Abstract
Aims:
To compare the effect of Manuka honey, chlorhexidine gluconate (0.2%) mouthwash and xylitol chewing gum on the dental plaque levels.
Materials and Methods:
Sixty healthy male dental students aged between 21 and 25 years (mean age 23.4 years) participated in the study. All the subjects received a professional prophylaxis at the start of the study, with the purpose of making the dentition 100% free of plaque and calculus. The subjects were then randomly divided into three groups, i.e. the Manuka honey group, the chlorhexidine gluconate mouthwash group and the xylitol chewing gum group. Rinsing with water or any other fluid after the procedure was not allowed as also any form of mechanical oral hygiene for all the subjects during the experimental period of 72 h. After the experimental period, the plaque was disclosed using disclosing solution and their scores were recorded at six sites per tooth using the Quigley and Hein plaque index modified by Turesky-Gilmore-Glickman.
Results:
The mean plaque scores for Groups I, II and III were 1.37, 1.35 and 1.57, respectively. The ANOVA revealed that between-group comparison was significant, with an F-value of 5.99 and a probability value of 0.004. The T-test was carried out to evaluate the inter-group significance, which revealed that the plaque inhibition by Manuka honey was similar to that of chlorhexidine mouthwash. Both Manuka honey and chlorhexidine mouthwash reduced plaque formation significantly, better than the xylitol chewing gum.
Conclusion:
Manuka honey and chlorhexidine mouthwash reduced plaque formation significantly better than xylitol chewing gum.
Keywords: Chlorhexidine, Manuka honey, plaque Score, xylitol
Introduction
The most prevalent infectious oral diseases in humans, caries and periodontal disease are associated with dental plaque. The removal of bacterial biofilm is a decisive component in the prevention and treatment of these diseases. The use of mechanical agents is a simple and cost-effective method that has been demonstrated to be efficient in the control of gingivitis.[1] The effectiveness of this method, however, is influenced by the individual's manual dexterity and motivation. Because of the difficulty to ensure adequate removal of plaque by mechanical means, there is a great interest in the use of antimicrobial agents to replace or to be adjuncts to the mechanical approaches.
Chlorhexidine (CHX) is one of the most effective antimicrobial agents for plaque control.[2,3] Rinsing for 60 s twice a day with 10 ml of 0.2% chlorhexidine gluconate solution in the absence of normal tooth cleaning inhibited plaque regrowth and the development of gingivitis.[4]
There is a large amount of evidence to support the use of xylitol in the form of chewing gum as an anti-caries and anti-plaque agent.[5] The chewing of any gum stimulates saliva flow, which increases the buffering capacity of saliva and, thus, neutralizes the reduction in plaque pH that normally follows eating.[6] The first chewing gum developed with the aim of reducing caries and improving oral health was released in Finland in 1975 and in the United States shortly thereafter. The first xylitol studies in humans, known as the Turku Sugar Studies, demonstrated the relationship between dental plaque and xylitol as well as the safety of xylitol for human consumption.[7] The most comprehensive study with xylitol gum, conducted in 1995, compared the effect on caries incidence for xylitol, sorbitol and sucrose consumption.[8] The group that received 100% xylitol gum five times/day had significantly lower levels of sucrose and free sialic acid in whole saliva than at baseline and significantly lower plaque index scores.
Honey has been used to treat infected wounds since as long as 2000 years before bacteria were discovered to be the cause of infection. The antibacterial property of honey was first recognized in 1892 by Van Ketel. It has often been assumed that this is due entirely to the osmotic effect of its high sugar content. The fact that the antibacterial properties of honey increased when diluted was clearly observed and reported in 1919.[9] The explanation for this apparent paradox came from the finding that honey contains an enzyme that produces hydrogen peroxide when diluted. This agent was referred to as “inhibine” prior to its identification as hydrogen peroxide.
