Abstract
Objective:
This study aimed at qualitatively evaluating the remineralization potential of casein phosphopeptide amorphous calcium phosphate on artificial early enamel lesions in an ex-vivo scenario by observing the treated tooth surface using a scanning electron microscope (SEM).
Materials and Methods:
This randomized study was conducted on 10 subjects undergoing orthodontic treatment with premolar extraction as part of their treatment. Artificial white lesions were created with the application of 37% phosphoric acid for 20 mins. Teeth were then divided into two groups: one experimental and the other control. Customised orthodontic band with a window was luted with intermediate restorative material in the experimental group whereas in the control group, band without a window was luted. The casein phosphopeptide amorphous calcium phosphate (GC TOOTH MOUSSE) paste was then applied on the window region of the experimental group for 3 mins thrice daily after meals for 14 days, whereas no paste was applied in the control group. After 14 days, teeth were extracted and viewed under an SEM.
Results:
The study groups showed remineralization of the lesions as compared with the control group in most of the samples.
Conclusion:
Casein phophopeptide could significantly remineralize the artificial enamel lesions in vivo.
Keywords: Enamel remineralisation, casein-phosphopeptide amorphous calcium phosphate
Introduction
Dental caries is the localized destruction of the tissues of the tooth by acids, particularly lactic acid, produced by the fermentation of dietary carbohydrates by bacteria in dental plaque.[1] Scientific advances in restorative materials and techniques, as well as in understanding the pathogenesis and prevention of caries, have led to more efficient oral health management.
Efforts have focused on reducing the risk of caries in patients and have highlighted the importance of a partnership approach between patients and dentists in order to ensure ultimate success in the caries control regimen.
A substantial literature now exists demonstrating an anticariogenic effect of diary products (milk, milk concentrates, powders and cheeses). This anitcariogenic effect has been attributed to the multiphosphoseryl-containing sequences of casein. These sequences can be released as casein phosphopeptide from an enzymatic digest of casein. The casein phosphopeptide has a remarkable ability to stabilize calcium phosphate in solution as amorphous calcium phosphate nanocomplexes, thereby allowing the formation of small casein phosphopeptide amorphous calcium phosphate clusters. It has been reported that the average time for remineralization after acid exposure is 14 days.
The proposed anticariogenic mechanism for CCP-ACP is by the localization of amorphous calcium phosphate on the tooth surface, which buffers the free calcium and phosphate ion activities, thereby helping to maintain a state of supersaturation with respect to the tooth enamel and, thus, preventing demineralization and enhancing remineralization.
Modern prospective caries studies require the same scenario as present in the oral cavity and, hence, ex vivo clinical trials can be performed for better evaluation.
This study aimed at qualitatively evaluating the effect of the CCP-ACP paste on remineralization of early enamel lesions in human enamel in an ex vivo environment using a scanning electron microscope (SEM).
Material and Methods
This randomized study was conducted in10 subjects undergoing orthodontic treatment with premolar extraction as part of their treatment. Approval for the study was taken from the Ragas Dental College Ethical Committee and informed consent was taken from all the subjects.
An intraoral examination confirmed that each subject had at least 22 natural teeth with no current caries activity, periodontal disease or other oral pathology. None of the subjects was using antibiotics or medications that could have affected the salivary flow rate. Artificial white lesions were created with the application of 37% phosphoric acid for 20 mins [Figure 1].
Figure 1.
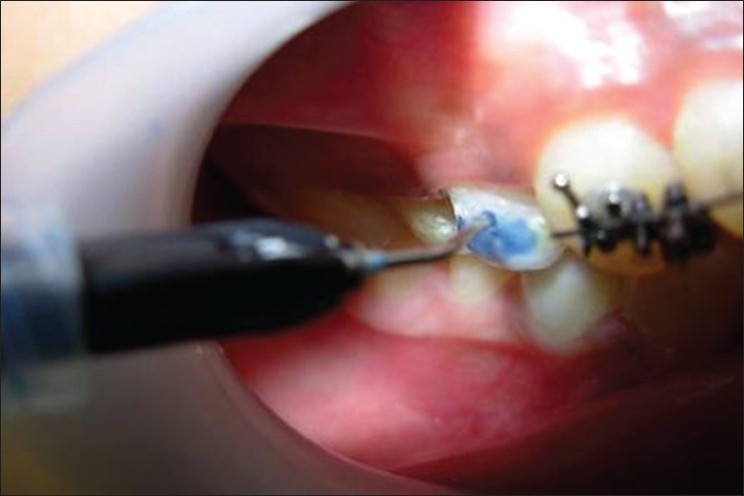
Acid etching performed for 20 min on 14 and 44
The teeth were then divided into two groups: one experimental group and the other control group. Customized orthodontic band with a window was luted with IRM in the experimental group, the dimensions of the window being 4mm × 3 mm, whereas in the control group, band without a window was luted [Figure 2].
Figure 2.
