Abstract
Background
F. hepatica infection is rare and mostly subclinical. Migration of juvenile forms of F. hepatica into the host's liver is accompanied by an inflammatory reaction followed by fibrosis and cirrhosis.
Objective
The aim of this study was to evaluate oxidative status by using a novel automated method in patients with Fasciola hepatica.
Methods
Twenty two patients with a diagnosis of F. hepatica and 26 healthy volunteers were enrolled in the study. Their Total antioxidant capacity status (TAC), total oxidant status (TOS) and catalase were measured in them and oxidative stress index (OSI) was calculated. These measurements were also taken for the control group and the values were compared.
Results
Plasma levels of total TOS and OSI were significantly increased in patients as compared with healthy controls (P<0.001, P<0.001, P=0.001, P=0.008) respectively. In contrast, TAC level was significantly lower in patients as compared with controls (p<0.05). There was no significant difference between the catalase results of the two groups (p>0.05).
Conclusion
Total oxidative status and OSI were increased and total antioxidative status capacity was decreased in patients with F. hepatica infeciton. A high oxidative stress occurs during F. hepatica infection, which may cause severe damage in both the liver.
Keywords: Fasciola hepatica, total antioxidant capacity, total oxidative status, oxidative stress index
Introduction
Fascioliasis is a zoonotic disease caused by F. hepatica. Humans become accidentally infected after eating aquatic plants on which encysted organisms are present or by drinking contaminated water. Over the period 1970–1990, 2594 human cases were reported in 42 countries, and the World Health Organisation now recognises fascioliasis as an important disease in humans.1 The infections are mostly subclinical.2
Diagnostic limitations and the fact that human fascioliasis is not a notifiable disease make it likely that the number of human cases is much greater than reflected in published reports. Recent papers estimate human infection of up to 2.4 million people or even higher in many countries, mainly in Asia and Africa.3,4 In our geographic region in Turkey, recent prevalence rates of fascioliasis were 6.1% and 14.5% in patients with eosinophilia and chronic urticaria, respectively.5 Migration of juvenile forms of F. hepatica into the host's liver is accompanied by an inflammatory reaction followed by fibrosis and cirrhosis.6 Some oxidative parameters have been studied in few human and animal patients with F. hepatica. 6,7,8
In this study, we evaluated the oxidative status in patients with F. hepatica and in healthy controls via measurement of serum oxidative stress markers, including total antioxidant capacity (TAC), total oxidative status (TOS), oxidative stress index (OSI), and catalase.
Method
The study protocol was approved by the local research committee for ethics. In total, 22 patients with fascioliasis and 26 healthy controls were included in this study. For each subject the diagnosis of fascioliasis was established serologically using a modified enzymelinked immunosorbent assay (ELISA) prepared with ES antigens and/or by finding F. hepatica eggs in stools. Radiological, clinical and laboratory parameters were subsequently evaluated. Venous blood samples of the patients before therapy were taken and stored at −80°C until the analysis of tests. The blood samples of the control group were also stored. Special care was taken to exclude subjects who were taking anabolic drugs, vitamins, or other antioxidants, or who were smokers. None of the subjects was following a special diet.
Measuring the total antioxidative capacity of plasma (TAC)
The total antioxidant status capacity (TAC) of the plasma was determined using a novel automated measurement method developed by Erel.9 In this method, hydroxyl radical, which is the most potent biological radical, is produced. The assay measures the antioxidative effect of the sample against potent free radical reactions that are initiated by the hydroxyl radical produced. The precision of the assay is excellent and is lower than 3%. The results are expressed as µmol Trolox equivalent/L.
Measuring total oxidant status (TOS)
The total oxidant status (TOS) of serum was determined using a novel automated measurement method as described previously.10 Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction was enhanced by the glycerol molecules present in the reaction medium. The ferric ion produced a colored complex with xylenol orange in acidic solution. The color intensity, which was measured spectrophotometrically, is proportional to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide, and the results were expressed in terms of micromolar hydrogen peroxide equivalents per liter (µmol H2O2 equivalent/L).
