Abstract
Homeostasis of metal ions such as Zn2+ is essential for proper brain function. Moreover, the list of psychiatric and neurodegenerative disorders involving a dysregulation of brain Zn2+-levels is long and steadily growing, including Parkinson’s and Alzheimer’s disease as well as schizophrenia, attention deficit and hyperactivity disorder, depression, amyotrophic lateral sclerosis, Down's syndrome, multiple sclerosis, Wilson’s disease and Pick’s disease. Furthermore, alterations in Zn2+-levels are seen in transient forebrain ischemia, seizures, traumatic brain injury and alcoholism. Thus, the possibility of altering Zn2+-levels within the brain is emerging as a new target for the prevention and treatment of psychiatric and neurological diseases. Although the role of Zn2+ in the brain has been extensively studied over the past decades, methods for controlled regulation and manipulation of Zn2+ concentrations within the brain are still in their infancy. Since the use of dietary Zn2+ supplementation and restriction has major limitations, new methods and alternative approaches are currently under investigation, such as the use of intracranial infusion of Zn2+ chelators or nanoparticle technologies to elevate or decrease intracellular Zn2+ levels. Therefore, this review briefly summarizes the role of Zn2+ in psychiatric and neurodegenerative diseases and highlights key findings and impediments of brain Zn2+-level manipulation. Furthermore, some methods and compounds, such as metal ion chelation, redistribution and supplementation that are used to control brain Zn2+-levels in order to treat brain disorders are evaluated.
Keywords: Alzheimer’s disease, ion chelators, nanoparticles, postsynaptic density, amyotrophic lateral sclerosis, zinc, nutrition, dietary zinc, epilepsy
1. INTRODUCTION
The metal-ion content in the brain is surprisingly high compared to other tissues [1], with Zn2+ and Fe2+ as prevalent metals. In particular, since the first discovery in 1955, Zn2+ has been known to be highly enriched in the hippocampus [2] and neocortical (150 – 200 µM [3, 4]) region of the mammalian brain [5]. Zn2+ is necessary for the maturation and function of the brain and a dysregulation of brain Zn2+-levels is seen in many psychiatric and neurological diseases like Parkinson’s [6–8] and Alzheimer’s disease [6, 9–11], schizophrenia [12, 13], ADHD [14–16], mood disorders [17–20], amyotrophic lateral sclerosis (ALS) [21], Down's syndrome [22], multiple sclerosis [23, 24], epilepsy [25–27], Wilson’s disease [28, 29] and Pick’s disease [30].
Zinc plays a role in synaptic transmission and serves as an endogenous neuromodulator. Moreover, Zn2+ is important for postsynaptic density (PSD) stability, such that PSDs contain a Zn2+ concentration of 4.1 nmol per mg protein [31, 32]. Zn2+ levels are also important for nucleic acid metabolism and brain microtubule growth [1]. Zn2+ is selectively stored in, and released from, glutamatergic presynaptic vesicles [33]. Vesicular Zn2+ that is co-released with neurotransmitters elevates Zn2+ concentrations in the synaptic cleft from approximately 10 nM to 300 µM [34, 35]. This Zn2+ then binds to neurotransmitter receptors [36], such as the NMDA subtype of glutamate receptor (NMDAR) [37, 38] or enters the postsynaptic cell via various routes, including ion channels [39]. Zn2+ deprivation affects Zn2+ homeostasis in the brain and the reduced levels of Zn2+ in the hippocampus lead to brain dysfunctions and learning impairment.
Over the past decades, a vigorous scientific debate has occurred on the role of Zn2+ within the healthy brain and especially in neurodegenerative disorders [40–48]. Therapies attempting to regulate Zn2+-levels by preventing release, blocking ion channels, supplementing Zn2+ and buffering Zn2+ concentration within the brain have played increasingly important roles in the treatment of diverse neurological and neuropsychiatric diseases [41]. Controlled and regional delivery of Zn2+ to the brain is highly desirable and an important direction for future research including the delivery of drugs. However, there are only few studies that deliver or delete Zn2+ in specific brain areas, and though some methods and compounds are available to achieve this task, the vast majority of studies manipulate Zn2+ levels by dietary supplementation or restriction. In the present review, we explore the role of Zn2+ in a variety of disorders and highlight recent therapeutic approaches designed to modulate Zn2+ levels in the brain. We also review strategies for modulating Zn2+ levels and delivery into the brain.
2. DYSREGULATION OF BRAIN Zn2+-LEVELS IN PSYCHIATRIC AND NEURODEGENERATIVE DISORDERS
2.1. Alzheimer’s Disease, Parkinson’s Disease and Pick’s Disease
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are two neurodegenerative disorders that occur in an age-related manner. AD is characterized by the abnormal intracellular accumulation of the amyloid beta (Aβ) protein [49] and/or its assembly into paired helical filaments and extracellular accumulation in plaques. Possible causes of AD include increased levels of oxidative stress in the AD brain, as well as the sequestration of Zn2+ ions within amyloid plaques. Intriguingly, Zn2+ can induce Aβ monomers to aggregate in different forms [50, 51], and is known to bind Aβ via its histidine imidazole rings and accumulate within senile plaques [52, 53]. This led Adlard et al. to propose that Aβ causes cognitive impairment by trapping synaptic Zn2+ rather than through direct toxicity [53]. Functionally, Zn2+ trapping by Aβ likely resembles phenotypes observed in loss of function studies of ZnT3 (a vesicular Zn2+ transporter) [53]. ZnT3 knockout mice exhibit a complete absence of Zn2+ from synaptic vesicles throughout the brain [54], and show dramatic synaptic and memory deficits similar to those seen in APP transgenic mice, a model for AD [53].
Intriguingly, serum Zn2+ concentrations were found to be significantly decreased in AD patients compared to control subjects [9]. Moreover, in an AD mouse model, Zn2+ supplementation greatly delayed hippocampal-dependent memory deficits and strongly reduced Aβ pathology in the hippocampus [55]. Given that increased brain Zn2+-levels enhance plaque formation, Zn2+ was regarded as disease promoting in the past. However, as clustering of Aβ is mediated by Zn2+ ions, drugs with metal chelating properties are expected to produce a significant reversal of plaque deposition in vitro and in vivo [43]. Yet, there is emerging evidence that Aβ plaques are actually non-toxic deposits within the brain that may even be protective compared to protofilamentous Aβ. Clearly additional research is needed to resolve the role of Zn2+ in Alzheimer’s disease and more completely explore the potential benefits of Zn2+ supplementation.
In Parkinson’s disease (PD), α-synuclein aggregates in intracellular inclusions called Lewy bodies, which are associated with the degeneration of dopaminergic neurons in the substantia nigra pars compacta. Patients with PD show a significant decrease in Zn2+ levels compared to control subjects [56]. Oxidative stress is implicated as a major causative factor for PD. However, oxidative stress is hard to separate from other facets of the degenerative processes, including mitochondrial dysfunction, excitotoxicity, nitric oxide toxicity and inflammation [57]. Intriguingly, altered production of nitric oxide is thought to directly influence Zn2+ levels. Moreover, in a Drosophila PD disease model, Zn2+ supplementation greatly improves the phenotype of the flies [58].
Pick's disease is a relatively rare form of dementia. Similar to AD and PD, Pick's disease is marked by the accumulation of randomly oriented filaments of tau proteins called “Pick bodies”. However, these Pick bodies differ markedly from neurofibrillary tangles associated with Alzheimer's disease [59]. Pick's disease eventually leads to the gradual shrinking of brain cells and is associated with changes in personality, including socially inappropriate behavior, poor decision-making skills and eventually a decline in memory as well as ability to speak coherently. Postmortem studies of patients with Pick’s disease revealed that the hippocampus had higher levels of atomic zinc, as well as stronger Timm’s staining – a method that detects Zn2+ and heavy metals [60], as compared to control or Alzheimer’s disease (AD) patients [30, 61]. Moreover, Pick’s disease patients have increased Zn2+ levels in blood cells and urine. Thus, in contrast to AD and PD, an excess of Zn2+ might contribute to the pathogenesis of Pick’s disease.
2.2. Depression
A correlation between Zn2+ deficiency and clinical depression has been demonstrated in both clinical studies and in animal models [17, 18]. Clinical depression is often accompanied by lower serum Zn2+ concentrations [19, 20, 62] and Zn2+ deficiency is able to cause depression- and anxiety-like behaviors in humans, whereas Zn2+ supplementation has been used to treat depression. Intriguingly, a correlation between Zn2+ deficiency and severity of depression has also been shown, such that patients suffering from major depression had significantly lower serum Zn2+ levels than non-depressed controls. Furthermore, patients with minor depression had intermediate levels of Zn2+ [63]. In a group of depressed female students, serum Zn2+ levels were inversely correlated with depression severity [64]. Moreover, severity of depressive symptoms and decreased serum Zn2+ concentration were also correlated in women with postpartum depression [65]. Intriguingly, very low doses of Zn2+ administered together with very low, otherwise ineffective doses of the antidepressant drugs imipramine or citalopram, enhanced their antidepressant-like effect [66, 67]. Additionally, dietary Zn2+ supplementation was shown to be potentially effective in reducing anger and depression based on the evaluation with the Profile of Moods State (POMS) in a group of women who underwent Zn2+ supplementation [68].
