Abstract
Aims:
This study was performed to compare the degree of post operative analgesia, patient compliance, and frequency of adverse events with the use of oral diclofenac tablets and transdermal diclofenac patch following multiple premolar extractions in patients undergoing orthodontic treatment.
Materials and Methods:
Twenty young pre-orthodontic patients requiring bilateral maxillary and mandibular first premolar extractions were selected for the study. The right maxillary and mandibular first premolars were extracted first and 50 mg oral diclofenac sodium tablets were prescribed to be taken thrice a day for three days. In the next appointment, the contralateral first premolars were extracted and a 100 mg transdermal diclofenac patch was applied once a day for three days. Pain relief and pain intensity with both the diclofenac formulations was recorded for each of the three postoperative days using 5-point Verbal Pain Intensity and Pain Relief Score Charts.
Results and Conclusions:
Statistical analyses revealed that there was a gradual increase in pain relief scores and a gradual decrease in pain intensity scores with the use of oral diclofenac tablets as well as with the transdermal patch. However, subjects reported that they were more comfortable using the transdermal patch particularly due to the once-a-day application and lesser frequency of systemic adverse effects. Results of this study indicate that the transdermal diclofenac patch provides as potent analgesia as the oral diclofenac tablets with the added advantage of better patient compliance and may be used for routine post extraction analgesia.
Keywords: Diclofenac sodium, post extraction analgesia, transdermal patch
Introduction
The premise of successful dental treatment is based not only on the correct operative technique but also on the prevention and management of post operative complications. Post extraction pain has often been a nemesis for dental surgeons, with clinicians perpetually striving for an analgesic modality that would provide profound analgesia and would be best tolerated by the patient, hence ensuring patient compliance.
Non-steroidal anti-inflammatory drugs (NSAIDs) are amongst the most widely used therapeutic class of analgesic compounds used to relieve post extraction pain.[1–3] Diclofenac sodium is a commonly prescribed NSAID, which exhibits anti-inflammatory, analgesic and anti-pyretic activity.
When used by the oral route, however, only about 50% of the absorbed dose of diclofenac becomes systemically available, due to the first pass metabolism. Also, due to the high plasma concentrations attained,[4,5] oral diclofenac carries the potential for significant adverse reactions, particularly those involving the gastrointestinal tract.[6,7]
Transdermal patches have in the recent past been developed as innovative topical delivery systems for diclofenac and other NSAIDs, offering the advantage of sustained drug delivery[8] with reduced incidence of systemic adverse effects due to lower plasma concentrations.[9,10]
The present study was carried out to compare and evaluate the post operative analgesia, adverse events, patient tolerability and compliance with the use of oral diclofenac sodium tablets and the diclofenac transdermal patch following multiple premolar extractions in patients undergoing orthodontic treatment. The bilateral extraction of maxillary and mandibular first premolars in young orthodontic patients provided a favorable setting to evaluate the two formulations since identical operative procedures could be performed in the same individuals on two different occasions, with the patients acting as their own control in this cross over trial.
Materials and Methods
Twenty young pre- orthodontic patients requiring bilateral maxillary and mandibular first premolar extractions were selected for the study. The subjects belonged to both sexes and were within the age range of 14 to 26 years, with a mean age of 17.5 years.
All the subjects chosen for the study had a healthy periodontal status with none of their teeth being extensively decayed or periapically involved. Subjects with history or clinical evidence of allergy to NSAIDs or those with active peptic ulceration within the last six months were excluded from the sample. Subjects undergoing treatment with other NSAIDs or any other analgesics or corticosteroids during the trial period and those with history of systemic diseases like bronchial asthma, epilepsy, and emotional and psychosomatic disorders were also excluded.
The ethical clearance for the study was provided by an institutionally approved ethical committee and all subjects were informed about the nature of the study and the probable side effects from the drugs being administered. A written informed consent was obtained from all subjects.
The right maxillary and mandibular first premolars were first extracted successively in the same appointment using a standardized armamentarium [Figure 1], following which 50 mg oral diclofenac sodium tablets were prescribed to be taken thrice a day for a period of three days (nine tablets). Each of the patients was given a Verbal Pain Intensity and Pain Relief Score Chart (both 5- point scales with values from 0 to 4) [Figure 2] for assessing pain intensity and pain relief for each of the three postoperative days.
