradation underlies robustness of morphogen gradients. Dev Cell. 2003;5:635–646. doi: 10.1016/s1534-5807(03)00292-2. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, González-Gaitán M. Gradient formation of the TGF-β homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Esinduy CB, Chang CC, Trosko JE, Ruch RJ. In vitro growth inhibition of neoplastically transformed cells by non-transformed cells: requirement for gap junctional intercellular communication. Carcinogenesis. 1995;16:915–921. doi: 10.1093/carcin/16.4.915. [DOI] [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, White B, Tyurina OV, Guner B, Larson T, Lee HY, Karlstrom RO, Kohtz JD. Synergistic and antagonistic roles of the Sonic hedgehog N- and C-terminal lipids. Development. 2004;131:4357–4370. doi: 10.1242/dev.01301. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Griffith J, Maurice MM, Vincent JP. In vivo role of lipid adducts on Wingless. J Cell Sci. 2008;121:1587–1592. doi: 10.1242/jcs.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A. Hedgehog morphogen: from secretion to reception. Trends Cell Biol. 2011 doi: 10.1016/j.tcb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. BMP1 controls TGFβ1 activation via cleavage of latent TGFβ-binding protein. Journal of Cell Biology. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O, Basler K. Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 2002;16:1055–1059. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell. 2002;2:667–676. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Glise B, Miller CA, Crozatier M, Halbisen MA, Wise S, Olson DJ, Vincent A, Blair SS. Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev Cell. 2005;8:255–266. doi: 10.1016/j.devcel.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Goetz JA, Singh S, Suber LM, Kull FJ, Robbins DJ. A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. J Biol Chem. 2006;281:4087–4093. doi: 10.1074/jbc.M511427200. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M. Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol. 2003;4:213–224. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Swales L, Bejsovec A, Skaer H, Martinez Arias A. Secretion and movement of wingless protein in the epidermis of the Drosophila embryo. Mech Dev. 1991;35:43–54. doi: 10.1016/0925-4773(91)90040-d. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Sierra J, Callejo A, Ibanez C, Guerrero I. The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Dev Cell. 2005;8:241–253. doi: 10.1016/j.devcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Greco V, Hannus M, Eaton S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Gregor T, Wieschaus EF, McGregor AP, Bialek W, Tank DW. Stability and nuclear dynamics of the bicoid morphogen gradient. Cell. 2007;130:141–152. doi: 10.1016/j.cell.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Harger P, Mitchell A, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371:487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- Hacker U, Lin X, Perrimon N. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–3573. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Heslip TR, Marsh JL, O’Connor MB. Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development. 1997;124:3055–3064. doi: 10.1242/dev.124.16.3055. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Khalsa O, O’Connor MB, Wharton KA. Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development. 1998;125:3977–3987. doi: 10.1242/dev.125.20.3977. [DOI] [PubMed] [Google Scholar]
- Hagemann AI, Xu X, Nentwich O, Hyvonen M, Smith JC. Rab5-mediated endocytosis of activin is not required for gene activation or long-range signalling in Xenopus. Development. 2009;136:2803–2813. doi: 10.1242/dev.034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Smith JC. Visualisation and quantification of morphogen gradient formation in the zebrafish. PLoS Biol. 2009;7:e1000101. doi: 10.1371/journal.pbio.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Denk W. 3D structural imaging of the brain with photons and electrons. Curr Opin Neurobiol. 2008;18:633–641. doi: 10.1016/j.conb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y. Left-right determination: involvement of molecular motor KIF3, cilia, and nodal flow. Cold Spring Harb Perspect Biol. 2009;1:a000802. doi: 10.1101/cshperspect.a000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Huang B, Babcock H, Zhuang X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell. 2010;143:1047–1058. doi: 10.1016/j.cell.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Litingtung Y, Chiang C. Ectopic sonic hedgehog signaling impairs telencephalic dorsal midline development: implication for human holoprosencephaly. Hum Mol Genet. 2007;16:1454–1468. doi: 10.1093/hmg/ddm096. [DOI] [PubMed] [Google Scholar]