Oxidative stress index (OSI)
The percentage ratio of the total peroxide level to the TAC was used as the oxidative stress index (OSI).11 The OSI was calculated as the total peroxide (in µmol/L) divided by the TAC (µmol Trolox equivalent/L) divided by 100. None of the subjects was taking any drug known to affect lipid or lipoprotein metabolism.
Determination of catalase activity
Catalase activity was determined by Goth's colorimetric method, in which supernatant was incubated in H2O2 substrate, and the enzymatic reaction stopped by the addition of ammonium molybdate. The intensity of the yellow complex formed by molybdate and H2O2 was measured at 405 nm.12
Statistical analysis
The SPSS 15 commercial software package was used for statistical analysis. All data were expressed as median ± interquartile range (Median±IQR). Kolmogorov-Smirnov test was used to determine the distribution of studied parameters. The comparisons of parameters were performed using Mann-whitney U test and a p value of < 0.05 was accepted as significant.
Results
Twenty two patients included in the study were suffering from acute fascioliasis. The ratio numbers of the females to males in patients and the control groups were 19/3 and 22/4 respectively. The mean age of the patients was 24.5±18.6 years (range 5–64years) while in the control group was it was 22.2±14.3 years (range 7–72 years). Regarding age and gender, no statistically significant difference was observed between the two groups (P>0.05).
All patients had a history of watercress ingestion. The ratios of malaise, abdominal pain, headache fever, sweating, dyspepsia, nausea-vomiting, itching, dyspnea and weight loss were 100%, 45.8%, 33.3%, 41.7%, 50%, 25%, 16.7%, 33.3%, 75% respectively.
The ELISA test was positive for all patients. 11 patients, the diagnosis of fascioliasis was made by the detection of parasite ova in the stool. Among the patients, the elevated white blood cell, erythrocyte sedimentation rate, gamma-glutamyl transpeptidase (GGT), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were observed in 63.63%, 45.45%, and 18.18%, 27.27%, 95.45% of the patients, respectively. All patients were given a single dose of 10 mg/kg triclabendazole.
There were statistically significant differences between the two groups for TAC, TOS and OSI enzyme activities (table). No statistically significant difference was observed between the two groups for catalase (P>0.05). The serum OSI levels are shown in figure 1.
Table 1.
Serum oxidative status of patients and healty controls
| Parameters | Patients (n=22) | Control (n= 26) | P value |
| Mean±SD | Mean±SD | ||
| TAC | 1.14±0. 13 | 1.29±0.15 | 0.029 |
| TOS | 136.40±37.80 | 10.40±2.43 | <0.001 |
| OSI | 10.8±3.31 | 0.81±0.28 | <0.001 |
| Catalase | 9.33±5.05 | 7.64±3.41 | 0.185 |
TAC: Total antioxidant capacity, TOS: Total oxidant status, OSI: Oxidative stress index
Figure 1.
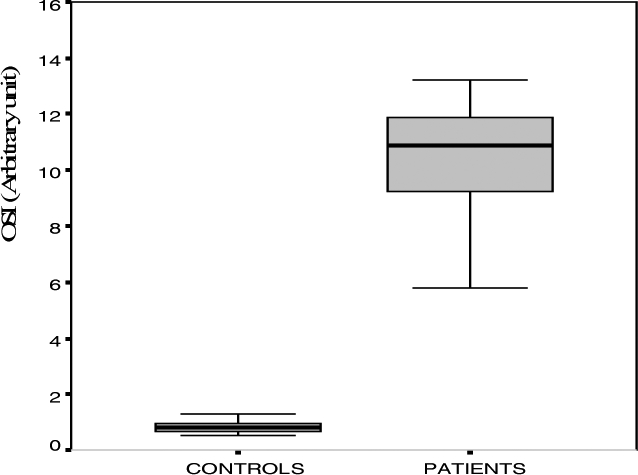
The OSI level of patients and control groups
Discussion
Free oxygen radicals cause protein oxidation, lipid peroxidation and DNA damage13. In some diseases, an increase in reactive oxygen species is not the primary cause of the illness; it develops secondarily to the primary disorder and is involved in pathogenesis14. There are some studies indicating that oxidative stres plays a role in the pathogenesis of viral hepatitis, sepsis, and bacterial infections15–19. Numerous studies demonstrated that in many infectious diseases, a variety of inflammatory cells are activated, which lead to production of reactive oxygen and nitrogen species to kill parasites.20,21 One of theparasites which inhabits in hepatic bile ducts is F. hepatica and causes the disease22.
