Abstract
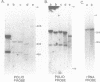
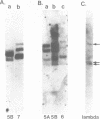
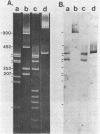
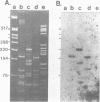
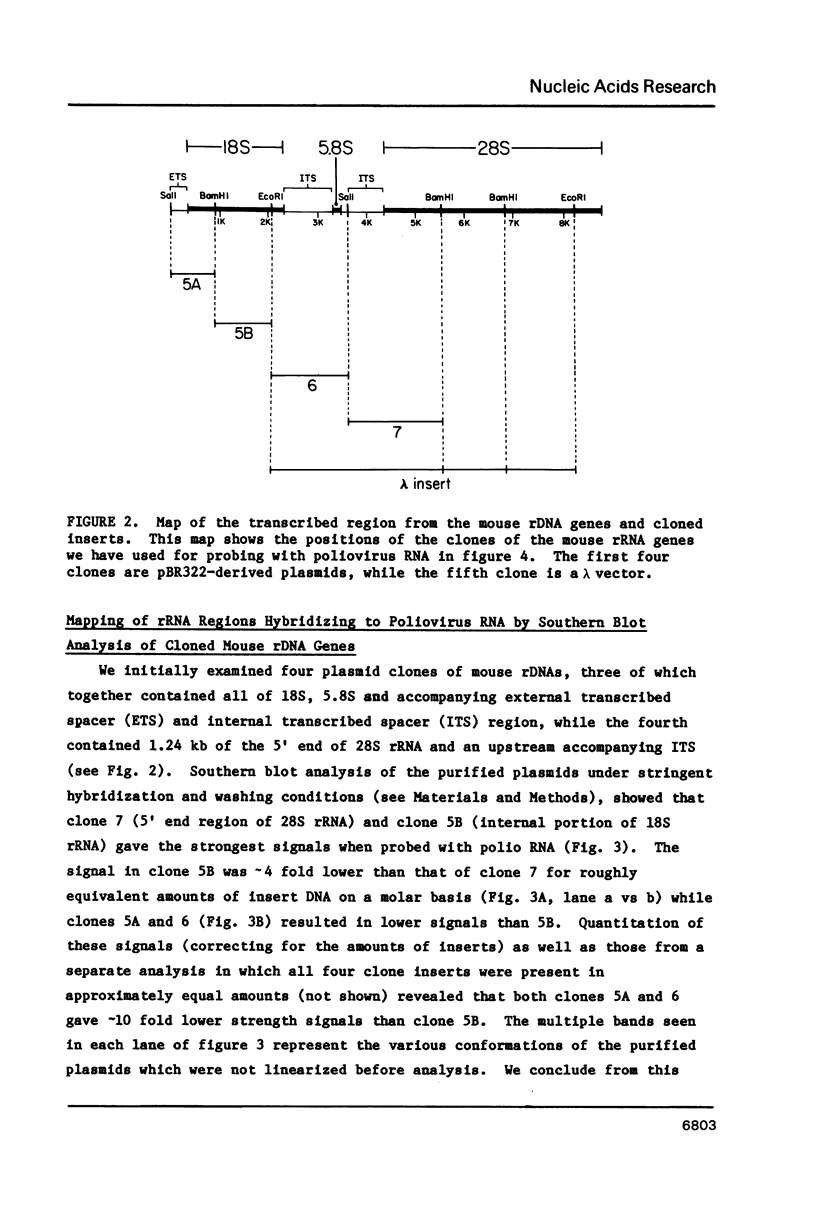
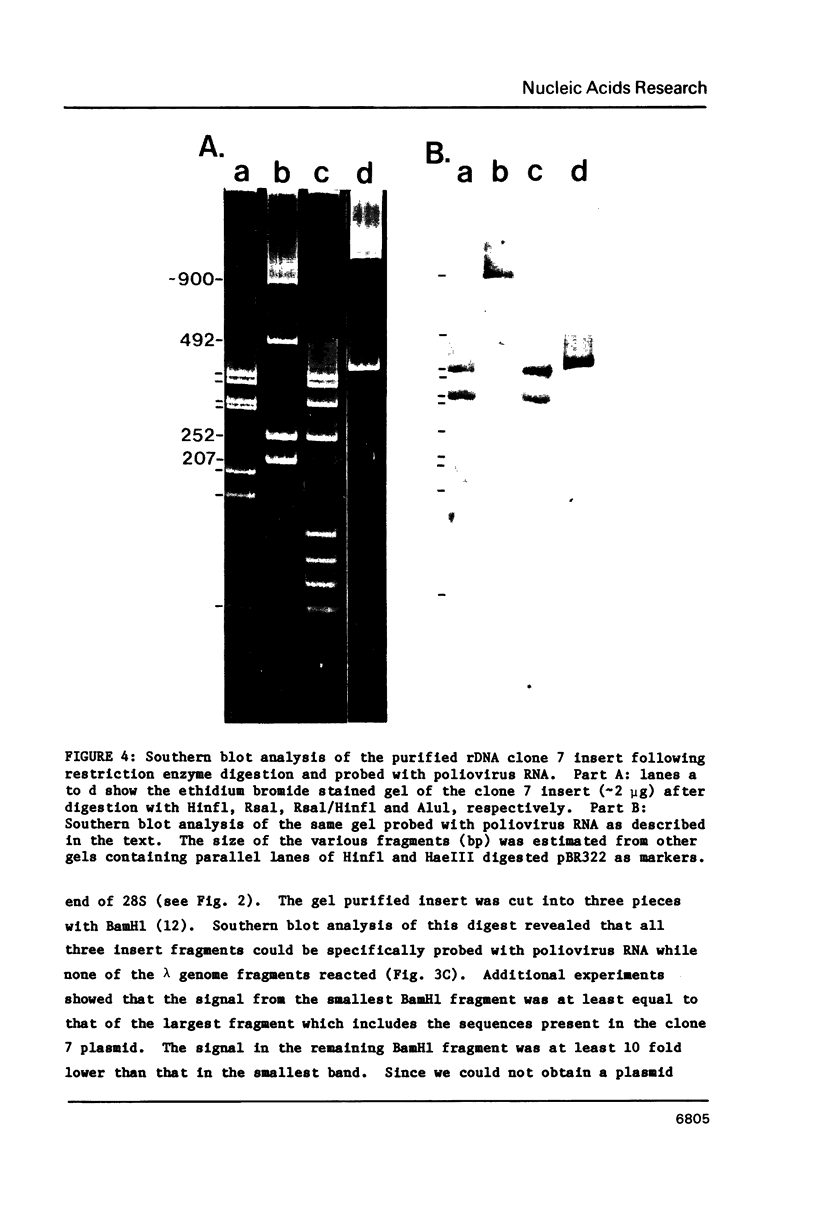
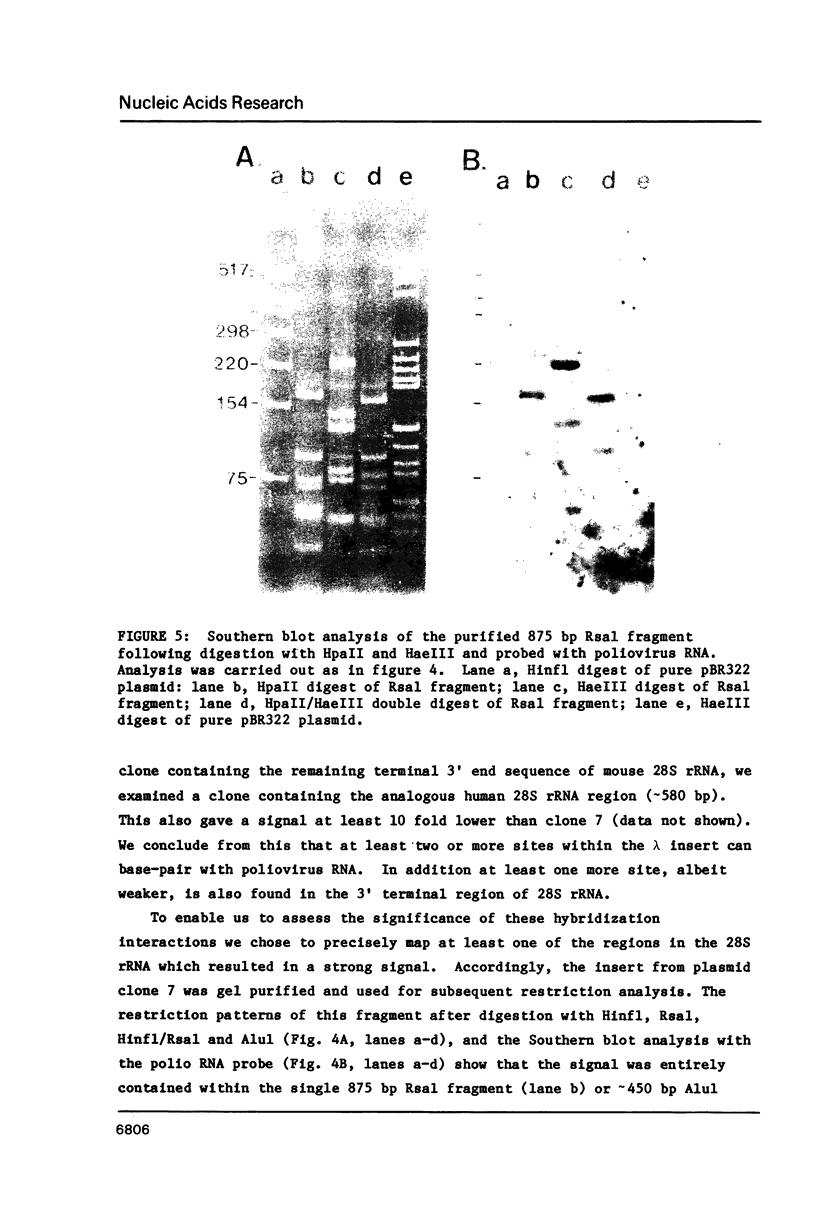
The RNA genome of poliovirus hybridizes to 28S and 18S rRNAs of higher eukaryotes under stringent conditions. The hybridization detected by Northern blot analyses is specific since little or no signal was detected for yeast or prokaryotic rRNAs or other major cellular RNAs. Southern blot analysis of DNA clones of mouse rRNA genes leads us to conclude that several regions of 28S rRNA, and at least one region in 18S rRNA, are involved in the hybridization to polio RNA, and that G/C regions are not responsible for this phenomenon. We have precisely mapped one of these hybridizing regions in both molecules. Computer analysis confirms that extensive intermolecular base-pairing (81 out of 104 contiguous bases in the rRNA strand) could be responsible for this one particular site of interaction (polio genome, bases 5075-5250; 28S rRNA, bases 1097-1200). We discuss the possible functional and/or evolutionary significance of this novel type of interaction.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V., Pettersson R. F., Baltimore D. An enzymatic activity in uninfected cells that cleaves the linkage between poliovirion RNA and the 5' terminal protein. Cell. 1978 Dec;15(4):1439–1446. doi: 10.1016/0092-8674(78)90067-3. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Evolution of RNA viruses. Ann N Y Acad Sci. 1980;354:492–497. doi: 10.1111/j.1749-6632.1980.tb27988.