In some honey treated with catalase to remove hydrogen peroxide activity, additional non-peroxide anti-bacterial factors have been identified, e.g. Manuka honey from New Zealand is associated with an unidentified phytochemical component. Manuka honey is a monofloral honey obtained from the species Leptospermum scoparium and has a long-standing reputation in New Zealand folklore for its antiseptic properties.[10]
Hence, the present study was undertaken to compare the effect of Manuka honey, chlorhexidine gluconate mouthwash (0.2%) and xylitol chewing gum on the dental plaque levels.
Materials and Methods
Sixty healthy male dental students aged between 21 and 25 years (mean age 23.4 years) participated in the study. The selection criteria were: no removable or fixed dental prosthetics, no extensive cervical restorations and a minimum of five evaluable teeth per quadrant. At the start of the study, all subjects were given oral and written instructions and information about the product and purpose of the study. The written consent for the study from the volunteers and the approval from the ethical committee of the institution were obtained.
The study was designed as a single-blind experiment. All the subjects received a professional prophylaxis at the start of the study, with the purpose of making the dentition 100% free of plaque and calculus. This was realized by using hand instruments and rotating cups and brushes with fluoride-free polishing paste. After the calculus was removed, the plaque was stained with an erythrosine disclosing solution applied with cotton buds. All visible plaque was removed. Next, the erythrosine disclosing solution was used again to make sure that all the plaque was removed. Finally, unwaxed floss was used for a professional interdental cleaning. The subjects were then randomly divided into three groups, i.e. the Manuka honey group, the chlorhexidine group and the xylitol chewing gum group.
In the Manuka honey group, the subjects were trained to apply the honey gently into the gingival sulcus of all the teeth, wait for 5 min and then repeat the procedure twice. The honey was applied twice a day after meals.
In the chlorhexidine group, the subjects received one bottle of 0.2% chlorhexidine and were trained to rinse twice a day with 10 ml for 60 s. After reaching the exact-rinsing time, the subjects had to expectorate the mouth rinse. A written instruction on how to use the mouthrinse was included.
In the xylitol chewing gum group, the subjects were instructed to chew the sugarless gum for 5 min, thrice a day after meals.
Rinsing with water or any other fluid after the procedure was not allowed as also any form of mechanical oral hygiene for all the subjects during the experimental period of 72 h.
After the experimental period, the plaque was disclosed using disclosing solution and the scores were recorded at six sites per tooth using the Quigley and Hein plaque index modified by Turesky-Gilmore-Glickman. All measurements were carried out under the same circumstances using the same batch of disclosing solution.
Results
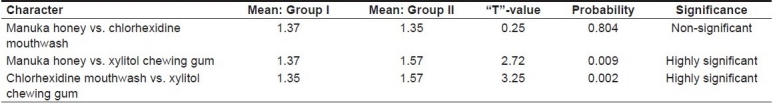
The mean plaque scores for Groups I, II and III were 1.37, 1.35 and 1.57, respectively, as shown in Table 1. ANOVA revealed that the between-group comparison was significant, with an F-value of 5.99 and a probability value of 0.004 [Table 2]. T test was performed to evaluate the inter-group significance, which revealed that the plaque inhibition by Manuka honey was similar to that of chlorhexidine mouthwash [Table 3]. Both Manuka honey and chlorhexidine mouthwash reduced the plaque formation significantly better than the xylitol chewing gum.
Table 1.
Mean and range of plaque scores in different groups
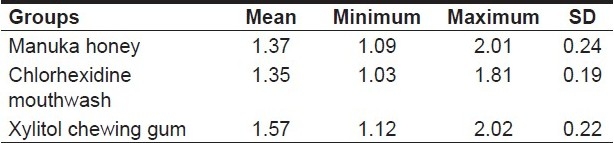
Table 2.
Analysis of variance for plaque scores of different groups
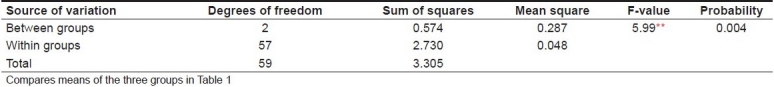
Table 3.