Figure 1.
Armamentarium used for premolar extractions
Figure 2.
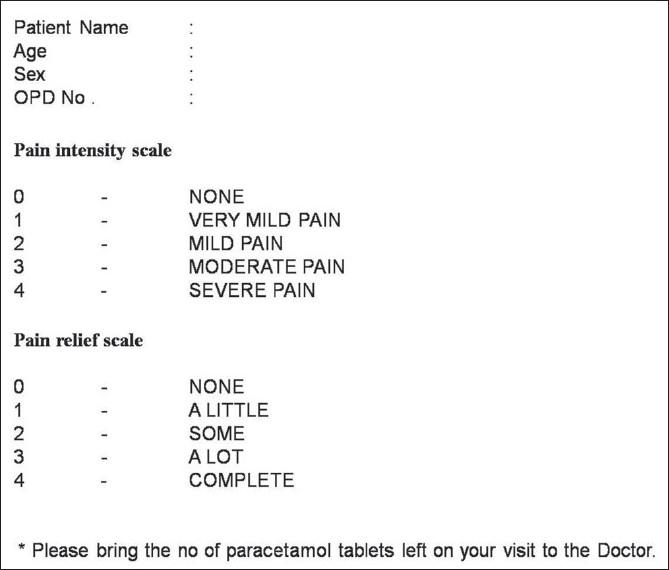
The verbal pain intensity and pain relief score chart
Paracetamol 500 mg tablets were permitted to be used as rescue medication and a total of nine tablets were provided to each of the patients for the three postoperative days. The patients were asked to maintain a record of the number of paracetamol tablets consumed on the pain assessment charts and to return the remaining tablets to operator on their next visit.
After three post operative days, another day was given to allow for the complete wash out of drug from the body and the patients were recalled and their verbal pain score charts were evaluated. The left maxillary and mandibular first premolars were then extracted and a 100 mg transdermal Diclofenac patch (Nu Patch - Zydus-Cadilla labs) was placed. The matrix controlled Diclofenac transdermal patch [Figures 3 and 4] is a flat and transparent transdermal delivery system (TDS) that provides continuous and systemic release of diclofenac and is designed to remain at the site of application for 24 hours. Each 50 sq. cm patch contains 100 mg of Diclofenac Diethylamine as its active ingredient. The device [Figure 4] consists of a polymer matrix that controls the release of the drug and an impermeable backing membrane that prevents the leaching of drug from the top. Adhesives fasten the device to the skin during use. The patch delivers a slow release of drug into the body over time, resulting in long-term effectiveness and added convenience.
Figure 3.

Transdermal diclofenac patch – 100 mg.
Figure 4.
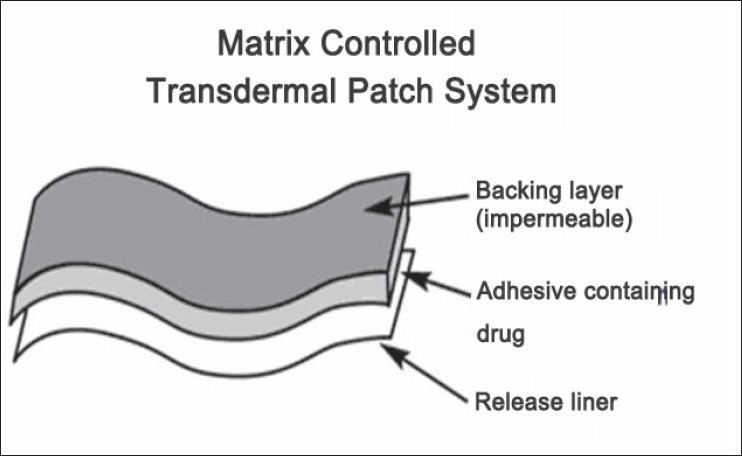
Design of a matrix controlled transdermal patch
On the each of the following two days, the patch was changed and a new one placed; thus placing a total of three patches over the three post operative days. Each successive application of the transdermal patch was made on a different hairless skin area [Figures 5 and 6]. The patients were permitted to use Paracetamol 500 mg tablets as rescue medication during the post operative period.
Figure 5.
Transdermal patch placed over the right shoulder
Figure 6.