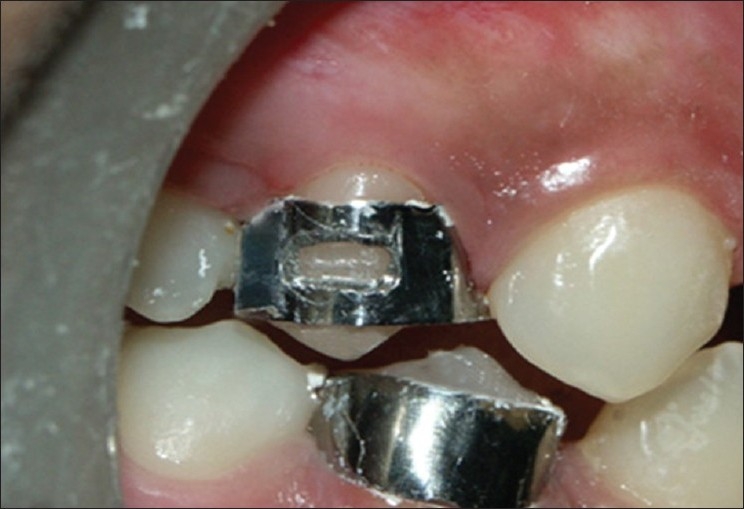
Upper premolar (14)-:experimental group; lower premolar (44)-:control group
The subjects were then called daily and CPP-ACP (GC TOOTH MOUSSE) paste was applied for 3 mins thrice daily after meals for 14 days on the part of the tooth exposed in the window. However, no paste was applied in the control group, which was fully covered with the band for 14 days [Figure 3].
Figure 3.
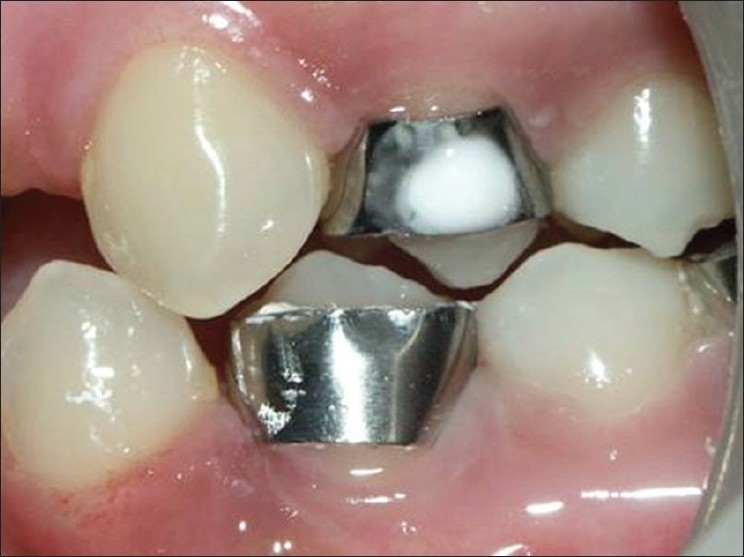
Application of GC TOOTH MOUSSE paste in the experimental-group(tooth-14)
For assessing demineralization and remineralization, the teeth were extracted after 14 days and embedded in an acrylic block. Horizontal 1mm-thick sections were made using hard tissue microtome and the sections were then examined under an SEM.
Results
SEM was used to determine the morphological variations between the treated surface and the untreated surface. Demineralization of the enamel surface was more pronounced in the control group and the enamel revealed SILVERSTONE TYPE-1 type of acid dissolution. In the experimental group, microscopic surface irregularities on the enamel were observed as adherent granules or globules. These represent the redeposited mineral phases following mobilization of calcium and phosphate from the CCP-ACP [Figures 4 a,b and 5 a,b].
Figure 4a.

Case1-control group (shows the demineralised enamel surface)
Figure 4b.
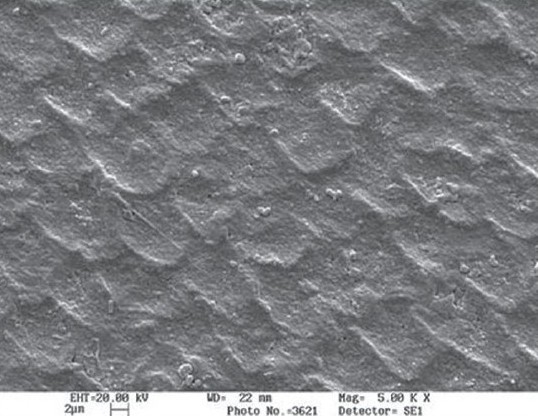
Case1-experimental-group (mineral phase deposited after application of CPP-ACP paste)
Figure 5a.

Case 2-control group (demineralised surface)
Figure 5b.

Case 2-Experimental group (after application of CPP-ACP paste)
Discussion
Although human extracted teeth are generally used for in vitro studies for the simulation of the oral environment, an ex -vivo clinical trial can be performed for better evaluation rather than simulating in -vivo conditions extra-orally.
The experimental parameters that must be considered in this type of experiment are the study design, the type of substrate and the method that is used to assess the mineral status. Each parameter should be carefully considered in relation to the objective of the research.[1]
This study was conducted on subjects undergoing orthodontic treatment with premolar extractions as part of their treatment in order to standardize the age group of the subjects and to produce artificial enamel lesions on non-carious teeth that could be later extracted for evaluation. The dimensions of the window were kept as 4 mm × 3 mm to standardize the size of the window. The band was luted with IRM so that no other source of remineralization could be present except for the CCP-ACP paste and the saliva.