2.3. ADHD and Schizophrenia
Attention deficit and hyperactivity disorder (ADHD) is, characterized by attention - problems and hyperactivity, with symptoms presenting usually before seven years of age [14]. Although there are many theories about the possible causes of ADHD, one of the prevalent theories involves impaired dopaminergic signaling. This is supported by the observation of beneficial effects found in patients treated with dopamine agonists like methylphenidate or amphetamines [14]. Intriguingly, many children with ADHD have lower Zn2+ levels compared to healthy children [16]. Moreover, Zn2+ supplementation, combined with methylphenidate, had favorable affects on the treatment of children with ADHD. Although a dysregulation of brain Zn2+-levels might not be a direct underlying cause of ADHD, the effects of Zn2+ supplementation as an ADHD therapy are currently being explored in ADHD children with low plasma Zn2+ concentrations.
Schizophrenia is a chronic psychiatric disorder characterized by a disruption in cognition and behavior that is likely caused by a combination of genetic and environmental factors that are not yet understood. In 1973, McLardy observed a 30% deficit of brain Zn2+ content in individuals with early onset schizophrenia [69]. Since then, other researchers have obtained similar results from postmortem patient brains showing dramatic decreases in hippocampal Zn2+ with up to a 50% reduction in schizophrenic patients [70].
2.4. Epilepsy
Zinc ions appear to play an ambiguous role in epilepsy. There is good reason to believe that excessive intracellular Zn2+ contributes to neurodegeneration [25] and that Zn2+ entry into neurons is facilitated by synaptic activity and mediates the selective neuronal cell death that occurs following global ischemic insults or prolonged seizures [47]. Indeed, some researchers have noted an increase in brain Zn2+ levels following a seizure in rats and mice. However, the contribution of Zn2+ to the etiology and manifestation of epileptic seizures is less clear [71]. Zn2+ can influence neuronal transmission by multiple mechanisms, such that Zn2+ may act to attenuate the GABA response, thereby eliciting hyperexcitability in neurons and ultimately triggering a seizure. Conversely, Zn2+ may also act as an inhibitory neurotransmitter decreasing voltage-dependent potassium currents and excitatory neurotransmitter release [72] increasing the likelihood of a seizure [73]. Additionally, intracellular modulatory affects of Zn2+ are likely to occur [74]. Not surprisingly, Zn2+ has been reported to have proconvulsant [75] as well as anticonvulsant [76] affects, such that seizure susceptibility is decreased by dietary Zn2+ supplementation in an epilepsy mouse model, and conversely increased by dietary Zn2+ deficiency [77]. Since these mice exhibit significant decreases in Zn2+ concentration within the hippocampal dentate gyrus [78], a role for Zn2+ dysregulation in the pathophysiology of convulsive seizures was suggested. However, systemic Zn2+ supplementation or depletion might lead to brain Zn2+ levels that are hard to predict and control, adversely affecting areas outside the seizure foci. Recent studies have elegantly used Zn2+ infusion into the hippocampus, hinting towards an anticonvulsant affect of Zn2+ in epilepsy [79]. This conclusion is consistent with studies showing that ZnT3 knockout-mice [80] and mice on a Zn2+-deficient diet [81,82] were more susceptible to kainic acid induced seizures. Taken together, a connection between Zn2+ and seizures is highly probable, though only a minority of studies suggests a proconvulsant effect of Zn2+. Clearly, additional studies are necessary to tease apart the apparent contradictions.
3. Zn2+ DEPLETION FROM AND DELIVERY TO THE BRAIN
3.1. Beneficial Effects of Zn2+ Supplementation
Several studies reported beneficial effects of Zn2+ supplementation in the treatment of neurodegeneration. Moreover, Zn2+ has been successfully employed to treat specific types of schizophrenia and Wilson’s disease, a disease characterized by psychosis and hallucinations caused by Cu2+ overload [83]. A Zn2+ supplement called “Ziman drops” (10% zinc sulfate with 0.5% manganese chloride) was administered to patients suffering from schizophrenia who had low serum Zn2+-levels [13]. Subsequent studies showed that along with normalizing biochemical abnormalities, the patients demonstrated significant mental and physical improvements [13]. Moreover, Zn2+ administration improved the efficacy of antidepressant drugs in depressed patients [84, 85]. Thus, Zn2+ supplementation may be important in the treatment of depression [86–91]. Furthermore, individuals with ADHD receiving a dose of 55 mg zinc sulfate per day, which is equivalent to 15 mg Zn2+, in addition to methylphenidate showed beneficial effects of Zn2+ supplementation, confirming the role of Zn2+ deficiency in the etiopathogenesis of ADHD [15]. However, 15 mg zinc per day is higher than the Recommended Dietary Allowance (RDA) for zinc, thereby raising the question of how much Zn2+ is needed to obtain beneficial affects in the treatment of psychiatric and neurodegenerative disorders. Moreover, although systemic Zn2+ supplementation was shown to have beneficial effects in the past, there is a fundamental flaw in the approach of dietary Zn2+ supplementation. Since the brain is protected from excessive Zn2+ and Zn2+ uptake into the brain is highly regulated (see discussion below), only patients with a preexisting Zn2+ deficiency might benefit from dietary Zn2+ supplementation. Thus, the Zn2+ status of patients needs to be evaluated if Zn2+ delivery into the brain by dietary supplementation is to become an effective therapeutic strategy. Although Zn2+ deficiency in humans is generally uncommon, elderly populations often consume insufficient amounts of Zn2+ [92], perhaps acting as a modifying factor in neurodegenerative diseases like Alzheimer’s and Parkinson’s.
3.2. Measurement of Zn2+ Levels
The most common approach for assessing Zn2+ levels is measuring serum or plasma Zn2+. In humans Zn2+ can be found in concentrations of 11 – 23 µM in blood [93] and 0.15 µM in cerebrospinal fluid [4]. Normal plasma concentrations range from about 80 to 120 µg/dL, whereas Zn2+ deficiency concentrations are less then 70 µg/dL. Additionally, Metal-lothionein levels in erythrocytes can be a useful index to measure the Zn2+ status in humans [94]. However, measuring serum Zn2+ levels does not reveal slight and/or temporally fluctuating alterations in extra- and/or intracellular Zn2+ levels in discrete brain areas. This might be a reason for some contradictory results in Zn2+ research in the past. For example, determining Zn2+ levels in the CSF from the area of abnormality (e.g., seizure focus) during neurosurgery might be a better approach, although it is seldom pursued. Thus, not only a controlled and region specific targeted Zn2+ delivery or depletion is desirable, but also a region specific evaluation of existing steady state Zn2+ levels.
3.3. Regulation of Zn2+ Levels
Several paradigms exist for inducing Zn2+ deficiency or supplementing Zn2+ in animal models like mice and rats. Zn2+ deficiency is usually caused by a dietary Zn2+ restriction. While control mice and rats are normally fed diets containing between 25 mg kg and 80 mg/kg Zn2+[95–101], Zn2+ deficiency is caused when the diet contains 0.5 mg/kg – 6 mg/kg Zn2+ [95, 99, 101]. Zn2+ overdoses can be reached at 100 mg/kg – 180 mg/kg [96, 97]. However, though Zn2+ overdoses might elevate serum Zn2+ levels, this doesn’t necessarily lead to elevated brain Zn2+ levels in healthy control animals. This is likely a consequence of reduced absorption of Zn2+ at higher doses that might occur due to the saturation of Zn2+ transport mechanisms [102]. Nonetheless, dietary Zn2+ supplementation is considered a treatment in Zn2+ deficient rats/mice. Another common method for Zn2+ supplementation is tap water, where usually doses between 75 mg to 132 mg ZnSO4 per L water are used [55]. The daily water intake by a 470 g rat is approximately 35 ml/day [103]. Intraperitoneal injection of zinc is used as an additional method in numerous studies, with injections between 3 mg/kg body weight/day – 10 mg/kg/day [95, 104, 105]. Zn2+ deficiency early in life can be induced by a Zn2+ deficient diet of pregnant animals. For example, female rats shifted to a Zn2+ deficient diet (0.5 mg Zn /kg diet) at day 19 of pregnancy will result in pups being fed Zn2+ deficient milk during lactation [106].
The time course of treatment highly depends on the research objective, as a consequence both, acute and chronic treatments have been used. Moreover, Zn2+ supplementation and deficiency manifest at different rates in different tissues. Intriguingly, in fasted rats, a single dose of Zn2+ containing the daily amount of Zn2+ intake led to a rise in plasma Zn2+ levels within an hour and a half [107]. However, plasma Zn2+ levels and brain Zn2+ levels might not directly correlate, for example, hippocampal mossy fiber Zn2+ levels were reduced in rats fed a Zn2+ deficient diet, but only after 90 days of treatment, not 28 days [108]. The average total brain Zn2+ concentration is approximately 150 mmol/l, which is about 10-fold serum Zn2+ levels [108]. However, the pool of free Zn2+ ions, with about 500 nmol/l in brain extracellular fluids, is very low [110]. Nevertheless, in general, brain Zn2+-levels can be affected by altering dietary Zn2+ concentrations [108].