Transdermal patch placed on the abdomen
The subjects were asked to report the intensity and pain relief on the verbal pain score chart for the three postoperative days, following which they were asked to submit the 5-point pain intensity scale chart and the 5-point pain relief scale chart for evaluation. The rescue medication tablets taken, if any, were noted and the patients were asked if they experienced any adverse effects such as gastric discomfort, nausea, vomiting, gastric acidity or burning sensation and dyspepsia, diarrhea, dizziness, pruritis etc. The data obtained from the study subjects were statistically evaluated using the Mann-Whitney U test.
All the premolar extractions were performed by the same operator, thus removing any operator-induced bias from the study. Since all the premolars extracted in the same patient were of comparable periodontal status, study bias was further negated.
Observations
The duly filled verbal pain intensity and pain relief score charts were collected from all the subjects and the data was analyzed statistically using the Mann-Whitney U nonparametric test.
An assessment of the intensity of pain following premolar extractions revealed that there was a gradual decrease in the pain intensity scores from day one to day three with both the oral diclofenac tablets as well as with the transdermal patch [Table 1, Graph 1].
Table 1.
Score on the pain intensity scale
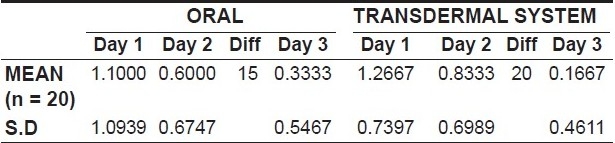
Graph 1.
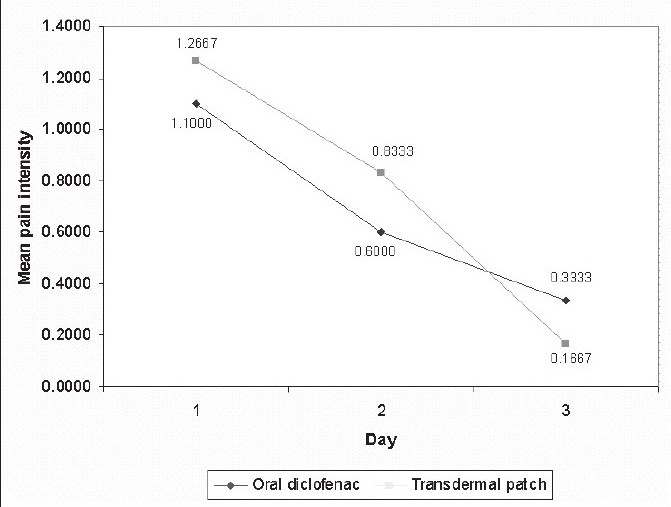
variation in the mean intesity from day 1 to day 3
On evaluating the variation in pain relief amongst the subjects, it was observed that all patients reported of complete or almost complete pain relief by the third day of therapy with either oral diclofenac tablets or transdermal diclofenac patch. In both groups of subjects, i.e, those taking oral diclofenac tablets and those in whom the transdermal patch was placed, there was a gradual increase in pain relief scores over the three post operative days [Table 2, Graph 2]
Table 2.
Score on the pain relief scale
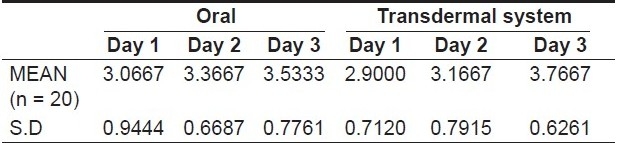
Graph 2.
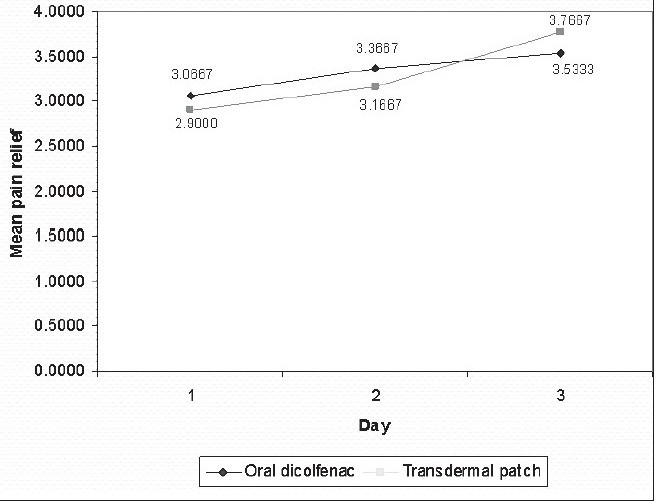
Variation in the mean pain relief from day 1 to day 3
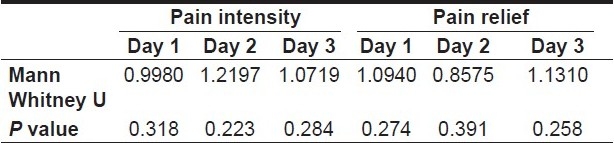
Statistical analysis using the Mann Whitney U test however revealed that the difference in the pain intensity as well as in the pain relief provided by the oral tablets and transdermal patch was not statistically significant [Table 3].