This study revealed that 10% CCP-ACP paste remineralized early enamel lesions in human enamel in -vivo and that significant alterations in the surface morphologies of the enamel were seen in 14 days of the experiment. As thrice application of the paste for 3 min for 14 days revealed remineralization, it could be inferred that the remineralization was dose dependent.[2–4]
The role of CCP-ACP has been described as localization of ACP on the tooth surface, which buffers the free calcium and phosphate ion activities, helping to maintain a state of supersaturation with respect to the enamel by suppressing demineralization and enhancing remineralization.[5,6]
The CCP-ACP has a substantial ability to stabilize calcium -phosphate in the solution. In neutral and alkaline supersaturated calcium and phosphate solutions, ACP nuclei are formed spontaneously. It is proposed that the peptide binds to the forming ACP nanoclusters producing a metastable solution preventing ACP growth to the critical size required for nucleation and phase transformation.[7,8]
The remineralization process involves diffusion of calcium and phosphate ions through the protein/water-filled pores of the carious surface of enamel into the body of the enamel lesion. Once in the body of the enamel lesion, these calcium and phosphate species increase the activities of the calcium and phosphate ions, thereby increasing the degree of supersaturation with respect to the hydroxyapatite crystals. The formation of hydroxyapatite in the lesion would lead to the generation of acid and phosphate, which would diffuse out of the lesion and down a concentration gradient.[9,10]
The CCP, by stabilizing the calcium phosphate in a metastable solution, facilitates high concentrations of calcium and phosphate ions, which can diffuse into the enamel subsurface lesions. The bound ACP, by being in dynamic equilibrium with free calcium and phosphate ions, will maintain the concentrations of the species involved in diffusion into the lesion. CPP-supported metastable calcium phosphate solutions are such efficient remineralizing solutions as they would consume the acid generated during enamel lesion and would aid in remineralization by generating more calcium and phosphate ions, thus maintaining their high concentration gradients into the lesion.[11,12]
The remineralization treatment regimen of 3 min twice-daily application was employed as per the manufacturer's recommendations to make it clinically relevant.
In the oral environment, host factors such as the mineral concentration of the tooth and pellicle and plaque formation can influence the progression of demineralization. Salivary factors such as the salivary flow rate, composition and buffering capacity might exert a protective action on the dental surfaces.[12] Enhancing the demineralization capability of saliva is important from the clinical point of view. Therefore, the influence of CCP-ACP on the prevention of demineralization observed in the present study would be clinically beneficial.
Conclusion
Within the limitations of this ex vivo study, the following conclusion was drawn:
The CCP-ACP paste was effective in preventing demineralization of the enamel more effectively. Further studies are needed to determine the amount of mineral loss or gain from the tooth under in vivo conditions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cummins D. The impact of research and development on the prevention of oral diseases in children and adolescents: An industry perspective. Pediatr Dent. 2006;28:118–27. [PubMed] [Google Scholar]
- 2.Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralisation of enamel subsurface lesions by sugar free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–70. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 3.Oshiro M, Yamaguchi K, Takamizawa T, Inage H. Effect of CCP-ACP Paste on tooth mineralization: An FE-SEM study. J Oral Sci. 2007;49:115–20. doi: 10.2334/josnusd.49.115. [DOI] [PubMed] [Google Scholar]
- 4.Rees J, Loyn T, Chadwick B. Pronamel and tooth mousse: An initial assessment of erosin prevention in vitro. J Dent. 2007;35:355–7. doi: 10.1016/j.jdent.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds EC. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: A review. Spec Care Dentist. 1998;18:8–16. doi: 10.1111/j.1754-4505.1998.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 6.Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC. Physicochemical characterization of casein phosphopeptide amorphous calcium phosphate nanocomplexes. J Biol Chem. 2005;280:15362–9. doi: 10.1074/jbc.M413504200. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds EC. Remineralisation of enamel subsurface lesions by casein phosphopeptide stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 8.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide- amorphous calcium phosphate on remineralization of artificial caries-like lesions: an in vitro study. Australian Dent J. 2008;53:34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 9.Gordon Nikiforuk. Understanding of dental caries-etiology mechanism & prevention basic clinical aspects volume 1 & 2 [Google Scholar]
- 10.Grenby TH, Andrews AT, Mistry M, Williams RJ. Dental caries protective agents in milk and milk products: investigations in vitro. J Dent. 2001;29:83–92. doi: 10.1016/s0300-5712(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 11.Cai F, Shen P, Morgan MV, Reynolds EC. Remnimeralization of enamel subsurface lesions in situ by sugar free lozenges containing casein phosphopeptide amorphous calcium phosphate. Australian Dent J. 2003;48:240–3. doi: 10.1111/j.1834-7819.2003.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 12.Marsh PD. Microbiologic aspects of dental plaque and dental caries. Dent Clini North Am. 1999;43:599–614. [PubMed] [Google Scholar]