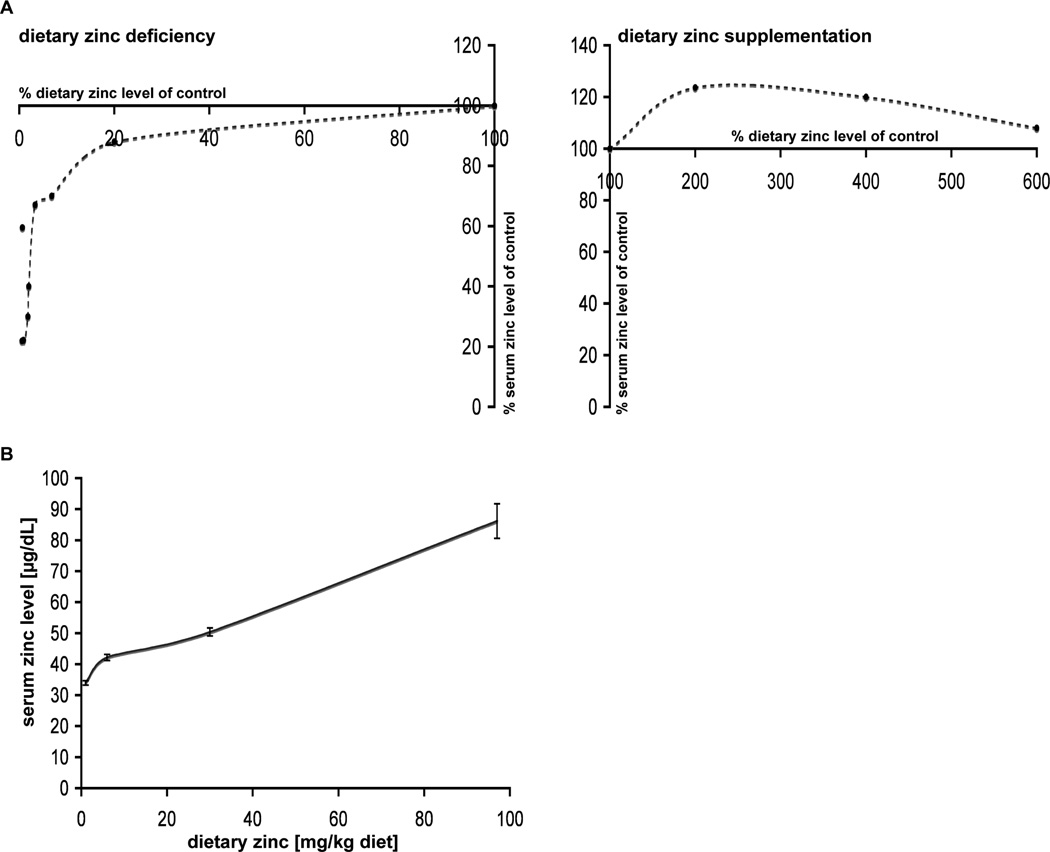
Based on several studies [95, 96, 98, 99, 105, 111–116], we calculated the change in Zn2+ levels based on measurements of serum and hepatic Zn2+ levels in rats treated for 28–42 days with dietary zinc deficiency versus Zn2+ supplementation. Our results revealed a steep increase in serum Zn2+ levels that can be observed in response to very low levels of dietary Zn2+ (0.57% dietary Zn2+ compared to controls with 21.9% serum Zn2+ levels and 6.8% dietary Zn2+ compared to controls with 70% serum Zn2+ levels of control animals) and milder Zn2+ deficiency (20% dietary Zn2+ compared to controls with 88% serum Zn2+ levels of control animals) Fig. (1A). However, at dietary Zn2+ levels between 20% and 100% compared to controls, the rate of increase in serum Zn2+ levels is much lower. Zn2+ supplementation, at 2-fold and 4-fold dietary Zn2+ concentrations, increases serum Zn2+ levels approximately 25% Fig. (1A). However, toxic side effects and the saturation of Zn2+ uptake mechanisms appear to set limits on further increases as seen when dietary Zn2+ is increased 6-fold. Here, only a 10% increase in serum Zn2+ levels is observed compared to controls. The dietary Zn2+ concentration in relation to serum Zn2+ levels in rats after 6 weeks of treatment again shows a steeper increase in serum Zn2+ concentration between 0.6 and 6 mg Zn /kg diet compared to higher dietary Zn2+ levels Fig. (1B).
Fig. (1).
Dietary zinc-concentration dependent increase or decrease in serum Zn2+ levels. A) Changes in serum Zn2+ levels induced by dietary zinc deficiency (left panel) or zinc supplementation (right panel) are calculated based on several studies (14–16,18, 22–28). Left panel: A steep increase in serum Zn2+ levels can be observed between very low levels of dietary Zn2+ and milder Zn2+ deficiency. In contrast, between 20% and 100% of dietary zinc compared to controls, the rate of increase in serum Zn2+ levels is much lower. Right panel: Zn2+ supplementation with 2-fold, 4-fold and 6-fold dietary zinc increases Zn2+ levels approximately 25% (2-fold and 4-fold) and decreases slightly with 6-fold (only 10% increase in serum Zn2+ levels compared to controls). B) Based on several studies, the dietary zinc concentration in relation to serum Zn2+ levels in rats after 6 weeks of treatment is shown. Between 0.6 and 6 mg Zinc/kg diet a steeper increase in serum Zn2+ concentration is seen.
Regarding treatment of human patients and the validity of animal models, one can calculate that Zn2+ supplementation between 70 mg/kg diet – 90 mg/kg diet is at the lower end of Zn2+ doses used for Zn2+ supplementation in many studies and translates to a human supplementation of 25 mg – 100 mg zinc per day for a 70 kg individual [103]. With a daily recommendation of about 11 mg and an absorption of 20 – 40% of zinc, these doses are within the same range as those used for Zn2+ supplementation in many commercial preparations [103] ranging from 15–100 mg zinc per tablet with a recommendation of 1 tablet per day. Since serum levels of less then 70 µg/dL suggest Zn2+ deficiency and serum Zn2+ levels of animal fed on Zn2+ deficient diet reached approximately 50 µg/dL in studies with very low levels of dietary Zn2+, mouse and rat animal models indeed reflect Zn2+ deficient states of humans. However, systemic Zn2+ supplementation or induced deficiency affects the whole body and local brain Zn2+ levels poorly reflect measured serum Zn2+ concentrations. This is a disadvantage to many studies on the function of Zn2+ within the central nervous system, besides other impediments.
4. IMPEDIMENTS TO Zn2+ DELIVERY INTO THE BRAIN
The daily zinc requirements for adult men and women were set at 9.4 mg and 6.8 mg, respectively, while 11 mg and 8 mg were set at RDAs, respectively [117]. This amount can be easily reached by dietary Zn2+ supplementation. The rate of absorption can be additionally increased if the Zn2+ supplement is taken along with vitamin B6 [118]. Specifically, 71% of dietary zinc was absorbed when rats were given 40 mg of vitamin B6 per kilogram of diet [112] compared to 46% with only 2 mg vitamin B6. Zn2+ excess appears to have almost no brain toxicity, although patients show some significant somatic effects [119, 120]. In rats fed 100 mg of zinc oxide (via gastric tube), Zn2+ has very little toxicity in the brain [121, 122, 13]. In humans, even massive zinc ingestion (12 grams over 2 days) was reported to produce only reversible lethargy [123] in terms of neurological symptoms. One explanation for this might be that the mammalian brain is protected from systemic factors by the blood-brain barrier (BBB). The BBB is comprised by a layer of specialized endothelial cells [124] and Zn2+ enters the brain at a constant rate when within the range of plasma Zn2+ concentrations occurring in healthy individuals. However, outside this range, the Zn2+ transport rate is significantly altered [125]. Unfortunately, many drugs are unable to cross the BBB [126]. The transmissivity of this epithelial structure is restricted by the presence of tight junctions, connecting the cerebral endothelial and epithelial cells of the choroids plexus. Moreover, glial cells found surrounding the surface of these capillaries cohere the endothelial cells, producing high electrical resistance compared to other systemic endothelia [127]. Studies of sodium and potassium transport by brain capillaries revealed that endothelial cells of the BBB contain distinct types of ion transport systems on the two sides of the capillary wall. This allows ions to be pumped across the capillary against an electrochemical gradient [128]. The BBB is highly restrictive for Zn2+, but transport does not require energy [125]. Thus, although the BBB protects the brain from excessive Zn2+, it creates a problem for the regulation of Zn2+ brain-levels with dietary Zn2+ supplementation, especially if the desired final Zn2+ level is higher than physiological levels restricted by BBB permeability. Additionally, the final Zn2+ concentration obtained by dietary Zn2+ supplementation is hard to predict given that other dietary factors can influence Zn2+ absorption. Inositol hexaphosphates and pentaphosphates present in food exert negative effects on Zn2+ absorption, as do iron and cadmium if given together in a supplement. Moreover, the amount of protein in a meal influences Zn2+ absorption, though some proteins may act differently. A few amino acids, such as histidine and methionine, as well as organic acids (e.g., citrate), have a positive effect on Zn2+ absorption and have been used along with Zn2+ supplements [102]. Thus, although dietary Zn2+ supplementation has been effective in some studies, a method for more targeted and controlled Zn2+ release within the brain is desirable.
Once inside the brain, the mechanisms that modulate the free Zn2+ pool are key to health and performance. Transition metals such as Zn2+ are maintained at low levels because an excess of free Zn2+ has been shown to be neurotoxic. 300–600 µM Zn2+ for 15 min induces rapid morphological changes in cultured mouse cortical neurons and leads to cell death of virtually all neurons within 24 h [110, 129, 130, 47]. If the concentration of Zn2+ is increased to 1 mM, an exposure time as short as 5 min suffices to destroy many cortical neurons [131]. Since Zn2+ is a small, hydrophilic, charged ion, which cannot cross biological membranes by passive diffusion, specialized mechanisms are required for its uptake. Indeed, free Zn2+ is distributed across the plasma membrane in a large gradient [132]. Maintenance of this electrochemical gradient requires an active transport system. A group of zinc transporter proteins termed “ZnTs” have been characterized as mediators of Zn2+ transport. Among these ZnT3 is important for synaptic vesicle Zn2+ uptake [80] and ZnT1, widely distributed throughout the brain, is associated with Zn2+ efflux [133, 134]. However, Zn2+ also readily enters neurons via glutamate receptors and voltage gated Ca2+ channels [132], consequently membrane depolarization substantially enhances the ability of Zn2+ to kill neurons [135].