Table 3.
Table showing the Mann-Whitney U test values for pain intensity and pain relief when comparing oral and transdermal diclofenac formulations
Diclofenac transdermal patch 100 mg used once daily was thus observed to be equally potent as oral diclofenac 150 mg daily, for post dental extraction analgesia.
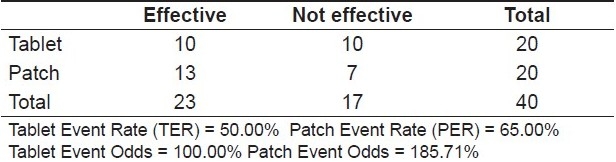
On comparing the difference in the levels of pain experienced from day one to day two for both oral and transdermal forms of treatment, it was observed that from day 1 to day 2, 50% of the patients prescribed oral diclofenac tablets reported of significant pain relief. On the other hand, amongst the patients in whom the transdermal patch was used, 65% reported of significant pain relief from day 1 to day 2 [Table 4, Graph 3]
Table 4.
Efficacy of the treatment
Graph 3.
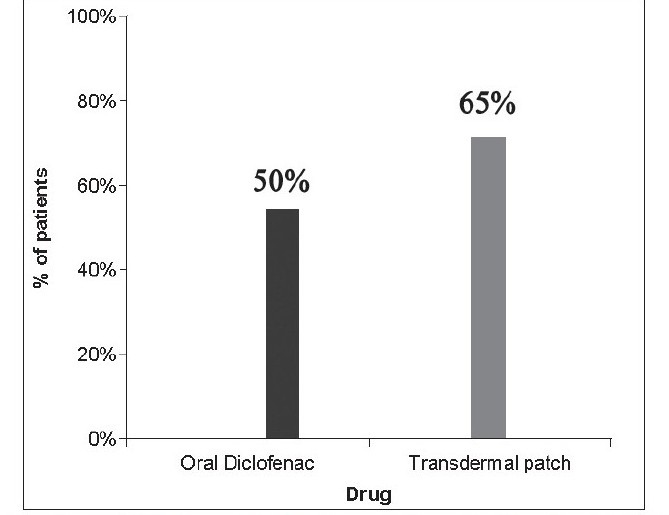
Distribution of patients reporting signifi cant pain relief from day 1 to day 2
All but one patient reported the transdermal patch to be a better modality in terms of ease of use and once-a-day application as compared to oral tablets.
None of the patients on oral diclofenac therapy consumed paracetamol tablets, whereas one patient, when on transdermal patch therapy, consumed a total of six paracetamol tablets for pain relief.
Two patients when on oral diclofenac tablets complained of gastric acidity and burning sensation. No adverse events were reported with the use of transdermal patch.
Discussion
Dealing with post-operative pain remains an arena for never ending research with better formulations and modalities continuously replacing obsolete ones. Post extraction pain has often been a nemesis for dental surgeons and patients alike due to the considerable degree of inflammatory response involved.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are amongst the most common analgesic agents used to relieve post operative dental pain. The efficacy of NSAIDs in reducing pain is largely a result of their capacity to inhibit cyclo-oxygenases 1 and 2 (COX-1 and COX-2), key enzymes in prostaglandin (PG) biosynthesis.[11]
Oral administration of NSAIDs, however, carries a risk of first pass metabolism with significant amount of the drug being lost before it is systemically absorbed. Oral NSAIDs are also known to cause several adverse effects, particularly gastro intestinal effects, which are dose dependant. Topical formulations of NSAIDs have been developed as alternate routes of drug administration, offering the advantage of local, enhanced drug delivery to the affected tissues with a lower incidence of systemic adverse effects. Topical NSAIDs have thus carved out a niche for themselves as therapeutic analgesic modalities with established benefits and lower incidence of adverse events.
