Abstract
Purpose
Curative surgery for patients with advanced or even early gastric cancer can be defined as resection of the stomach and dissection of the first and second level lymph nodes, including the greater omentum. The aim of this study was to evaluate the short- and long- term outcomes of partial omentectomy (PO) as compared with complete omentectomy (CO).
Materials and Methods
Seventeen consecutive open distal gastrectomies with POs were initially performed between February and July in 2006. The patients' clinicopathologic data and post-operative outcomes were retrospectively compared with 20 patients who underwent open distal gastrectomies with COs for early gastric cancer in 2005.
Results
The operation time in PO group was significantly shorter than that in CO group (142.4 minutes vs. 165.0 minutes, p=0.018). The serum albumin concentration on the first post-operative day in PO group was significantly higher than CO group (3.8 g/dL vs. 3.5 g/dL, p=0.018). Three postoperative minor complications were successfully managed with conservative treatment. Median follow-up period between PO and CO was 38.1 and 37.7 months. All patients were alive without recurrence until December 30, 2009.
Conclusion
PO during open radical distal gastrectomy can be considered a more useful procedure than CO for treating early gastric cancer. To document the long-term technical and oncologic safety of this procedure, a large-scale prospective randomized trial will be needed.
Keywords: Partial omentectomy, gastric cancer, post-operative outcome, comparative study
INTRODUCTION
The greater omentum is the largest peritoneal fold and contains areas with high concentrations of immune cells that may aid in the removal of foreign materials and bacteria. The greater omentum becomes densely adherent to intraperitoneal sites of inflammation, often preventing free peritonitis during instances of intestinal gangrene or perforation. The greater omentum is also frequently involved in intra-abdominal dissemination of gastrointestinal or ovarian malignancies, either facilitating primary spread or being a site of recurrent cancer after surgical treatment.1,2
While curative surgery for patients with advanced or even early gastric cancer can be defined as resection of the stomach and dissection of the first and second level lymph nodes, including the greater omentum,3 early gastric cancer (pT1, mucosa or submucosa) is known to be one of the gastrointestinal malignancies which have satisfactory prognosis, with 5-year survival rates of >90% by appropriate surgical treatment.4-6 Only 10% of early gastric cancer patients have metastatic lymph nodes, which are primarily located in the perigastric area (first level lymph nodes).7 Therefore, many physicians have been interested in a variety of limited surgeries,8-11 including local resection, such as endoscopic submucosal dissection, segmental resection, pylorus-preserving gastrectomy, and sentinel node navigation surgery, for treating early gastric cancer. Even though Japanese guidelines for treatment of gastric cancer3 is still considered controversial in the west, recent studies have demonstrated that western surgeons can be trained to perform dissection of first and second level lymph nodes named D2 lymphadenectomy on western patients with low postoperative morbidity and mortality.12,13
Recently, laparoscopy-assisted gastrectomy (LAG) has become an attractive treatment alternative in the East14-17 and some regions of the west18,19 as one of the minimally invasive options for early gastric cancer. First large-scale prospective randomized multicenter study of laparoscopic versus open gastrectomy for gastric cancer has been on-going by the Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) group since January 2006.14 Favorable interim result of KLASS trial was published.20 Regarding surgical techniques, LAG for gastric cancer is equivalent to open surgery except for the extent of the omentectomy. Realistically, a significant number of laparoscopic surgeons are performing partial omentectomies,21-23 instead of complete omentectomies,19,24,25 during curative gastric surgery for treating early gastric cancer.
However, no reports on partial omentectomies during gastric cancer surgery have been published. The aim of this study was to evaluate the short- and long-term outcomes of partial omentectomy as compared with complete omentectomy.
MATERIALS AND METHODS
Laparoscopy-assisted distal gastrectomy (LADG) with partial omentectomy was first performed in our institute in 1998. Seventeen consecutive open distal gastrectomies with 962partial omentectomies were initially performed between February and July in 2006 by one surgeon (Kim MC). The patients' clinicopathologic data and post-operative outcomes were retrospectively compared with 20 patients who underwent conventional open distal gastrectomies with complete omentectomies for early gastric cancer in 2005. The clinicopathologic data included the patients' age, gender, body mass index, co-morbidities, pT stage, pN stage, the number of retrieved lymph nodes and the extent of lymph node dissection. The post-operative outcomes consisted of operation times, quantity of post-operative transfusions, serum albumin level on the first post-operative day, time to first flatus, complications, postoperative hospital stay, median follow-up duration, and recurrence.