In order to investigate the effect of F. hepatica on oxidative status, we measured TAC, TOS, OSI and catalase. To the best of our knowledge, there is no prior study investigating these biomarkers of oxidant and antioxidant defense in patients with fascioliasis. Reactive oxygen species (ROS) are produced during many metabolic and physiological processes. Organisms have several antioxidant systems that prevent harmful effects of these ROS. Under certain conditions, antioxidants mechanisms are impaired and/or ROS are increased so antioxidant mechanisms may be deficient to prevent oxidative damage completely. As a result, oxidative stress develops23,24.
Although the level of serum oxidant and antioxidant components can be measured separately, these measurements are time consuming, expensive, impractical and labour intensive. Various measurement methods have been developed to measure total antioxidant status, but there is not yet an accepted reference method25. Antioxidant activities indicate the antioxidant characteristics of only one antioxidant, whereas total antioxidant status represents the sum of all antioxidant characteristics. In this study, a novel automated colorimetric measurement method for TAC and TOS developed by Erel was used. This method is advantageous since it is simple, very cheap, reliable, and very sensitive9,10. Kaya S. et al. have investigated the relationship between fascioliasis and serum malondialdehyde (MDA) levels, superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) activities. They reported that oxidative stress has an important role in the pathogenesis of fascioliasis and the persistence of this oxidative stress can be one of the underlying factors in the pathogenesis of the chronic disease.5 In an experimental F. Hepatica infection in rats, liver tissue antioxidant ability was decreased whereas oxidant capacity was increased, which represents alterations in liver structure and functions.6 In our patints liver function tests (ALT, GGT, ALP), OSI and TOS capacity were increased significantly while TAC was decreased. This condition showsthat parasite causes severedamage in liver, which couldn't have the ability to give antioxidative response. Studies have reported that catalase is a major intracellular antioxidant enzyme. 6,26,27 In our study, catalase activity was found to be unimportantl in fascioliasis since no statistically significant difference was available between patients and control groups (p>0.05). Rehim WM et al. in their study have reported that antioxidative support treatment may be helpful in elimination of F hepatica.7 A decreased TAC and no increased catalase in our study necessitates the need for supportive antioxidative teratment. Similar findings were reported by others such as Kaya et al. in fascioliasis, 6 Melek et al. and Serefhanoglu et al. in brucellosis. 18,28
In the present study TOS and OSI were significantly higher in the patients than controls. OSI is the ratio of the total plasma peroxide level to TAC, and it is an indicator of oxidative stress. It has been suggested that OSI may reflect the state of oxidative status in fascioliasis. Oxidative stress has an important role in the pathogenesis of fascioliasis.
Conclusion
A decreased TAC and increased TOS and OSI levels may lead to considerable oxidative stress in patients with F. hepatica infection. A high oxidative stress occurs during F. hepatica infection, which causes severe damage in the liver and body.