Transdermal systems for NSAIDs are an innovative delivery mechanism replacing oral and other traditional forms of drug administration. The drug contained in the transdermal patch enters the body through skin and ultimately diffuses into capillaries for systemic delivery. The steady permeation of drug across the skin allows for more consistent serum drug levels, often a goal of therapy.[12,13]
In the present study, diclofenac was used as analgesic, both in its oral and transdermal form, following multiple first premolar extractions in patients undergoing orthodontic treatment. Diclofenac is an NSAID, which exhibits anti-inflammatory, analgesic, and anti-pyretic activity and has been routinely used as an analgesic following dental extractions.
The two formulations of diclofenac used in this study were oral Diclofenac 50 mg tablets to be taken thrice a day and 100 mg transdermal Diclofenac patch (Zydus-Cadilla labs), which is designed to remain at the site of application for 24 hours. The 50-sq. cm patch used in the study contains 100 mg of Diclofenac Diethylamine as its active agent and allows for sustained release of the drug.
The analgesic efficacy, safety profile, and tolerability of both oral and transdermal forms of diclofenac were evaluated in orthodontic patients undergoing bilateral extraction of maxillary and mandibular first premolars. The maxillary and mandibular premolars on one side were removed in the first appointment and those on the contra lateral side were extracted on the next appointment. This setting allowed the comparison of the two forms of the drug in a similar clinical situation, with the oral tablets being prescribed on the first post extraction appointment and the transdermal patch placed in the subsequent appointment. All subjects were from a similar age group and had good periodontal status. Also, since similar surgical procedures were performed for extractions on both sides and the perception of pain was also similar, bilateral orthodontic extractions allowed two similar procedures to be carried out on two different occasions, with the patients acting as their own controls.
The transdermal diclofenac patch 100 mg used once daily was found to be as potent as oral diclofenac 150 mg daily for post dental extraction analgesia. These findings are similar to those of Funk et al,[14] who reported that when used in patients with post operative shoulder pain, both oral and transdermal diclofenac showed similar analgesic efficacy. Diclofenac patches have also been reported to provide efficient analgesia following laparoscopic surgery.[15]
In the present study, patients using the transdermal patch reported a statistically and clinically significant reduction in pain scores, similar to those achieved with oral diclofenac tablets. However, from day 1 to day 2, 50% of the patients who were prescribed oral diclofenac tablets reported of significant pain relief, while amongst those with the transdermal patch, 65% reported of significant pain relief in the first two postoperative days.
In terms of safety, the patch was well tolerated and did not cause any local or systemic adverse effects whereas two patients on oral diclofenac therapy reported with gastric acidity and nausea. Agarwal et al,[16] when using the transdermal diclofenac patch for the attenuation of venous cannulation, reported the occurrence of a localized erythematous rash or pruritis at the site of application of the transdermal patch. This finding is contrary to those in the present study perhaps due to the fact that each successive application of the diclofenac patch was done at a different site.
The safety profile of diclofenac patches has also been emphasized by Mason et al,[17] in their systematic review on the use of topical NSAIDs in the UK and by studies reporting the use of the diclofenac trandermal patch in osteo arthritis[18] as well as in in sports-related injuries.[19]
Conclusions
The transdermal diclofenac patch seems to be a promising analgesic modality for the management of mild to moderate pain following dental extractions, given the evidence of its established analgesic potency with a lower incidence of systemic adverse effects. Transdermal diclofenac therapy may have a role to play in post-traumatic pain, perhaps with an increased strength of the analgesic drug in the transdermal patch. However, longer clinical trials with a larger sample need to be conducted before the real scope of the transdermal diclofenac patch can be clearly defined.