“T”-values for plaque scores of the different groups
Discussion
Dental plaque is considered to be a complex, metabolically interconnected, highly organized bacterial system consisting of dense masses of microorganisms embedded in an inter-microbial matrix. The pellicle, which is an organic bacteria-free film, deposits on the tooth surfaces within nanoseconds after vigorous tooth brushing or polishing. Then, the bacteria start to colonize the tooth surface. The dental plaque, in sufficient concentration, can disturb the host–parasite relationship and cause dental caries and periodontal disease. The plaque thickness differs depending on the locally prevailing oral cleansing forces, oral hygiene and other factors such as salivary components.[11]
Chlorhexidine gluconate (0.2%) is retained in the oral cavity and is progressively desorbed in bacteriostatic concentrations 8–12 h after rinsing.[12] The recent studies suggest that the chlorhexidine molecule, being dicationic, attaches to the pellicle by one cation leaving the other free to interact with bacteria attempting to colonize the tooth surface.[13] Chlorhexidine gluconate has not been proven to reduce the subgingival calculus and, in some studies, it actually increased the deposits. When combined with xylitol, a synergistic effect has been observed to enhance the efficacy.[14]
Xylitol is not fermented by cariogenic plaque bacteria and, thus, does not lower the pH of plaque. Xylitol reduces the accumulation of plaque on the tooth surface. Because the plaque pH does not drop when xylitol-sweetened gum is chewed, remineralisation is enhanced. Regular chewing of xylitol-sweetened gum has specific inhibiting effects on the growth of mutans streptococci in the mouth. This suggests that there may be permanent reductions in oral mutans streptococci levels from this practice.[5]
Xylitol currently is available in many forms such as gums, mints, chewable tablets, lozenges, toothpastes, mouthwashes and cough mixtures.[15] Xylitol chewing gum has been shown to be effective as a preventive agent; however, the usefulness of other xylitol products that have not been studied is uncertain and cannot be recommended at this time because both the delivery system and the dose/frequency of use impact the effectiveness of the products.
A community trial was conducted in a total of 921 children. The oral health status in the xylitol group was a little bit better than that in the control group. The use of xylitol can therefore be recommended, especially if the personnel do not have the possibility of supervising the brushing.[16]
The maximum effect of sugarless gum chewing occurs when it is chewed three times a day directly after meals.[5]
Manuka honey has a phytochemical component and a low hydrogen peroxide component. The non-peroxide antibacterial activity of typical Manuka honey was tested against seven species of bacteria and compared with typical honey with a hydrogen peroxide component. The minimum inhibitory concentration MIC of honey was found to range from 1.8 to 10.8%.[10] Types of honey differ greatly in their antimicrobial potency, varying as much as a 100-fold. The research has shown that honey not only stops the growth of dental plaque bacteria but also reduces the amount of acid produced, which stops the bacteria from producing dextran. The factors involved in gingivitis and periodontal diseases are very similar to those in inflamed and infected wounds. The honey rapidly clears bacteria from infected wounds, even when the infection is deep seated. However, unlike some other antiseptics, honey is gentler on tissue. The potent and anti-inflammatory property of the honey rapidly reduces the pain and inflammation. Honey also has a marked stimulatory effect on the growth of cells that repair the tissues damaged by infection.