References
- 1.Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull WHO. 1999;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- 2.Maguire JH. Trematodes (Schistosomiasis) and other flukes. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 2005. pp. 3276–3285. [Google Scholar]
- 3.Rim HJ, Farag HF, Sommani S, Cross JH. Food-borne trematodes: ignored or emerging? Parasitol Today. 1994;10:207–209. [Google Scholar]
- 4.Hopkins DR. Homing in on helminths. Am J Trop Med Hyg. 1992;46:626–634. doi: 10.4269/ajtmh.1992.46.626. [DOI] [PubMed] [Google Scholar]
- 5.Kaya S, Sütçü R, Cetin ES, Aridogan BC, Deliba° N, Demirci M. Lipid peroxidation level and antioxidant enzyme activities in the blood of patients with acute and chronic fascioliasis. Int J Infect Dis. 2007;11(3):251–255. doi: 10.1016/j.ijid.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Kolodziejczyk L, Siemieniuk E, Skrzydlewska E. Antioxidant potential of rat liver in experimental infection with Fasciola hepatica. Parasitol Res. 2005;96(6):367–372. doi: 10.1007/s00436-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 7.Rehim WM, Sharaf IA, Hishmat M, el-Toukhy MA, Rawash NA, Fouad HN. Antioxidant capacity in Fasciola hepatica patients before and after treatment with triclabendazole alone or in combination with ascorbic acid (vitamin C) and tocofersolan (vitamin E) Arzneimittelforschung. 2003;53:214–220. doi: 10.1055/s-0031-1297097. [DOI] [PubMed] [Google Scholar]
- 8.Maffei Facino R, Carini M, Genchi C, Tofanetti O, Casciarri I. Participation of lipid peroxidation in the loss of hepatic drug metabolizing activities in experimental fascioliasis in the rat. Pharmacol Res. 1989;21:549–560. doi: 10.1016/1043-6618(89)90196-5. [DOI] [PubMed] [Google Scholar]
- 9.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–566. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 12.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 13.Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O. Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res. 2005;585:71–78. doi: 10.1016/j.mrgentox.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 15.Horoz M, Bolukbas C, Bolukbas FF, Aslan M, Koylu AO, Selek S, et al. Oxidative stress inhepatitis C infectedend-stage renal disease subjects. BMC Infect Dis. 2006;6:114. doi: 10.1186/1471-2334-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draganov D, Teiber J, Watson C. PON1 andoxidative stres in human sepsis and an animal model of sepsis. Adv Exp Med Biol. 2010;660:89–97. doi: 10.1007/978-1-60761-350-3_9. [DOI] [PubMed] [Google Scholar]
- 17.Bayraktar N, Kilic S, Bayraktar MR, Aksoy N. Lipid peroxidation and antioxidant enzyme activities in cancerous bladder tissue and their relation with bacterial infection: a controlled clinical study. J Clin Lab Anal. 2010;24:25–30. doi: 10.1002/jcla.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serefhanoglu K, Taksin A, Turan H, Timurkaynak FE, Arslan H, Erel O. Evaluation of oxidative status in patients with brucellosis. Braz J Infect Dis. 2009;13:249–251. doi: 10.1590/s1413-86702009000400001. [DOI] [PubMed] [Google Scholar]
- 19.Karaagac L, Koruk ST, Koruk I, Aksoy N. Decreasing oxidative stress in response to treatment in patients with brucellosis: could it be used to monitor treatment? Int J Infect Dis. 2011 Mar 2; doi: 10.1016/j.ijid.2011.01.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Gantt KR, Goldman TL, McCormick ML, et al. Oxidative response of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- 21.Murray HW, Teitelbaum RF. l-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992;165:513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- 22.Karahocagil MK, et al. A familial outbreak of fascioliasis in Eastern Anatolia: A report with review of literature. Acta Trop. doi: 10.1016/j.Acta tropica 2008.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3. Oxford: Oxford Science Publications; 1999. [Google Scholar]
- 24.Aruoma OI. Characterization of drugs as antioxidant prophylactics. Free Radic Biol Med. 1996;20:675–705. doi: 10.1016/0891-5849(95)02110-8. [DOI] [PubMed] [Google Scholar]
- 25.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309–1315. [PubMed] [Google Scholar]
- 26.Aslan M, Kosecik M, Horoz M, et al. Assessment of paraoxonase and arylesterase activities in patients with iron deficiency anemia. Atherosclerosis. 2007;191:397–402. doi: 10.1016/j.atherosclerosis.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Ece A, Gürkan F, Kervancioglu M, et al. Oxidative stress, inflammation and early cardiovascular damage in children with chronic renal failure. Pediatr Nephrol. 2006;21:545–552. doi: 10.1007/s00467-006-0039-0. [DOI] [PubMed] [Google Scholar]
- 28.Melek IM, Erdogan S, Celik S, et al. Evaluation of oxidative stres and inflammation in long term Brucella melitensis infection. Molecular and Cellular Biochemistry. 2006;293:203–209. doi: 10.1007/s11010-006-9243-2. [DOI] [PubMed] [Google Scholar]