Acknowledgments
The authors wish to acknowledge Prof. Shridhar D Baliga, KLE Institute of Dental Sciences, Belgaum for his academic support in the study
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sachs CJ. Oral Analgesics for Acute Nonspecific Pain. Am Fam Physician. 2005;71:913–8. [PubMed] [Google Scholar]
- 2.Vaile JH, Davis P. Topical NSAIDs for Musculoskeletal Conditions- A Review of the Literature. Drugs. 1998;56:783–99. doi: 10.2165/00003495-199856050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Zuniga JR, Phillips CL, Shugars D, Lyon JA, Peroutka SJ, Swarbrick J, et al. Analgesic Safety and Efficacy of Diclofenac Sodium Softgels on Postoperative Third Molar Extraction Pain. J Oral Maxillofac Surg. 2004;62:806–15. doi: 10.1016/j.joms.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 4.The Royal College of Anaesthetists. Guidelines for the Use of Non-Steroidal Anti-Infl ammatory Drugs in the Perioperative Period. 1998 Mar [Google Scholar]
- 5.Heyneman CA, Lawless-Liday C, Wall GC. Oral versus Topical NSAIDs in Rheumatic Diseases - A Comparison. Drugs. 2000;60:555–74. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Naesdal J, Brown K. NSAID-Associated Adverse Effects and Acid Control Aids to Prevent Them: A Review of Current Treatment Options. Drug Saf. 2006;29:119–32. doi: 10.2165/00002018-200629020-00002. [DOI] [PubMed] [Google Scholar]
- 7.Roth SH, Shainhouse JZ. Efficacy and Safety of a Topical Diclofenac Solution (Pennsaid) in the Treatment of Primary Osteoarthritis of the Knee: A Randomized, Double-Blind, Vehicle-Controlled Clinical Trial. Arch Intern Med. 2004;164:2017–23. doi: 10.1001/archinte.164.18.2017. [DOI] [PubMed] [Google Scholar]
- 8.Hadgraft J, Lane ME. Passive Transdermal Drug Delivery Systems.Recent Considerations and Advances. Am J Drug Deliv. 2006;4:153–60. [Google Scholar]
- 9.Cranney A, O’Donnell S. Topical Diclofenac improved pain and physical function with no systemic side effects in primary osteoarthritis of the knee. Evid Based Med. 2005;10:81. [Google Scholar]
- 10.Radbruch L. Buprenorphine TDS: Use in daily practice, benefits for patients. Int J Clin Pract Suppl. 2003;133:19–22. [PubMed] [Google Scholar]
- 11.Burian M, Tegeder I, Seegel M, Geisslinger G. Peripheral and central antihyperalgesic effects of Diclofenac in a model of human inflammatory pain. Clin Pharmacol Ther. 2003;74:113–20. doi: 10.1016/S0009-9236(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 12.Mazieres B. Topical Ketoprofen Patch. Drugs R D. 2005;6:337–44. doi: 10.2165/00126839-200506060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Tracy H. Breaking Barriers in Transdermal Drug Delivery. JAMA. 2005;293:2083. doi: 10.1001/jama.293.17.2083. [DOI] [PubMed] [Google Scholar]
- 14.Funk L, Umaar R, Molajo A. Diclofenac patches for postoperative shoulder pain. Int J Shoulder Surg. 2008;2:47–8. doi: 10.4103/0973-6042.41035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alessandri F, Lijoi D, Mistrangelo E, Nicoletti A, Crosa M, Ragni N. Topical Diclofenac patch for postoperative wound pain in laparoscopic gynecologic surgery: A randomized study. J Minim Invasive Gynecol. 2006;13:195–200. doi: 10.1016/j.jmig.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Dhiraaj S, Kumar A, Singhal V, Singh U. Evaluation of a Diclofenac transdermal patch for the attenuation of venous cannulation pain: A prospective, randomised, double-blind, placebo-controlled study. Anaesthesia. 2006;61:360–2. doi: 10.1111/j.1365-2044.2006.04538.x. [DOI] [PubMed] [Google Scholar]
- 17.Mason L, Moore R A, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for acute pain: A meta-analysis. BMC Family Practice. 2004;5:10. doi: 10.1186/1471-2296-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruhlmann P, Michel BA. Topical diclofenac patch in patients with knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Clin Exp Rheumatol. 2003;21:193–8. [PubMed] [Google Scholar]
- 19.Predel HG, Koll R, Pabst H, Dieter R, Gallacchi G, Giannetti B, et al. Diclofenac patch for topical treatment of acute impact injuries: A randomised, double blind, placebo controlled, multicentre study. Br J Sports Med. 2004;38:318–23. doi: 10.1136/bjsm.2003.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]


