Summary
The functions of T lymphocytes are regulated by transcription factors controlling gene expression. New studies indicate that the transcriptional repressor Blimp-1 promotes the development of short-lived effector cells and regulates clonal exhaustion during acute and persistent infections, respectively.
The B cell transcriptional repressor Blimp-1 (B lymphocyte induced maturation protein-1), encoded by the Prdm1 gene locus, is known as a decision maker in the fate of B cells by regulating genes promoting B cell terminal differentiation into plasma cells but not into memory B cells (Shaffer et al, 2002; Shapiro-Shelef, et al., 2003). More recent studies, including three in this issue of Immunity, indicate that Blimp-1 may provide similar functions for CD8 T cells, by promoting the terminal differentiation of most into short-lived cytotoxic T lymphocytes (CTL) rather than long-lived central memory (CM) T cells (Kallies, et al., 2009; Rutishauser, et al., 2009; Shin, et al., 2009). Although originally defined in B cells, Blimp-1 is important for the development of multiple lineages during embryogenesis and promotes terminal differentiation of skin keratinocytes (John and Garrett-Sinha, 2009). Mice with T cell-specific deletions in Blimp-1 develop severe immunopathology, indicating a requirement for Blimp-1 in normal T cell homeostasis (Kallies, et al., 2006). Blimp-1 is induced in T cells by IL-2 and other cytokines, but its expression represses IL2 gene transcription (Martins, et al., 2008). Thus, it can serve in a negative feedback process, altering T cell gene expression.
How then, does the activity of Blimp-1 act on the T cell response to viral infection? Viruses are notoriously strong stimulators of CD8+ CTL, which can lyse virus-infected targets and control viral replication by secreting anti-viral cytokines like interferon (IFN)γ. Virus-specific T cells undergo a programmed expansion and differentiation into effector cells and thereafter contract in numbers due to apoptosis, leaving a smaller but stable subpopulation of memory T cells that can vigorously respond to re-infection at a later date (Harty and Badinovac, 2008). T cells activated during the acute infection can express different antigens that predict their fate. For example, mouse memory CD8 T cells are more likely derived from T cells expressing high levels of the IL-7 receptor (CD127) and low levels of the natural killer cell receptor KLRG1, whereas short-lived effector cells are more likely to be CD127low, KLRG1high (Parish and Kaech, 2009). Overwhelming viral loads, however, can drive T cells either into a clonal deletion by apoptosis or into a functional exhaustion associated with the expression of T cell inhibitory receptors, such as PD-1, LAG-3, 2B4, and CD160 (Blackburn, et al. 2009). These new studies indicate that Blimp-1 is an important regulator of all of these fates.
Rutishauser, et al. (2009) examined the role of Blimp-1 during the CD8 T cell response of mice acutely infected with lymphocytic choriomeningitis virus (LCMV), strain Armstrong. Using mice where the gene for yellow fluorescent protein (YFP) was under the control of Blimp-1 promotor elements, they found high expression in most cells during the acute response and lower expression in memory cells after resolution of infection. Blimp-1 mRNA, protein, and YFP reporter expression were high in the CD127lowKLRG1high population of short lived effector cells and low in the memory precursor CD127highKLRG1low cells. In the memory state, Blimp-1 expression remained elevated for some time in effector memory (EM) phenotype cells (CD127lowKLRG1highCD27lowCD62Llow) but low in the more frequent CM phenotype cells (CD127highKLRG1lowCD27highCD62Lhigh), which are considered highly proliferative on antigenic re-challenge. This suggests that Blimp1 expression distinguishes the effector cell lineage from the CM lineage. These authors crossed mice carrying the loxP-flanked Blimp1 (Pdrm1) gene with mice carrying cre-recombinase under control of the Granzyme B promoter, such that T cells would delete the Blimp1 gene when they became activated during infection. These Blimp-1 knock-out (KO) mice had reduced frequencies of short lived effector cells and a reduced contraction phase, coupled with enhanced overall survival of virus-specific CD8 T cells.