Once inside the cell, a number of conserved proteins are known to bind Zn2+, among them metallothioneins [136, 137], but several other proteins actively buffer Zn2+, including glutathione. Approximately 90% of the total brain Zn2+ is bound to endogenous proteins [4]. This binding is reversible, and recent studies have shown that Zn2+ is released from metallothioneins in response to nitric oxide (NO) [138] and oxidized glutathione (GSSG). Thus, these intracellular Zn2+ stores might be interesting targets for new drug development.
The intracellular bound Zn2+ influences many cellular functions and pathways. An astonishing number (over 300) of enzymes require Zn2+ for their functions [46] and Zn2+ has catalytic, coactive (or cocatalytic) and structural [139, 140] importance. Taken together, Zn2+ ions play important roles in regulating biological functions including the activity of transcriptional factors, oxidative stress response, DNA repair and DNA transcription. In the CNS, Zn2+ has been additionally found to influence NMDAR mediated signaling [141] and might even mediate higher cognitive functions such as learning and memory and plasticity of emotional networks [41, 142, 143]. Depending on a variety of factors, including protein turnover and Zn2+ binding affinity, Zn2+ deficiency most likely affects some Zn2+ dependent processes more than others, and thus Zn2+ deficiency versus Zn2+ supplementation will have several outcomes that have to be carefully monitored.
Despite most studies aiming to influence the function of neurons by Zn2+ deficiency and supplementation, glial cells might play a modifying role. Little is known about the uptake of Zn2+ in glial cells. However, prolonged exposure to high concentrations of free Zn2+ causes glial cell death. These cells express ZnT1 transporters and metallothioneins. For future studies on the contribution of glial cells to Zn2+ homeostasis within the brain, a mechanism to trigger Zn2+ increase in neurons versus glial cells would be desirable.
5. METHODS AND COMPOUNDS SUITABLE FOR REGULATION OF BRAIN Zn2+-LEVELS
5.1. Dietary Zinc Supplementation
Dietary Zn2+ supplementation has been the method of choice for years and has been widely used. Zinc can be administered in the form of oral tablets, lozenges or sprays. Common compounds used for Zn2+ supplementation include zinc oxide, zinc sulfate, zinc acetate, zinc chloride and zinc gluconate. However, these compounds differ in the amount of Zn2+ provided as well as in absorption properties. Zinc oxide for example has 78% Zn but is poorly absorbed [144], Zinc carbonate has 52.1%, zinc chloride 48%, sulfate monohydrate 36.4% and zinc gluconate has only 14.3% Zn. While zinc chloride, zinc sulfate and zinc acetate are all very soluble, zinc carbonate and zinc oxide are fairly insoluble. The tolerable maximum intake level for zinc has been set at 40 mg daily, which is mostly restricted by side effects caused by the resulting copper deficiency [145]. Gastric irritation is another common side effect. Zinc acetate is one of the best-tolerated Zn2+ preparations by the digestive system. All Zn2+ supplements should be taken on empty stomach, without simultaneously consummation of other mineral supplements. The main site of absorption of Zn2+ found in dietary supplements is the proximal small intestine, most likely the jejunum. Zn2+ is absorbed into enterocytes by carrier - mediated processes. Several endogenous substances are thought to serve as ligands for Zn2+ that can enhance absorption. Such substances include citric acid, picolinic acid, prostaglandins and amino acids like histidine and cysteine [146]. Glutathione may also serve as ligand. Amino acid ligands help to maintain the solubility of Zn2+ in the gastrointestinal tract. Once absorbed, Zn2+ passes into portal blood and is transported while loosely bound to albumin or immunoglobulin G until it reaches the BBB [146]. However, uptake through the BBB is mediated by an active transportation-process and therefore final Zn2+ concentrations reached in the brain are hard to control or predict. This is the major disadvantage of dietary Zn2+ supplementation and future studies will have to consider alternative ways of Zn2+ delivery to the brain. Unfortunately, to date, no drug that selectively increases brain Zn2+-levels is available. However, a study by Czerniak and Haim revealed that phenothiazine derivatives like chlorpromazine, thioridazine and perphenazine are able to increase the total brain Zn2+ uptake in Zn2+ supplemented rats and mice [147]. Interestingly, dietary Zn2+ supplemented with L-histidine improved short-term memory function in Zn2+ deficient animals more efficiently than dietary Zn2+ chloride supplementation alone [148]. Histidine may be involved in Zn2+ transport across the BBB and mediate Zn2+ increase by binding to Zn2+ from plasma proteins [149]. Furthermore N-acetyl cysteine, a FDA approved precursor of glutathione might increase glutathione levels [150]. Glutathione together with glutathione disulfide enhances delivery of Zn2+ to cells.
5.2. Alternative Ways of Zinc Supplementation
For a more targeted Zn2+ delivery to the brain, systemic Zn2+ supplementation by dietary application is an inadequate method. Alternative methods can achieve Zn2+ level regulation by intracranial delivery of therapeutic compounds via implanted cannulas, devices or drug-releasing polymers. A recent study used Alzet® pumps implanted in rat brains, releasing up to 1 mM ZnCl2, at a rate of 0.25 µl/h continuously into the hippocampal hilus for 4 weeks [79]. Furthermore, guide cannulae can be implanted bilaterally into the dorsal hippocampus or lateral ventricle to deliver Zn2+ or Zn2+ chelators [151]. Another strategy for a more targeted Zn2+ delivery is intralumbar injection or of Zn2+-supplements directly into the cerebrospinal fluid (CSF). Thus, immediate high CSF drug concentrations can be reached. The CSF freely exchanges molecules with the extracellular fluid of the brain parenchyma [152]. However, this delivery method faces some difficulties like slow rate of substance distribution within the CSF and increased intracranial pressure [152]. Although these techniques are available, they are unfortunately seldom used in the field of Zn2+ supplementation.
Recently, novel nanoparticulate systems were developed that might be used to deliver Zn2+ ions through the BBB and thus directly into the brain [130]. This approach dramatically improves control of desired Zn2+ concentration in the brain and makes it possible to increase Zn2+ levels above those restricted by physiological BBB uptake. Nanoparticle injection is a non-invasive method for drug delivery to the CNS that has a high rate of efficiency (13–15% of the injected dose) [153]. Nanoparticulate drug carriers made of Polylactide-co-glycolide (PLGA) or polylactide (PLA) polymers are biodegradable, biocompatible and FDA-approved. They can be specifically modified with ligands and have been demonstrated to cross the BBB, thereby representing an important tool for future treatment of neurological diseases [154]. Moreover, nanoparticles can be targeted to specific antigen-representing cells, which are subsequently taken up by these cells [130]. Once endocytosed, these nanoparticles were found to degrade constantly over time releasing their content into the cell soma. This strategy thus provides a new opportunity to delivery Zn2+ to specific cells within the brain, though their usefulness in vivo has yet to be proven. Nanoparticles themselves are not toxic to cells in vitro, even at concentrations higher than those used for delivering Zn2+ [130] in in vitro studies. Thus, the properties of these Zn2+ loaded Nanoparticles should provide a new therapeutic strategy for altering Zn2+ levels in patients with neuropsychological disorders.
5.3. Zn2+ Chelation
Recent studies have focused on the treatment of patients with metal chelating drugs [155–157]. Therapeutic benefits from chelation of Zn2+ ions might result from an impairment of their ability to mediate protein folding, clustering or a reduction of redox processes. Brain delivery of Zn2+ chelators can be achieved by oral ingestion or by intravenous infusions, i.e. of EDTA, as some Zn2+ chelators can directly penetrate cell membranes or be enticed to do so by esterification or by acquisition of a nonpolar state following complex formation with metals [158]. For example, the zinc-binding 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol) is a chelator that is able to cross the BBB when given at doses of up to 80 mg/day to patients [159]. It also markedly reduced synaptic targeting of Aβ oligomers [160]. PBT2, a derivative of clioquinol, is currently under investigation as a potential treatment for AD. Similarly, it was shown that N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) and bathocuproine disulfonic acid (BC) are able to dissolve Aβ deposits in postmortem AD brain samples [161]. However current research hints to a higher toxicity of fibrillary Aβ compared to Aβ plaques. Thus, a major application for Zn2+ chelators like pyrithione, inositol hexakisphosphate and CaEDTA might be after traumatic brain injury and seizures, where chelation of excessive neuronal Zn2+ might decrease neurotoxicity [162]. However, CaEDTA is cell impermeable and studies by Lavoie et al. suggest that only intracellular but not extracellular Zn2+ chelators might influence seizure-induced Zn2+ accumulation [74].
The use of Zn2+ chelators in general is experiencing difficulties. Although there are high affinity Zn2+ chelators available, all Zn2+ chelators show additional affinity for copper ions and less for calcium and magnesium ions. Thus, side effects caused by the chelation of other important divalent metal ions in the brain and body tissues are unavoidable. Thus, similar to Zn2+ supplementation, a controlled release within specific brain areas is highly desired.