- Huang X, Litingtung Y, Chiang C. Region-specific requirement for cholesterol modification of sonic hedgehog in patterning the telencephalon and spinal cord. Development. 2007;134:2095–2105. doi: 10.1242/dev.000729. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- Hurtig J, Chiu DT, Onfelt B. Intercellular nanotubes: insights from imaging studies and beyond. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:260–276. doi: 10.1002/wnan.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, Stuermer CA, Basler K. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J. 2008;27:509–521. doi: 10.1038/sj.emboj.7601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicheva A, Pantazis P, Bollenbach T, Kalaidzidis Y, Bittig T, Jülicher F, González-Gaitán M. Kinetics of morphogen gradient formation. Science. 2007;315:521–525. doi: 10.1126/science.1135774. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc Natl Acad Sci U S A. 2010;107:22344–22349. doi: 10.1073/pnas.1013145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmit A, Koyama T, Dejima K, Hayashi Y, Kamimura K, Nakato H. Drosophila heparan sulfate 6-O endosulfatase regulates Wingless morphogen gradient formation. Dev Biol. 2010;345:204–214. doi: 10.1016/j.ydbio.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott JE. Effect of localized photoperiod on spinach. Proc. Am. Soc. Hortic. Sci. 1934;31:152–154. [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell. 2004;7:503–512. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kruse K, Pantazis P, Bollenbach T, Jülicher F, González-Gaitán M. Dpp gradient formation by dynamin-dependent endocytosis: receptor trafficking and the diffusion model. Development. 2004;131:4843–4856. doi: 10.1242/dev.01335. [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515–523. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007;128:245–256. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lander AD, Lo WC, Nie Q, Wan FY. The measure of success: constraints, objectives, and tradeoffs in morphogen-mediated patterning. Cold Spring Harb Perspect Biol. 2009;1:a002022. doi: 10.1101/cshperspect.a002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen HP, Ploug T, Prats C, Tavare JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes. 2006;55:1300–1306. doi: 10.2337/db05-1216. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F. Notch signaling: endocytosis makes delta signal better. Curr Biol. 2003;13:R273–275. doi: 10.1016/s0960-9822(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev Cell. 2003;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Joubin K, Giraldez AJ, Ben-Haim N, Beck S, Chen Y, Schier AF, Constam DB. Nodal stability determines signaling range. Curr Biol. 2005;15:31–36. doi: 10.1016/j.cub.2004.12.062. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development. 1998;125:4901–4907. doi: 10.1242/dev.125.24.4901. [DOI] [PubMed] [Google Scholar]
- Lenhard M, Laux T. Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development. 2003;130:3163–3173. doi: 10.1242/dev.00525. [DOI] [PubMed] [Google Scholar]
- Lewis J. From signals to patterns: space, time, and mathematics in developmental biology. Science. 2008;322:399–403. doi: 10.1126/science.1166154. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Litingtung Y, Chiang C. Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc Natl Acad Sci U S A. 2006;103:6548–6553. doi: 10.1073/pnas.0600124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9:417–422. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. Journal of Cell Biology. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. Journal of Cell Biology. 2006;173:949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lim TM, Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal. 2010;3:ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- Lopez-Bernal A, Alcantara E, Testi L, Villalobos FJ. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010;30:1536–1544. doi: 10.1093/treephys/tpq095. [DOI] [PubMed] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-β from fibroblast-conditioned medium. Journal of Cell Biology. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, et al. Conversion of Mechanical Force into TGF-β-Mediated Biochemical Signals. Curr Biol. 2011 doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhijani K, Kalyani C, Srividya T, Shashidhara LS. Modulation of Decapentaplegic gradient during haltere specification in Drosophila. Dev Biol. 2007;302:243–255. doi: 10.1016/j.ydbio.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Marjoram L, Wright C. Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left-right asymmetry in Xenopus. Development. 2011;138:475–485. doi: 10.1242/dev.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- McDowell N, Gurdon JB, Grainger DJ. Formation of a functional morphogen gradient by a passive process in tissue from the early Xenopus embryo. Int J Dev Biol. 2001;45:199–207. [PubMed] [Google Scholar]
- Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res. 2000;60:3989–3999. [PubMed] [Google Scholar]
- Miller J, Fraser SE, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121:2501–2511. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science. 2010;328:872–875. doi: 10.1126/science.1187959. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Neuropeptide signaling near and far: how localized and timed is the action of neuropeptides in brain circuits? Invert Neurosci. 2009;9:57–75. doi: 10.1007/s10158-009-0090-1. [DOI] [PubMed] [Google Scholar]
- Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, Rot A, Thelen M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Neumann S, Coudreuse DY, van der Westhuyzen DR, Eckhardt ER, Korswagen HC, Schmitz G, Sprong H. Mammalian Wnt3a is released on lipoprotein particles. Traffic. 2009;10:334–343. doi: 10.1111/j.1600-0854.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- Nichols JT, Miyamoto A, Weinmaster G. Notch signaling--constantly on the move. Traffic. 2007;8:959–969. doi: 10.1111/j.1600-0854.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, et al. Fibrillin-1 and -2 differentially modulate endogenous TGF-β and BMP bioavailability during bone formation. Journal of Cell Biology. 2010;190:1107–1121. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-β binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-β. Journal of Cell Biology. 1997;136:1151–1163. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12:205–209. doi: 10.1016/s0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- Ohlig S, Farshi P, Pickhinke U, van den Boom J, Höing S, Jakuschev S, Hoffmann D, Dreier R, Schöler HR, Dierker T, et al. Sonic Hedgehog shedding results in functional activation of the solubilized protein. Dev Cell. 2011 doi: 10.1016/j.devcel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Oki S, Hashimoto R, Okui Y, Shen MM, Mekada E, Otani H, Saijoh Y, Hamada H. Sulfated glycosaminoglycans are necessary for Nodal signal transmission from the node to the left lateral plate in the mouse embryo. Development. 2007;134:3893–3904. doi: 10.1242/dev.009464. [DOI] [PubMed] [Google Scholar]
- Palacios-Prado N, Bukauskas FF. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc Natl Acad Sci U S A. 2009;106:14855–14860. doi: 10.1073/pnas.0901923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panáková D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Alexandre C, Calleja M, Vincent JP. The progeny of wingless-expressing cells deliver the signal at a distance in Drosophila embryos. Curr Biol. 2000;10:321–324. doi: 10.1016/s0960-9822(00)00381-x. [DOI] [PubMed] [Google Scholar]
- Phillips R, Kondev J, Theriot J. Physical Biology of the Cell. Garland Science. 2009 [Google Scholar]
- Pizette S, Rabouille C, Cohen SM, Therond P. Glycosphingolipids control the extracellular gradient of the Drosophila EGFR ligand Gurken. Development. 2009;136:551–561. doi: 10.1242/dev.031104. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4:814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Ebner A, Affolter M. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell. 2002;2:677–683. doi: 10.1016/s1534-5807(02)00171-5. [DOI] [PubMed] [Google Scholar]
- Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- Robert HS, Friml J. Auxin and other signals on the move in plants. Nat Chem Biol. 2009;5:325–332. doi: 10.1038/nchembio.170. [DOI] [PubMed] [Google Scholar]
- Rojo E, Sharma VK, Kovaleva V, Raikhel NV, Fletcher JC. CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell. 2002;14:969–977. doi: 10.1105/tpc.002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4:e7140. doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the Drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Scalettar BA. How neurosecretory vesicles release their cargo. Neuroscientist. 2006;12:164–176. doi: 10.1177/1073858405284258. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Brand M. Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr Biol. 2004;14:1834–1841. doi: 10.1016/j.cub.2004.09.084. [DOI] [PubMed] [Google Scholar]
- Schreml S, Landthaler M, Schaferling M, Babilas P. A new star on the H(2) O(2) rizon of wound healing? Exp Dermatol. 2011;20:229–231. doi: 10.1111/j.1600-0625.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of Transforming Growth Factor β (TGFβ) Superfamily Members Specify Different Functions: EXTRACELLULAR MATRIX INTERACTIONS AND GROWTH FACTOR BIOAVAILABILITY. J Biol Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Yanofsky MF, Weigel D. Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science. 2000;289:779–782. doi: 10.1126/science.289.5480.779. [DOI] [PubMed] [Google Scholar]
- Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–420. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo BZ, Barkai N. EGF receptor signaling - a quantitative view. Curr Biol. 2007;17:R1038–1041. doi: 10.1016/j.cub.2007.10.062. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120:873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorten PR, McMahon CD, Soboleva TK. Insulin transport within skeletal muscle transverse tubule networks. Biophys J. 2007;93:3001–3007. doi: 10.1529/biophysj.107.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer J, Treisman JE. Lipid-modified morphogens: functions of fats. Curr Opin Genet Dev. 2009;19:308–314. doi: 10.1016/j.gde.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson SL, Kenny AJ. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987;241:237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Su T, Bryant DM, Luton F, Verges M, Ulrich SM, Hansen KC, Datta A, Eastburn DJ, Burlingame AL, Shokat KM, et al. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2010;12:1143–1153. doi: 10.1038/ncb2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Szuperak M, Salah S, Meyer EJ, Nagarajan U, Ikmi A, Gibson MC. Feedback regulation of Drosophila BMP signaling by the novel extracellular protein larval translucida. Development. 2011;138:715–724. doi: 10.1242/dev.059477. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Sakuma R, Nakamura T, Hamada H, Saijoh Y. Long-range action of Nodal requires interaction with GDF1. Genes Dev. 2007;21:3272–3282. doi: 10.1101/gad.1623907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM. Extracellular control of TGFβ signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- The I, Bellaiche Y, Perrimon N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol Cell. 1999;4:633–639. doi: 10.1016/s1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- Tian J, Andree B, Jones CM, Sampath K. The pro-domain of the zebrafish Nodal-related protein Cyclops regulates its signaling activities. Development. 2008;135:2649–2658. doi: 10.1242/dev.019794. [DOI] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van der Zee M, Stockhammer O, von Levetzow C, Nunes da Fonseca R, Roth S. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc Natl Acad Sci U S A. 2006;103:16307–16312. doi: 10.1073/pnas.0605154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautrin J. The synaptomatrix: a solid though dynamic contact disconnecting transmissions from exocytotic events. Neurochem Int. 2010;57:85–96. doi: 10.1016/j.neuint.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Veening JG, Barendregt HP. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1. doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Dubois L. Morphogen transport along epithelia, an integrated trafficking problem. Dev Cell. 2002;3:615–623. doi: 10.1016/s1534-5807(02)00323-4. [DOI] [PubMed] [Google Scholar]
- Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, Pyrowolakis G. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol. 2010;12:611–617. doi: 10.1038/ncb2064. [DOI] [PubMed] [Google Scholar]
- Vyas N, Goswami D, Manonmani A, Sharma P, Ranganath HA, VijayRaghavan K, Shashidhara LS, Sowdhamini R, Mayor S. Nanoscale organization of hedgehog is essential for long-range signaling. Cell. 2008;133:1214–1227. doi: 10.1016/j.cell.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Jülicher F, González-Gaitán M. Dynamics of Dpp signaling and proliferation control. Science. 2011;331:1154–1159. doi: 10.1126/science.1200037. [DOI] [PubMed] [Google Scholar]
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Whale TA, Wilson HL, Tikoo SK, Babiuk LA, Griebel PJ. Pivotal Advance: passively acquired membrane proteins alter the functional capacity of bovine polymorphonuclear cells. J Leukoc Biol. 2006;80:481–491. doi: 10.1189/jlb.0206078. [DOI] [PubMed] [Google Scholar]
- White RJ, Nie Q, Lander AD, Schilling TF. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Williams PH, Hagemann A, González-Gaitán M, Smith JC. Visualizing long-range movement of the morphogen Xnr2 in the Xenopus embryo. Curr Biol. 2004;14:1916–1923. doi: 10.1016/j.cub.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Windler SL, Bilder D. Endocytic internalization routes required for delta/notch signaling. Curr Biol. 2010;20:538–543. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windt CW, Vergeldt FJ, de Jager PA, van As H. MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ. 2006;29:1715–1729. doi: 10.1111/j.1365-3040.2006.01544.x. [DOI] [PubMed] [Google Scholar]
- Wizenmann A, Brunet I, Lam JS, Sonnier L, Beurdeley M, Zarbalis K, Weisenhorn-Vogt D, Weinl C, Dwivedy A, Joliot A, et al. Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo. Neuron. 2009;64:355–366. doi: 10.1016/j.neuron.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Gerlach N, Schuh R. Drosophila tracheal system formation involves FGF-dependent cell extensions contacting bridge-cells. EMBO Rep. 2002;3:563–568. doi: 10.1093/embo-reports/kvf115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, et al. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. Diffusible gradients are out - an interview with Lewis Wolpert. Int J Dev Biol. 2009;53:659–662. doi: 10.1387/ijdb.072559mr. Interviewed by Richardson, Michael K. [DOI] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Y, Feng Y, Lin SC, Lin X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell. 2009;17:470–481. doi: 10.1016/j.devcel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Belenkaya T, Lin X. Sulfated is a negative feedback regulator of wingless in Drosophila. Dev Dyn. 2011;240:640–648. doi: 10.1002/dvdy.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SR, Burkhardt M, Nowak M, Ries J, Petrasek Z, Scholpp S, Schwille P, Brand M. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 2009;461:533–536. doi: 10.1038/nature08391. [DOI] [PubMed] [Google Scholar]
- Zeevaart JA. Florigen coming of age after 70 years. Plant Cell. 2006;18:1783–1789. doi: 10.1105/tpc.106.043513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Goetz JA, Suber LM, Scott WJ, Jr., Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]