During the acute LCMV infection Blimp-1 deficiency yielded populations of CD8 T cells that could produce relatively normal levels of IFNγ and TNF, but they produced less Granzyme B, involved in cytotoxicity, and much more IL-2 than wild type (WT) cells. The relatively low IL-2 in Blimp-1+ cells and high amounts in Blimp-1- cells is consistent with Blimp-1 being a transcriptional repressor of the IL2 gene (Martins et al., 2008). These same functional properties of Blimp-1 KO T cells were also noted in the studies of Kallies et al. (2009) and Shin et al. (2009), and all three groups correlated Blimp-1 expression with short lived effector cells and the absence of Blimp-1 expression with the generation of CM cells.
Shin et al (2009) compared Blimp1 expression in T cells from mice undergoing acute LCMV-strain Armstrong infection to those from mice undergoing a persistent infection established by high dose inoculation with the highly disseminating clone 13 variant of LCMV. In both infections Blimp-1 mRNA or Blimp-1-YFP was elevated initially, but Blimp-1 expression then became especially elevated during the persistent infection. Blimp-1 was most highly expressed in T cells specific for the LCMV-encoded NP396 peptide, and these are usually the first T cells to exhaust or undergo apoptosis during persistent LCMV infection. Cells undergoing clonal exhaustion during persistent infection express high levels of PD-1, and the PD-1high cells expressed 2-3-fold more Blimp-1 mRNA than did a subset expressing lower levels of PD-1. In general, expression of Blimp1-YFP was highest in CD8 T cells expressing T cell exhaustion-associated inhibitory receptors, such as PD-1, Lag-3, 2B4, and CD160. LCMV-specific T cells from persistently infected Blimp-1-deficient mice expressed low levels of inhibitory receptors, resisted clonal exhaustion, and were at higher frequencies than those in infected WT mice, and many expressed high levels of CD127 and CD62L, consistent with a CM phenotype. Decreased inhibitory receptor expression on T cells in the absence of Blimp-1 might predict that Blimp-1 KO mice would clear persistent LCMV quickly, but this did not happen. Blimp-1 was also needed for effector cell function, and Blimp-1 KO T cells had reduced cytotoxicity against viral peptide-coated targets. However, “haploinsufficient” mice, constructed to have only one copy of the Blimp1 gene, had reduced expression of Blimp-1 and cleared LCMV better, presumably because this intermediate level of Blimp-1 allowed for effector cell function without up-regulating inhibitory receptors.
Kallies et al made chimeric mice with an immune system derived from fetal liver cells of mice whose Blimp1 gene was inactivated by a green fluorescent protein knock-in (Blimp1gfp), and found that those mice suffered severely from an influenza A virus (IAV) infection that was tolerated in control mice. Bone marrow chimeric mice were next made with a combination of Blimp1gfp/gfp (KO) and Blimp1+/+ (WT control), and the presence of the WT cells allowed for enhanced resistance to IAV and the opportunity to compare the responses of KO to WT cells under similar conditions of viral load. These, Blimp-1 KO T cells in IAV-challenged chimeric mice lacked the short-lived effector phenotype, and highly expressed the memory cell transcription factor Eomesodermin and the memory cell-associated transcriptional repressor Bcl-6, also detected in the molecular screens of Ruitshauser, et al. (2009). After resolution of infection a high proportion of Blimp-1 KO cells were CD127highCD62Lhigh, consistent with a CM phenotype. Thus, all three studies indicate that Blimp-1 expression is associated with effector cells and its absence is associated with long lasting CM cells or their precursors.
The Kallies study also detected a potentially important cell migration phenotype. After a primary IAV infection of chimeric mice, the Blimp-1 KO CD8 T cell frequency was relatively normal in the spleen but significantly reduced in the lungs, in comparison to WT cells. On IAV challenge of mice previously immunized with another IAV strain, the KO T cells were elevated in the spleen but again low in the lung, compared to WT controls. These experiments suggested that Blimp-1 was needed for trafficking into the lung, and indeed, in the Blimp-1 KO cells the expression of the lung homing chemokine receptor, CCR5, was reduced, and expression of CCR7, important for recruitment into the spleen and lymph nodes, was elevated.