6. CONCLUSIONS
Based on the studies presented above, Zn2+ deficiency is surprisingly prevalent in patients with psychiatric and neurodegenerative disorders. However, in most cases, the mechanisms that lead to the decrease in Zn2+ levels remain elusive. Moreover, it is likely that the observed deficiencies do not share a common pathway and might occur in discrete brain areas. Unfortunately, most findings in Zn2+ research are based on the measurement of serum Zn2+ levels that are inefficient to detect slight and/or temporally fluctuations of Zn2+ in specific brain areas and more sensitive and direct approaches to detect brain Zn2+ levels should be considered for future studies. Thus, it is hard to predict how the brain responds to systemic Zn2+ supplementation on a cellular level in each clinical case discussed above.
Moreover, dietary Zn2+ supplementation might only be useful in restoring Zn2+ levels to those of control subjects. Thus it might not be effective if Zn2+ levels within specific brain regions are impaired due to shifts of zinc ions between different pools: i.e. aberrant proteins with Zn2+ binding sites. Clearly, the evaluation of Zn2+ levels in patients will be necessary to assess the possibility of Zn2+ delivery to the brain. Importantly, it is not entirely impossible that at least some of the studies in Zn2+ brain-research have been poorly designed and analyzed, creating ambiguous results. However, new methods like implanted osmotic pumps or BBB crossing nanoparticle carriers might allow researchers to increase brain Zn2+ above levels restricted by BBB uptake and help to evaluate existing data and the often contradictory results in the light of a more directed approach of brain Zn2+-level regulation.
Although Zn2+ supplementation has been shown to be useful on its own, a combination of drug treatment with Zn2+ supplementation might be more promising. Zn2+ chelation on the other hand should only be considered in cases where toxic Zn2+ brain-levels are expected to cause cell death. In Alzheimer´s disease for example, restoring Zn2+ balance in a narrow range might lead to beneficial effects. While Zn2+ chelators will dissolve Aβ plaques, they might create more toxic protofibrillary Aβ and cause memory problems on their own. Zn2+ supplementation however might face difficulties in early stages of the disease since Zn2+ ions shift from synaptic stores towards Aβ, but are not depleted from the brain. Only in late stages of Alzheimer’s disease, the demand of Aβ for Zn2+ ions might generate a measurable Zn2+ deficiency. Although this deficiency might be compensated by Zn2+ supplementation, one should be cautious to supplement only equimolar amounts of Zn2+ to the loss caused from uptake by Aβ since higher Zn2+-levels might promote surplus generation of Aβ. Thus, controlled and region specific targeted Zn2+ delivery to the brain is highly desirable and will be an important goal in future research for drug delivery to the brain.
ACKNOWLEDGEMENTS
AMG has been supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG), MR by an NRSA fellowship (NS066786-025) and CCG by the National Institutes of Health (P01 NS053862; R21 MH091471).
REFERENCES
- 1.Pfeiffer CC, Braverman ER. Zinc, the brain and behavior. Biol. Psychiatry. 1982;17(4):513–532. [PubMed] [Google Scholar]
- 2.Maske H. Über den topochemischen Nachweis von Zink im Ammonshorn verschiedener Säugetiere. Die Naturwissenschaften. 1955;424 [Google Scholar]
- 3.Ebadi M. Metallothioneins and other zinc-binding proteins in brain. Methods Enzymol. 1991;205:363–387. doi: 10.1016/0076-6879(91)05119-g. [DOI] [PubMed] [Google Scholar]
- 4.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 5.Ehmann WD, Markesbery WR, Alauddin M, Hossain TI, Brubaker EH. Brain trace elements in Alzheimer's disease. Neurotoxicology. 1986;7(1):195–206. [PubMed] [Google Scholar]
- 6.Brewer GJ, Kanzer SH, Zimmerman EA, Molho ES, Celmins DF, Heckman SM, Dick R. Subclinical zinc deficiency in Alzheimer's disease and Parkinson's disease. Am. J. Alzheimers Dis. Other Demen. 2010;25(7):572–575. doi: 10.1177/1533317510382283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed SS, Santosh W. Metallomic profiling and linkage map analysis of early Parkinson's disease: a new insight to aluminum marker for the possible diagnosis. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011252. e11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi GA, Qureshi AA, Memon SA, Parvez SH. Impact of selenium, iron, copper and zinc in on/off Parkinson's patients on L-dopa therapy. J. Neural Transm. Suppl. 2006;(71):229–236. doi: 10.1007/978-3-211-33328-0_24. [DOI] [PubMed] [Google Scholar]
- 9.Baum L, Chan IH, Cheung SK, Goggins WB, Mok V, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Zee BC, Cheng W, Chan MH, Szeto S, Lui V, Tsoh J, Bush AI, Lam CW, Kwok T. Serum zinc is decreased in Alzheimer's disease and serum arsenic correlates positively with cognitive ability. Biometals. 2010;23(1):173–179. doi: 10.1007/s10534-009-9277-5. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, Robertson JD, Markesbery WR, Lovell MA. Serum zinc in the progression of Alzheimer's disease. J. Alzheimers Dis. 2008;15(3):443–450. doi: 10.3233/jad-2008-15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vural H, Demirin H, Kara Y, Eren I, Delibas N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer's disease. J. Trace Elem. Med. Biol. 2010;24(3):169–173. doi: 10.1016/j.jtemb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Rahman A, Azad MA, Hossain I, Qusar MM, Bari W, Begum F, Huq SM, Hasnat A. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol. Trace Elem. Res. 2009;127(2):102–108. doi: 10.1007/s12011-008-8230-8. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer CC, LaMola S. Zinc and Manganese in the Schizophrenias. Journal of Orthomolecular Psychiatry. 1999;14(1):28–48. [Google Scholar]
- 14.Biederman J. Advancing the neuroscience of ADHD: Attention-Deficit/Hyperactivity Disorder: A Selective Overview. Biol. Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Arnold LE, Disilvestro RA, Bozzolo D, Bozzolo H, Crowl L, Fernandez S, Ramadan Y, Thompson S, Mo X, Abdel-Rasoul M, Joseph E. Zinc for attention-deficit/hyperactivity disorder: placebo-controlled double-blind pilot trial alone and combined with amphetamine. J. Child Adolesc. Psychopharmacol. 2011;21(1):1–19. doi: 10.1089/cap.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepping P, Huber M. Role of zinc in the pathogenesis of attention-deficit hyperactivity disorder: implications for research and treatment. CNS Drugs. 2010;24(9):721–728. doi: 10.2165/11537610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Levenson CW. Zinc: The New Antidepressant? Nutrition Reviews. 2006;64(1):39–42. doi: 10.1111/j.1753-4887.2006.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 18.Cope EC, Levenson CW. Role of zinc in the development and treatment of mood disorders. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13(6):685–689. doi: 10.1097/MCO.0b013e32833df61a. [DOI] [PubMed] [Google Scholar]
- 19.Manser WWT, Khan MA, Hasan KZ. Trace element studies on Karachi population. Part IV: blood copper, zinc, magnesium and lead levels in psychiatric patients with depression, mental retardation and seisure disorders. J. Pakistan Med. Assoc. 1989;39:269–274. [PubMed] [Google Scholar]
- 20.McLoughlin IJ, Hodge SJ. Zinc in depressive disorder. Acta. Psychiatr. Scand. 1990;82:451–453. doi: 10.1111/j.1600-0447.1990.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 21.Vinceti M, Bergomi M, Nacci G, Pietrini V, Ferrari A, Fortini K, Guidetti D, Sola P, Rocchi E, Mancia D, Vivoli G. Erythrocyte zinc, copper, and copper/zinc superoxide dismutase and risk of sporadic amyotrophic lateral sclerosis: a population-based case-control study. Amyotroph. Lateral. Scler. Other Motor Neuron Disord. 2002;3:208–214. doi: 10.1080/146608202760839006. [DOI] [PubMed] [Google Scholar]
- 22.Yenigun A, Ozkinay F, Cogulu O, Coker C, Cetiner N, Ozden G, Aksu O, Ozkinay C. Hair zinc level in Down syndrome. Down Syndrome Research and Practice. 2004;9(2):53–57. doi: 10.3104/reports.292. [DOI] [PubMed] [Google Scholar]
- 23.Ho SY, Catalanotto FA, Lisak RP, Dore-Duffy P. Zinc in multiple sclerosis. II: Correlation with disease activity and elevated plasma membrane-bound zinc in erythrocytes from patients with multiple sclerosis. Ann. Neurol. 1986;20:712–715. doi: 10.1002/ana.410200610. [DOI] [PubMed] [Google Scholar]
- 24.Dore-Duffy P, Catalanotto F, Donaldson JO, Ostrom KM, Testa MA. Zinc in multiple sclerosis. Ann. Neurol. 1983;14:450–454. doi: 10.1002/ana.410140409. [DOI] [PubMed] [Google Scholar]