By analogy with the familiar sunscreen protection factor rating (SPF), a “UMF” rating is used on the labels of Manuka honey (“UMF” is the “unique Manuka factor” – the non-peroxide antibacterial activity). The numbers used in the “UMF” rating are the concentration of phenol with the same antibacterial activity as the honey. (E.g. “UMF 15” honey has the same activity against the S. aureus test species as a solution of 15% phenol.) “UMF” was registered as a trademark by the producers of the active Manuka honey to stop its misuse with testing standards having to be complied with for permission to be granted for use of the trademark. It has been shown that Manuka honey with an antibacterial activity rated Unique Manuka Factor (UMF ×15) could be used to reduce dental plaque and clinical levels of gingivitis. When using honey, consideration needs to be given to its quality and antibacterial activity.[10]
However, the results of the present study suggest that Manuka honey has a potential therapeutic role in the treatment of gingivitis and periodontal disease. Further evidence and understanding of its therapeutic and chemical properties is needed to optimize its use in the clinical management of periodontal disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Beltrami M, Bickel M, Baehni PC. The effect of Supragingival plaque control on the composition of the subgingival microflora in human periodontitis. J Clin Periodontol. 1987;14:161–4. doi: 10.1111/j.1600-051x.1987.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 2.Bral M, Brownstein CN. Antimicrobial agents in the prevention and treatment of periodontal diseases. Dent Clin North Am. 1988;32:217–41. [PubMed] [Google Scholar]
- 3.Lang NP, Brecx MC. Chlorhexidine digluconate - an agent for chemical plaque control and prevention of gingival inflammation. J Periodontal Res. 1986;21:74–89. [Google Scholar]
- 4.Loe H, Schiott CR. The effect of mouthrinse and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 5.Burt BA. The use of sorbitol and xylitol sweetened chewing gum in caries control. J Am Dent Assoc. 2006;137:190–6. doi: 10.14219/jada.archive.2006.0144. [DOI] [PubMed] [Google Scholar]
- 6.Birkhed D, Svensäter G, Edwardsson S. Cariological studies of individuals with long-term sorbitol consumption. Caries Res. 1990;24:220–3. doi: 10.1159/000261269. [DOI] [PubMed] [Google Scholar]
- 7.Scheinin A, Makinen KK, Ylitalo K. Turku sugar studies. V. Final report on the effect of sucrose, fructose and xylitol diets on caries incidence in man. Acta Odontol Scand. 1976;34:179–216. doi: 10.3109/00016357608997711. [DOI] [PubMed] [Google Scholar]
- 8.Mäkinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR, Jr, et al. Xylitol chewing gums and caries rates: A 40-month cohort study. J Dent Res. 1995;74:1904–13. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- 9.Sackett WG. Honey as a carrier of intestinal diseases. Bull Colorado State Univ Agric Exp Stn. 1919;252:1–18. [Google Scholar]
- 10.English HK, Pack AR, Molan PC. The effects of Manuka Honey on Plaque and Gingivitis: A Pilot Study. J Int Acad Periodontol. 2004;6:63–7. [PubMed] [Google Scholar]
- 11.Koparal E, Tütüncü R. Investigation of Plaque Formation by Scanning Electron Microscopy. Turk J Med Sci. 2000;30:119–24. [Google Scholar]
- 12.Rolla G, Löe H, Schiott CR. Retention of chlorhexidine in the human oral cavity. Arch Oral Biol. 1971;16:1109–16. doi: 10.1016/0003-9969(71)90215-9. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins S, Addy M, Wade W. In vivo the mechanism of action of chlorhexidine: a study of plaque growth on enamel inserts. J Clin Periodontol. 1988;15:415–24. doi: 10.1111/j.1600-051x.1988.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 14.Decker EM, Maier G, Axmann D, Brecx M, von Ohle C. Effect of xylitol/chlorhexidine versus xylitol or chlorhexidine as single rinses on initial biofilm formation of cariogenic streptococci. Quintessence Int. 2008;39:17–22. [PubMed] [Google Scholar]
- 15.Lynch H, Milgrom P. Xylitol and dental caries: An overview for clinicians. J Calif Dent Assoc. 2003;31:205–9. [PubMed] [Google Scholar]
- 16.Kovari H, Pienihakkinen K, Alanen P. Use of xylitol chewing gum in daycare centers: a follow-up study in Savonlinna, Finland. Acta Odontol Scand. 2003;61:367–70. doi: 10.1080/00016350310007806. [DOI] [PubMed] [Google Scholar]