Together these reports indicate that Blimp-1 promotes the generation of short-lived effector T cells, of clonally exhausted T cells, and of the migration of T cells out of the spleen and lymph nodes and into peripheral tissues. Blimp-1 expression does permit the generation of some longer lived EM cells, but its absence allows for the generation of long term CM cells, which are thought to have higher proliferative potential on secondary challenge. Blimp-1 is a transcriptional repressor of IL-2 (Martins, et al, 2008), and the generation of polyfunctional T cells having the ability to make IL-2 has been correlated with more protective antiviral responses in vaccine studies (Seder et al., 2008). What remains unclear is why some T cells express Blimp-1 and others do not, but cytokines can stimulate its expression. The contraction phase of T cell responses is less dramatic when T cells are stimulated in low inflammatory environments (Harty and Badinovac, 2008). Perhaps high levels of inflammatory cytokines promote the expression of Blimp-1 and the generation of short lived effector cells, whose expansion and then loss would cause a greater contraction.
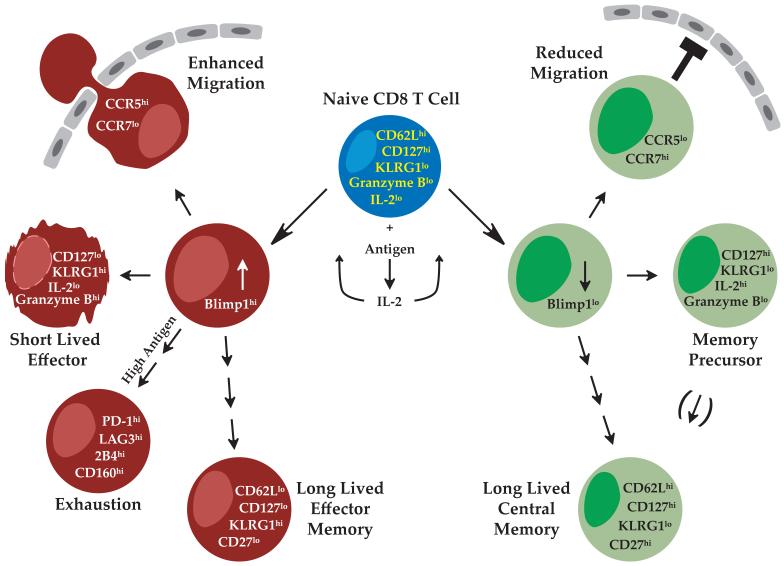
Figure 1. Blimp-1 and the fates of T cells during viral infections.
This figure portrays fates of CD8 T cells that either do or do not express Blimp-1 during a viral infection. Naïve T cells sensing antigen will secrete IL-2 and other cytokines and ultimately enter different differentiation pathways. IL-2 can stimulate expression of Blimp-1, which, in turn, will shut down IL-2 expression. Blimp-1+ cells can migrate into peripheral tissues, become short-lived effector cells under conditions of low antigen load, and become clonally exhausted under conditions of high antigen load. Some survive longer term as effector memory cells. Cells lacking Blimp-1 migrate relatively poorly into the periphery, produce IL-2, and are most of the memory cell precursors, evolving to become central memory cells.
Acknowledgements
This work was supported by United States National Institutes of Health Research grants R37 AI017672 and AI081675. The opinions expressed are those of the author and not necessarily that of the NIH. I thank Keith A. Daniels for preparing the figure, and Drs. Eva Szomolanyi-Tsuda and Stephen Waggoner for reviewing the manuscript.
References
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping T cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- John SA, Garrett-Sinha LA. Blimp1: a conserved transcriptional repressor critical for differentiation of many tissues. Exp Cell Res. 2009;315:1077–1084. doi: 10.1016/j.yexcr.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp1 is required for the differentiation of protective effector T cells and memory responses. Immunity. 2009 doi: 10.1016/j.immuni.2009.06.021. ????? [DOI] [PubMed] [Google Scholar]
- Martins GA, Cimmino L, Liao J, Magnusdottir E, Calame K. Blimp-1 directly represses IL-2 and the IL-2 activator Fos, attenuating T cell proliferation and survival. J Exp Med. 2008;205:1959–1965. doi: 10.1084/jem.20080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish IA, Kaech SM. Diversity in T cell differentiation. Curr Opinion Immunol. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Blimp-1 promotes terminal differentiation of virus-specific CD8 T cells and represses the acquisition of central memory T cell properties. Immunity. 2009 doi: 10.1016/j.immuni.2009.05.014. ????? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin K-I, Kyo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin K-I, McHezer-Williams LJ, Liao J, MeHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8 T cell exhaustion during chronic viral infection. Immunity. 2009 doi: 10.1016/j.immuni.2009.06.019. ?????? [DOI] [PMC free article] [PubMed] [Google Scholar]