- 25.Qian J, Xu K, Yoo J, Chen TT, Andrews G, Noebels JL. Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. 2011;31(1):97–104. doi: 10.1523/JNEUROSCI.5162-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayatzadeh H, Nouri M, Ghasemi M, Kebriaeezadeh A, Mehr SE, Dehpour AR. Effect of metal chelating agents on pentylenetetrazole-induced seizure threshold in cholestatic mice. Seizure. 2009;18(1):51–56. doi: 10.1016/j.seizure.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Foresti ML, Arisi GM, Fernandes A, Tilelli CQ, Garcia-Cairasco N. Chelatable zinc modulates excitability and seizure duration in the amygdala rapid kindling model. Epilepsy Res. 2008;79(2–3):166–172. doi: 10.1016/j.eplepsyres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Huster D. Wilson disease. Best Pract. Res. Clin. Gastroenterol. 2010;24(5):531–539. doi: 10.1016/j.bpg.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Horvath J, Beris P, Giostra E, Martin PY, Burkhard PR. Zinc-induced copper deficiency in Wilson disease. J. Neurol. Neurosurg. Psychiatry. 2010;81(12):1410–1411. doi: 10.1136/jnnp.2009.188896. [DOI] [PubMed] [Google Scholar]
- 30.Constantinidis J, Richard J, Tissot R. Pick’s disease and zinc metabolism. Rev. Neurol. 1977;133:685–696. [PubMed] [Google Scholar]
- 31.Jan HH, Chen IT, Tsai YY, Chang YC. Structural role of zinc ions bound to postsynaptic densities. J. Neurochem. 2002;83(3):525–534. doi: 10.1046/j.1471-4159.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 32.Grabrucker AM, Knight MJ, Proepper C, Bockmann J, Joubert M, Rowan M, Nienhaus GU, Garner CC, Bowie JU, Kreutz MR, Gundelfinger ED, Boeckers TM. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011;30(3):569–581. doi: 10.1038/emboj.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 34.Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 35.Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- 36.Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog. Neurobiol. 1994;42(3):393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 37.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 38.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 39.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 1997;17(24):9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdette SC, Lippard SJ. Meeting of the minds: metalloneurochemistry. Proc. Natl. Acad. Sci. U S A. 2003;100(7):3605–3610. doi: 10.1073/pnas.0637711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bitanihirwe BK, Cunningham MG. Zinc: the brain's dark horse. Synapse. 2009;63(11):1029–1049. doi: 10.1002/syn.20683. [DOI] [PubMed] [Google Scholar]
- 42.Mocchegiani E, Bertoni-Freddari C, Marcellini F, Malavolta M. Brain, aging and neurodegeneration: role of zinc ion availability. Prog. Neurobiol. 2005;75(6):367–390. doi: 10.1016/j.pneurobio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Cuajungco MP, Fagét KY. Zinc takes the center stage: its paradoxical role in Alzheimer's disease. Brain Res. Brain Res. Rev. 2003;41(1):44–56. doi: 10.1016/s0165-0173(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 44.Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J. Neural. Transm. 2010 doi: 10.1007/s00702-010-0470-z. [ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 2010;345(1–2):91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 46.Takeda A. Zinc homeostasis and functions of zinc in the brain. Biometals. 2001;14(3–4):343–351. doi: 10.1023/a:1012982123386. [DOI] [PubMed] [Google Scholar]
- 47.Choi DW, Koh JY. Zinc and brain injury. Annu. Rev. Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 48.Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10(5):432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- 49.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J. Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodali R, Williams AD, Chemuru S, Wetzel R. Abeta(1–40) forms five distinct amyloid structures whose beta-sheet contents and fibril stabilities are correlated. J. Mol. Biol. 2010;401(3):503–517. doi: 10.1016/j.jmb.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara T, Hiura Y, Kawahito O, Yasuzawa M, Kawashiro K. Selection of novel structural zinc sites from a random peptide library. FEBS Lett. 2003;555(2):317–321. doi: 10.1016/s0014-5793(03)01266-3. [DOI] [PubMed] [Google Scholar]
- 53.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease? J. Neurosci. 2010;30(5):1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. U S A. 1999;96(4):1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corona C, Masciopinto F, Silvestri E, Del Viscovo A, Lattanzio R, La Sorda R, Ciavardelli D, Goglia F, Piantelli M, Canzoniero LMT, Sensi SL. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death and Disease. 2010;1:1–8. doi: 10.1038/cddis.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forsleff L, Schauss AG, Bier ID, Stuart S. Evidence of functional zinc deficiency in Parkinson's disease. J. Altern. Complement. Med. 1999;5(1):57–64. doi: 10.1089/acm.1999.5.57. [DOI] [PubMed] [Google Scholar]
- 57.Jenner P. Oxidative stress in Parkinson's disease. Ann. Neurol. 2003;53:26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 58.Saini N, Schaffner W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biol. Chem. 2010;391(5):513–518. doi: 10.1515/BC.2010.052. [DOI] [PubMed] [Google Scholar]
- 59.Murayama S, Mori H, Ihara Y, Tomonaga M. Immunocytochemical and ultrastructural studies of Pick's disease. Ann. Neurol. 1990;27(4):394–405. doi: 10.1002/ana.410270407. [DOI] [PubMed] [Google Scholar]
- 60.Timm F. Histochemistry of zinc. Dtsch. Z. Gesamte Gerichtl. Med. 1958;47:428–431. [PubMed] [Google Scholar]
- 61.Constantinidis J, Tissot R. Role of glutamate and zinc in the hippocampal lesions of Pick's disease. Adv. Biochem. Psychopharmacol. 1981;27:413–422. [PubMed] [Google Scholar]
- 62.Nowak G, Szewczyk B. Mechanism contributing to antidepressant zinc actions. Pol. J. Pharmacol. 2002;54:587–592. [PubMed] [Google Scholar]
- 63.Maes M, D’Haese PC, Scharpe S, D’Hondt PD, Cosyns P, De Broe ME. Hypozincemia in depression. J. Affect Disord. 1994;31:135–140. doi: 10.1016/0165-0327(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 64.Amani R, Saeidi S, Nazari Z, Nematpour S. Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. Biol. Trace Elem. Res. 2010;137(2):150–158. doi: 10.1007/s12011-009-8572-x. [DOI] [PubMed] [Google Scholar]
- 65.Wójcik J, Dudek D, Schlegel-Zawadzka M, Grabowska M, Marcinek A, Florek E, Piekoszewski W, Nowak RJ, Opoka W, Nowak G. Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol. Rep. 2006;58(4):571–576. [PubMed] [Google Scholar]
- 66.Kroczka B, Brañski P, Palucha A, Pilc A, Nowak G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res. Bull. 2001;55:297–300. doi: 10.1016/s0361-9230(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 67.Szewczyk B, Brañski P, Wieroñska JM, Palucha A, Pilc A, Nowak G. Interaction of zinc with antidepressants in the mouse forced swimming test. Pol. J. Pharmacol. 2002;54:681–685. [PubMed] [Google Scholar]
- 68.Sawada T, Yokoi K. Effect of zinc supplementation on mood states in young women: a pilot study. Eur. J. Clin. Nutr. 2010;64(3):331–333. doi: 10.1038/ejcn.2009.158. [DOI] [PubMed] [Google Scholar]
- 69.Mclardy T. Hippocampal Zinc in Chronic Alcoholism and Schizophrenia. IRCS Med. Sci. 1973;2:1010. [Google Scholar]
- 70.Kimura I, Kumura J. Preliminary Reports on the Metabolism of Trace Elements in Neuro Psychiatric Diseases. 1. Zinc in Schizophrenia. Proc. Jap. Acad. Sci. 1965:943. [Google Scholar]
- 71.Dudek FE. Zinc and Epileptogenesis. Epilepsy Curr. 2001;1(2):66–70. doi: 10.1046/j.1535-7597.2001.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian Y, Yang Z, Zhang T. Zinc ion as modulator effects on excitability and synaptic transmission in hippocampal CA1 neurons in Wistar rats. Neurosci. Res. 2010;68(3):167–175. doi: 10.1016/j.neures.2010.07.2030. [DOI] [PubMed] [Google Scholar]
- 73.Cuajungco MP, Lees GJ. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol. Dis. 1997;(3–4):137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- 74.Lavoie N, Peralta MR, Chiasson M, Lafortune K, Pellegrini L, Seress L, Tóth K. Extracellular chelation of zinc does not affect hippocampal excitability and seizure-induced cell death in rats. J. Physiol. 2007;578(1):275–289. doi: 10.1113/jphysiol.2006.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pei Y, Zhao D, Huang J, Cao L. Zinc-induced seizures: a new experimental model of epilepsy. Epilepsia. 1983;24(2):169–176. doi: 10.1111/j.1528-1157.1983.tb04876.x. [DOI] [PubMed] [Google Scholar]
- 76.Williamson A, Spencer D. Zinc reduces dentate granule cell hyperexcitability in epileptic humans. Neuroreport. 1995;6(11):1562–1564. doi: 10.1097/00001756-199507310-00024. [DOI] [PubMed] [Google Scholar]
- 77.Fukahori M, Itoh M. Effects of dietary zinc status on seizure susceptibility and hippocampal zinc content in the El (epilepsy) mouse. Brain Res. 1990;529(1–2):16–22. doi: 10.1016/0006-8993(90)90806-m. [DOI] [PubMed] [Google Scholar]