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L. H., Rabin B., Schlessinger D. Multiple ribosomal RNA cleavage pathways in mammalian cells. Nucleic Acids Res. 1981 Oct 10;9(19):4951–4966. doi: 10.1093/nar/9.19.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M. Nucleic acid aggregation geometry and the possible evolutionary origin of ribosomes and the genetic code. J Mol Biol. 1982 Dec 5;162(2):379–391. doi: 10.1016/0022-2836(82)90533-2. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Wimmer E. Identification of a new neutralization antigenic site on poliovirus coat protein VP2. J Virol. 1984 Nov;52(2):719–721. doi: 10.1128/jvi.52.2.719-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg H., Schoulaker R. Sequence homologies between ribosomal and phage RNAs: a proposed molecular basis for RNA phage parasitism. J Mol Biol. 1976 Sep 25;106(3):709–730. doi: 10.1016/0022-2836(76)90260-6. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Goldberg G., Bowman L. H., Steinmetz D., Schlessinger D. Mouse rDNA: sequences and evolutionary analysis of spacer and mature RNA regions. Mol Cell Biol. 1983 Aug;3(8):1488–1500. doi: 10.1128/mcb.3.8.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassouna N., Michot B., Bachellerie J. P. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 1984 Apr 25;12(8):3563–3583. doi: 10.1093/nar/12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Jacobson A. B., Good L., Simonetti J., Zuker M. Some simple computational methods to improve the folding of large RNAs. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):45–52. doi: 10.1093/nar/12.1part1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Hyman R. W. Specious hybridization between herpes simplex virus DNA and human cellular DNA. Virology. 