- 78.Fukahori M, Itoh M, Oomagari K, Kawasaki H. Zinc content in discrete hippocampal and amygdaloid areas of the epilepsy (El) mouse and normal mice. Brain Res. 1988;455(2):381–384. doi: 10.1016/0006-8993(88)90099-6. [DOI] [PubMed] [Google Scholar]
- 79.Elsas SM, Hazany S, Gregory WL, Mody I. Hippocampal zinc infusion delays the development of afterdischarges and seizures in a kindling model of epilepsy. Epilepsia. 2009;50(4):870–879. doi: 10.1111/j.1528-1167.2008.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000;39(2):153–169. doi: 10.1016/s0920-1211(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 81.Barry-Sterman M, Shouse MN, Fairchild MD, Belsito O. Kindled seizure induction alters and is altered by zinc absorption. Brain Res. 1986;383(1–2):382–386. doi: 10.1016/0006-8993(86)90045-4. [DOI] [PubMed] [Google Scholar]
- 82.Takeda A, Hirate M, Tamano H, Nisibaba D, Oku N. Susceptibility to kainate-induced seizures under dietary zinc deficiency. J. Neurochem. 2003;85(6):1575–1580. doi: 10.1046/j.1471-4159.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- 83.Hoogenraad TU, Van den Hamer CJ, Koevoet R, Korver EG. Oral zinc in Wilson's disease. Lancet. 1978;2(8102):1262. doi: 10.1016/s0140-6736(78)92141-4. [DOI] [PubMed] [Google Scholar]
- 84.Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008;95(3):365–369. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 85.Nowak G, Szewczyk B, Pilc A. Zinc and depression. An update. Pharmacol. Rep. 2005;57(6):713–718. [PubMed] [Google Scholar]
- 86.Whittle N, Lubec G, Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36(1):147–158. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- 87.Kroczka B, Brañski P, Palucha A, Pilc A, Nowak G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res. Bull. 2001;55:297–300. doi: 10.1016/s0361-9230(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 88.Kroczka B, Ziêba A, Dudek D, Pilc A, Nowak G. Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Pol. J. Pharmacol. 2002;52:403–406. [PubMed] [Google Scholar]
- 89.Nowak G, Szewczyk B, Wieroñska JM, Brañski P, Palucha A, Pilc A, Sadlik K, Piekoszewski W. Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res. Bull. 2003;61:159–164. doi: 10.1016/s0361-9230(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 90.Rosa AO, Lin J, Calixto JB, Santos AR, Rodrigues AL. Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav. Brain Res. 2003;144:87–93. doi: 10.1016/s0166-4328(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 91.Cunha MP, Machado DG, Bettio LE, Capra JC, Rodrigues AL. Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(8):1913–1920. doi: 10.1016/j.pnpbp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Salgueiro MJ, Zubillaga M, Lysionek A, Cremaschi G, Goldman CG, Caro R, De Paoli T, Hager A, Weill R, Boccio J. Zinc status and immune system relationship: a review. Biol. Trace Elem. Res. 2000;76(3):193–205. doi: 10.1385/BTER:76:3:193. [DOI] [PubMed] [Google Scholar]
- 93.Jacob RA. In: Fundamentals of Clinical Chemistry. Tietz NW, editor. Philadelphia: Saunders W.B.; 1987. pp. 517–532. [Google Scholar]
- 94.Grider A, Bailey LB, Cousins RJ. Erythrocyte metallothionein as an index of zinc status in humans. Proc. Natl. Acad. Sci. U S A. 1990;87(4):1259–1262. doi: 10.1073/pnas.87.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sunar F, Baltaci AK, Ergene N, Mogulkoc R. Zinc deficiency and supplementation in ovariectomized rats: their effect on serum estrogen and progesterone levels and their relation to calcium and phosphorus. Pak. J. Pharm. Sci. 2009;22(2):150–154. [PubMed] [Google Scholar]
- 96.Shah BG, Giroux A, Belonje B, Jones JD. Optimal level of zinc supplementation of young rats fed rapeseed protein concentrate. J. Agric. Food Chem. 1979;27(2):387–389. doi: 10.1021/jf60222a055. [DOI] [PubMed] [Google Scholar]
- 97.Moore JB, Blanchard RK, Cousins RJ. Dietary zinc modulates gene expression in murine thymus: results from a comprehensive differential display screening. Proc. Natl. Acad. Sci. U S A. 2003;100(7):3883–3888. doi: 10.1073/pnas.0330670100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J. Nutr. 2009;139(9):1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui L, Blanchard RK, Cousins RJ. Dietary zinc deficiency increases uroguanylin accumulation in rat kidney. Kidney Int. 2001;59(4):1424–1431. doi: 10.1046/j.1523-1755.2001.0590041424.x. [DOI] [PubMed] [Google Scholar]
- 100.Peters JM, Wiley LM, Zidenberg-Cherr S, Keen CL. Influence of periconceptional zinc deficiency on embryonic plasma membrane function in mice. Teratog. Carcinog. Mutagen. 1993;13(1):15–21. doi: 10.1002/tcm.1770130103. [DOI] [PubMed] [Google Scholar]
- 101.Vruwink KG, Keen CL, Gershwin ME, Hurley LS. Studies of nutrition and autoimmunity. Failure of zinc deprivation to alter autoantibody production when initiated in disease-established mice. J. Nutr. 1987;117(1):177–182. doi: 10.1093/jn/117.1.177. [DOI] [PubMed] [Google Scholar]
- 102.Lönnerdal B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000;30:1378–1383. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 103.Fong LY, Jiang Y, Rawahneh ML, Smalley KJ, Croce CM, Farber JL, Huebner K. Zinc supplementation suppresses 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr004. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Irato P, Sturniolo GC, Giacon G, Magro A, D'Inca R, Mestriner C, Albergoni V. Effect of zinc supplementation on metallothionein, copper, and zinc concentration in various tissues of copper-loaded rats. Biol. Trace Elem. Res. 1996;51(1):87–96. doi: 10.1007/BF02790151. [DOI] [PubMed] [Google Scholar]
- 105.Baltaci AK, Mogulkoc R, Halifeoglu I. Effects of zinc deficiency and supplementation on plasma leptin levels in rats. Biol. Trace Elem. Res. 2005;104(1):41–46. doi: 10.1385/BTER:104:1:041. [DOI] [PubMed] [Google Scholar]
- 106.Dreosti IE, Manuel SJ, Buckley RA, Fraser FJ, Record IR. The effect of late prenatal and/or early postnatal zinc deficiency on the development and some biochemical aspects of the cerebellum and hippocampus in rats. Life Sci. 1981;28(19):2133–2141. doi: 10.1016/0024-3205(81)90620-2. [DOI] [PubMed] [Google Scholar]
- 107.Dreosti IE, Record IR, Manuel SJ, Buckley RA. High plasma zinc levels following oral dosing in rats and the incorporation of 3H-thymidine into deoxyribonucleic acid in rat fetuses. Res. Commun. Chem. Pathol. Pharmacol. 1981;31(3):503–513. [PubMed] [Google Scholar]
- 108.Wensink J, Lenglet WJ, Vis RD, Van den Hamer CJ. The effect of dietary zinc deficiency on the mossy fiber zinc content of the rat hippocampus. A microbeam PIXE study. Particle Induced X-Ray Emission. Histochemistry. 1987;87(1):65–69. doi: 10.1007/BF00518726. [DOI] [PubMed] [Google Scholar]
- 109.Takeda A, Suzuki M, Oku N. Possible involvement of plasma histidine in differential brain permeability to zinc and cadmium. Biometals. 2002;15:371–375. doi: 10.1023/a:1020256018861. [DOI] [PubMed] [Google Scholar]
- 110.Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 111.Reeves PG, O'Dell BL. Zinc deficiency in rats and angiotensin-converting enzyme activity: comparative effects on lung and testis. J. Nutr. 1988;118(5):622–626. doi: 10.1093/jn/118.5.622. [DOI] [PubMed] [Google Scholar]
- 112.Watanabe T, Arakawa T, Fukuda T, Higuchi K, Kobayashi K. Zinc deficiency delays gastric ulcer healing in rats. Dig. Dis. Sci. 1995;40(6):1340–1344. doi: 10.1007/BF02065548. [DOI] [PubMed] [Google Scholar]
- 113.Orbak R, Kara C, Ozbek E, Tezel A, Demir T. Effects of zinc deficiency on oral and periodontal diseases in rats. J. Periodontal. Res. 2007;42(2):138–143. doi: 10.1111/j.1600-0765.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 114.King LE, Frentzel JW, Mann JJ, Fraker PJ. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005;24(6):494–502. doi: 10.1080/07315724.2005.10719495. [DOI] [PubMed] [Google Scholar]
- 115.Sun D, Zhang L, Wang Y, Wang X, Hu X, Cui FA, Kong F. Regulation of zinc transporters by dietary zinc supplement in breast cancer. Mol. Biol. Rep. 2007;34(4):241–247. doi: 10.1007/s11033-007-9082-6. [DOI] [PubMed] [Google Scholar]
- 116.Kudo H, Doi Y, Nishino T, Nara S, Hamasaki K, Fujimoto S. Dietary zinc deficiency decreases glutathione S-transferase expression in the rat olfactory epithelium. J. Nutr. 2000;130(1):38–44. doi: 10.1093/jn/130.1.38. [DOI] [PubMed] [Google Scholar]
- 117.Institute of Medicine, Food and Nutrition Board. Washington, DC: National Academy Press; 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. [PubMed] [Google Scholar]
- 118.Evans GW, Johnson EC. Growth Stimulating Effect of Picolinic Acid added to Rat Diets. Proceedings of the Society for Experimental Biology and Medicine. 1980;165:457. doi: 10.3181/00379727-165-41004. [DOI] [PubMed] [Google Scholar]
- 119.Pfeiffer CC, Papaioannou R, Sohler A. Effect of Chronic Zinc Intoxication on Copper Levels, Blood Formation and Polyamines. The Journal of Orthomolecular Psychiatry. 1980;9:79–89. [Google Scholar]