1983 Dec;131(2):555–560. doi: 10.1016/0042-6822(83)90521-4. [DOI] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. doi: 10.1016/0092-8674(82)90454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Wilbur W. J., Smith T. F., Waterman M. S. On the statistical significance of nucleic acid similarities. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):215–226. doi: 10.1093/nar/12.1part1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClain K., Stewart M., Sullivan M., Maizel J. V., Jr Ribosomal binding sites on poliovirus RNA. Virology. 1981 Aug;113(1):150–167. doi: 10.1016/0042-6822(81)90144-6. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Holland J. J., Perrault J. Generation of defective interfering particles in picornaviruses. Virology. 1980 Jan 30;100(2):408–418. doi: 10.1016/0042-6822(80)90532-2. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Michot B., Bachellerie J. P., Raynal F. Sequence and secondary structure of mouse 28S rRNA 5'terminal domain. Organisation of the 5.8S-28S rRNA complex. Nucleic Acids Res. 1982 Sep 11;10(17):5273–5283. doi: 10.1093/nar/10.17.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Semler B. L., Omilianowski D. R., Anderson C. W., Wimmer E., Rueckert R. R. Protein processing map of poliovirus. J Virol. 1984 Mar;49(3):873–880. doi: 10.1128/jvi.49.3.873-880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Clinton G. M., McClure M. A. RNP template of vesicular stomatitis virus regulates transcription and replication functions. Cell. 1983 Nov;35(1):175–185. doi: 10.1016/0092-8674(83)90220-9. [DOI] [PubMed] [Google Scholar]
- Perrault J., Semler B. L. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H. U., Joseph E., Ullmann A., Danchin A. Formylation of initiator tRNA methionine in procaryotic protein synthesis: in vivo polarity in lactose operon expression. J Bacteriol. 1978 Aug;135(2):453–459. doi: 10.1128/jb.135.2.453-459.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen R. D., Staprans S. I., Shaw S. B., Spector D. H. Sequences in human cytomegalovirus which hybridize with the avian retrovirus oncogene v-myc are G + C rich and do not hybridize with the human c-myc gene. Mol Cell Biol. 1985 Jun;5(6):1525–1530. doi: 10.1128/mcb.5.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reanney D. C. The evolution of RNA viruses. Annu Rev Microbiol. 1982;36:47–73. doi: 10.1146/annurev.mi.36.100182.000403. [DOI] [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Zakharyev V. M., Krayev A. S., Skryabin K. G., Bayev A. A. The structure of the yeast ribosomal RNA genes. I. The complete nucleotide sequence of the 18S ribosomal RNA gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Dec 11;8(23):5779–5794. doi: 10.1093/nar/8.23.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Dorner A. J., Wimmer E. Production of infectious poliovirus from cloned cDNA is dramatically increased by SV40 transcription and replication signals. Nucleic Acids Res. 1984 Jun 25;12(12):5123–5141. doi: 10.1093/nar/12.12.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Devine C. S., Cohn S. M., Lieberman M. W. Quantitative electrophoretic transfer of DNA from polyacrylamide or agarose gels to nitrocellulose. Anal Biochem. 1984 Feb;137(1):120–124. doi: 10.1016/0003-2697(84)90356-7. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]