- 120.Snyder DR. Neurological, Microscopic and Enzyme-Hisochemical Assessment of Zinc Pyrithione Toxicity. Fd. Cosmet. Toxicol. 1979;17:651. doi: 10.1016/0015-6264(79)90126-3. [DOI] [PubMed] [Google Scholar]
- 121.Kozik MB. The Effect of ZnCI2 Ingestion on the Activity of Various Phosphatases and Esterases of the Rat Brain. Activ. Nerv. Sup. 1979;21:277. [Google Scholar]
- 122.Kozik MB, Maziarz L, Godlewski A. Morphological and Histochemical Changes Occurring in the Brain of Rats Fed Large Doses of Zinc Oxide. Folia Histochemica et Cytochemiczi. 1980;18:201. [PubMed] [Google Scholar]
- 123.Murphy JV. Intoxication following ingestion of elemental zinc. JAMA. 1970;212:2119–2120. [PubMed] [Google Scholar]
- 124.Davson H, Segal MB. Physiology of the CSF and blood– brain barriers. Florida: CRC Press; 1995. [Google Scholar]
- 125.Bobilya DJ, Guerin JL, Rowe DJ. Zinc transport across an in vitro blood-brain barrier model. The Journal of Trace Elements in Experimental Medicine. 1997;10:9–18. [Google Scholar]
- 126.Pardridge WM. Blood Brain Barrier drug targeting: the future of brain drug development. Mol. Interv. 2003;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 127.Burke M, Langer R, Brim H. Central Nervous System: Drug Delivery to Treat. New York: Wiley & Sons; 1999. pp. 184–212. [Google Scholar]
- 128.Betz AL. Transport of ions across the blood-brain barrier. Fed. Proc. 1986;45(7):2050–2054. [PubMed] [Google Scholar]
- 129.Yokoyama M, Koh J, Choi DW. Brief exposure to zinc is toxic to cortical neurons. Neurosci. Lett. 1986;71:351–355. doi: 10.1016/0304-3940(86)90646-4. [DOI] [PubMed] [Google Scholar]
- 130.Grabrucker AM, Garner CC, Boeckers TM, Bondioli L, Ruozi B, Forni F, Vandelli MA, Tosi G. Development of novel Zn2+ loaded nanoparticles designed for cell-type targeted drug release in CNS neurons: in vitro evidences. Plos One. 2011 doi: 10.1371/journal.pone.0017851. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Choi DW, Yokoyama M, Koh J. Zinc neurotoxicity in cortical cell culture. Neuroscience. 1988;24(1):67–79. doi: 10.1016/0306-4522(88)90312-0. [DOI] [PubMed] [Google Scholar]
- 132.Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J. Biol. Chem. 1995;270(6):2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 133.Tsuda M, Imaizumi K, Katayama T, Kitagawa K, Wanaka A, Tohyama M, Takagi T. Expression of zinc transporter gene, ZnT-1, is induced after transient forebrain ischemia in the gerbil. J. Neurosci. 1997;17(17):6678–6684. doi: 10.1523/JNEUROSCI.17-17-06678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McMahon RJ, Cousins RJ. Mammalian zinc transporters. J. Nutr. 1998;128(4):667–670. doi: 10.1093/jn/128.4.667. [DOI] [PubMed] [Google Scholar]
- 135.Weiss JH, Hartley DM, Koh JY, Choi DW. AMPA receptor activation potentiates zinc neurotoxicity. Neuron. 1993;10(1):43–49. doi: 10.1016/0896-6273(93)90240-r. [DOI] [PubMed] [Google Scholar]
- 136.Aschner M. The functional significance of brain metallothioneins. FASEB J. 1996;10(10):1129–1136. doi: 10.1096/fasebj.10.10.8751715. [DOI] [PubMed] [Google Scholar]
- 137.Coyle P, Hubert CA, Philcox JC, Rofe AM. Importance of storage conditions for the stability of zinc- and cadmium-induced metallothionein. Biol. Trace Elem. Res. 2001;81(3):269–278. doi: 10.1385/BTER:81:3:269. [DOI] [PubMed] [Google Scholar]
- 138.Lin W, Mohandas B, Fontaine CP, Colvin RA. Release of intracellular Zn(2+) in cultured neurons after brief exposure to low concentrations of exogenous nitric oxide. Biometals. 2007;20(6):891–901. doi: 10.1007/s10534-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 139.Vallee BL, Auld DS. Active zinc binding sites of zinc metalloenzymes. Matrix Suppl. 1992;1:5–19. [PubMed] [Google Scholar]
- 140.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73(1):79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 141.Hashemzadeh-Gargari H, Guilarte TR. Divalent cations modulate N-methyl-D-aspartate receptor function at the glycine site. J. Pharmacol. Exp. Ther. 1999;290(3):1356–1362. [PubMed] [Google Scholar]
- 142.Cunningham MG, Ames HM, Christensen MK, Sorensen JC. Zincergic innervation of medial prefrontal cortex by basolateral projection neurons. Neuroreport. 2007;18(6):531–535. doi: 10.1097/WNR.0b013e328091c212. [DOI] [PubMed] [Google Scholar]
- 143.Kodirov SA, Takizawa S, Joseph J, Kandel ER, Shumyatsky GP, Bolshakov VY. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc. Natl. Acad. Sci. U S A. 2006;103(41):15218–15223. doi: 10.1073/pnas.0607131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salgueiro MJ, Boccio J. Zinc intake versus zinc absorption: a bioavailability factor. Nutrition. 2002;18(4):354. doi: 10.1016/s0899-9007(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 145.Dickinson A. The Benefits of Nutritional Supplements. Council for Responsible Nutrition; 2002. Recommended Intakes of Vitamins and Essential Minerals. [Google Scholar]
- 146.Gropper SS, Smith JL, Groff JL. Advanced nutrition and human metabolism. Bemont: Wadsworth; 2009. pp. 498–491. [Google Scholar]
- 147.Czerniak P, Haim DB. Phenothiazine derivatives and brain zinc. Turnover radioactive isotope study. Arch. Neurol. 1971;24(6):555–560. doi: 10.1001/archneur.1971.00480360089012. [DOI] [PubMed] [Google Scholar]
- 148.Keller KA, Chu Y, Grider A, Coffield JA. Supplementation with L-histidine during dietary zinc repletion improves short-term memory in zinc-restricted young adult male rats. J. Nutr. 2000;130(6):1633–1640. doi: 10.1093/jn/130.6.1633. [DOI] [PubMed] [Google Scholar]
- 149.Takeda A, Suzuki M, Oku N. Possible involvement of plasma histidine in differential brain permeability to zinc and cadmium. Biometals. 2002;15(4):371–375. doi: 10.1023/a:1020256018861. [DOI] [PubMed] [Google Scholar]
- 150.Joshi D, Mittal DK, Shukla S, Srivastav AK. Therapeutic potential of N-acetyl cysteine with antioxidants (Zn and Se) supplementation against dimethylmercury toxicity in male albino rats. Exp. Toxicol. Pathol. 2010 doi: 10.1016/j.etp.2010.07.001. [ahead of print] [DOI] [PubMed] [Google Scholar]
- 151.Daumas S, Halley H, Lassalle JM. Disruption of hippocampal CA3 network: effects on episodic-like memory processing in C57BL/6J mice. Eur. J. Neurosci. 2004;20(2):597–600. doi: 10.1111/j.1460-9568.2004.03484.x. [DOI] [PubMed] [Google Scholar]
- 152.Ringe K, Walz CM, Sabel BA. In: Nanoparticle Drug Delivery to the Brain. Encyclopedia of Nanoscience and Nanotechnology. Nalwa HS, editor. Vol. 7. Los Angeles: American Scientific Publishers; 2004. pp. 91–104. [Google Scholar]
- 153.Veroni AV, Tosi G, Tacchi R, Randelli MA, Bertolini A, Costantino L. Nanoparticles as drug delivery agents specific for CNS: in vivo biodistribution. Nanomedicine. 2009;5(4):369–377. doi: 10.1016/j.nano.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 154.Costantino L, Gandolfi F, Tosi G, Rivasi F, Vandelli MA, Forni F. Peptide-derivatized biodegradable nanoparticles able to cross the blood–brain barrier. Journal of Controlled Release. 2005;108:84–96. doi: 10.1016/j.jconrel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 155.Bush AI. Metal complexing agents as therapies for Alzheimer's disease. Neurobiol. Aging. 2002;23(6):1031–1038. doi: 10.1016/s0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 156.Doraiswamy PM, Finefrock AE. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 2004;3(7):431–434. doi: 10.1016/S1474-4422(04)00809-9. [DOI] [PubMed] [Google Scholar]
- 157.Gaeta A, Hider RC. The crucial role of metal ions in neurode-generation: the basis for a promising therapeutic strategy. Br. J. Pharmacol. 2005;146(8):1041–1059. doi: 10.1038/sj.bjp.0706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cuajungco MP, Fagét KY, Huang X, Tanzi RE, Bush AI. Metal chelation as a potential therapy for Alzheimer's disease. Ann. N Y Acad. Sci. 2000;920:292–304. doi: 10.1111/j.1749-6632.2000.tb06938.x. [DOI] [PubMed] [Google Scholar]
- 159.Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, Sjögren M, Wallin A, Xilinas M, Gottfries CG. Treatment of Alzheimer's disease with clioquinol. Dement. Geriatr. Cogn. Disord. 2001;12(6):408–414. doi: 10.1159/000051288. [DOI] [PubMed] [Google Scholar]
- 160.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30(3):665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 161.Cherny RA, Legg J-T, McLean CA, Fairlie DP, Huang X, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI. Aqueous dissolution of Alzheimer's disease Aβ amyloid deposits by biometal depletion. J. Biol. Chem. 1999;274:23223–23228. doi: 10.1074/jbc.274.33.23223. [DOI] [PubMed] [Google Scholar]
- 162.Hellmich HL, Frederickson CJ, DeWitt DS, Saban R, Parsley MO, Stephenson R, Velasco M, Uchida T, Shimamura M, Prough DS. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci. Lett. 2004;355(3):221–225. doi: 10.1016/j.neulet.2003.10.074. [DOI] [PubMed] [Google Scholar]