Surgical procedure of partial omentectomy
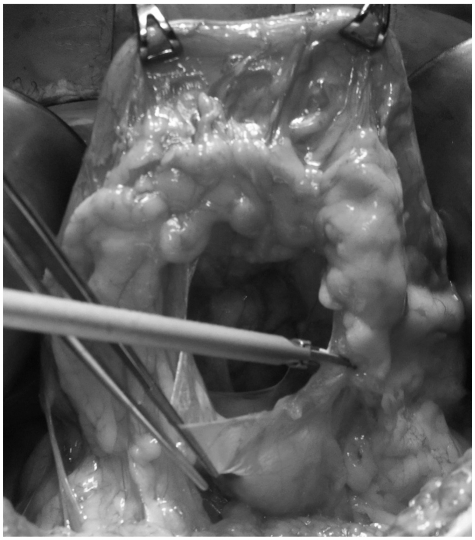
While the anterior wall of the stomach was pulled in the cranial direction by two Babcock clamps, the lesser sac was opened by dividing the greater omentum 4-5 cm from the gastroepiploic arcade using ultrasonic shears toward the most distal short gastric vessel (Fig. 1). One or two short gastric vessels were divided at their origin, including lymph nodes (all 4d and some 4sb). The right side of the greater omentum attaches itself to the mesentery of the transverse colon. This fusion is an exact plan of dissection for a right partial omentectomy. After adequate exposure of the anterior surface of the pancreas, the right gastroepiploic vessels were carefully identified and divided at their origin without any disturbance of the gastric lymphatic basin.
Fig. 1.
Partial omentectomy during gastric cancer surgery for early gastric cancer. The gastroepiploic vessel arcade which contains perigastric lymph nodes is not disturbed.
Perioperative management
Patients in both groups were managed by a standardized clinical pathway as follows: 1) no nasogastric intubation or pre-operative mechanical bowel preparation; 2) one closed suction drain near the anastomotic site; 3) sips of water 48 hrs after the operation; 4) a clear liquid diet on postoperative day 3 without regard to the first bowel movement; and 5) hospital discharge is recommended when patients tolerate a soft diet and pain after post-operative day 4. All patients received a continuous intravenous injection of mixed analgesics for 3-4 days after surgery.
Statistical analysis
Data were collected by reviewing the medical records and the Dong-A gastric cancer database. All statistical analyses were performed by SPSS software (basic and advanced program, version 11.0; SPSS Inc., Chicago, IL, USA) and GraphPad InStat® (version 3.06, GraphPad Software, Inc., La Jolla, CA, USA). A p<0.05 was considered to indicate statistical significance.
RESULTS
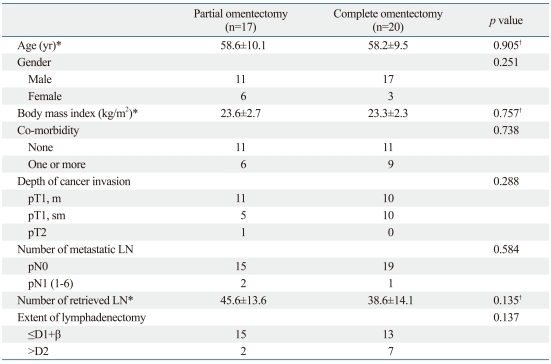
There were no intra-operative or post-operative complications related to the extent of omentectomy. Table 1 summarizes the patients' clinicopathologic characteristics, which were not significantly different in both groups. Of 17 patients in the partial omentectomy group, 2 patients had 1 and 3 metastatic lymph nodes, respectively. These metastatic lymph nodes were localized in the perigastric area (first level lymph node). The number of lymph nodes which should affect accurate staging was retrieved in both groups.
Table 1.
Clinicopathologic Characteristics of Partial Omentectomy and Complete Omentectomy Group
D1+β, D1+no 7, 8a, and 9; D2, D1+β+no 11p, 12a, and 14v; LN, lymph node.
Fisher's exact test for other variables.
*All values are the mean and standard deviation.
†Unpaired t-test.
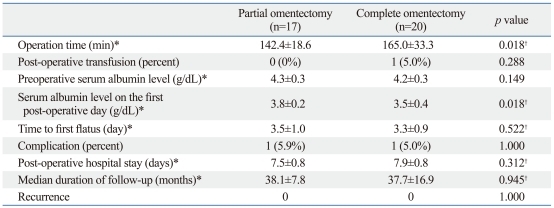
The post-operative outcomes with patients in both groups are listed in Table 2. As shown, the operation time in the partial omentectomy group was significantly shorter than complete omentectomy group (142 vs. 165 minutes, p=0.0176). The serum albumin concentration on the first post-operative day in the partial omentectomy group was significantly higher than the complete omentectomy group (3.8 vs. 3.5 g/dL, p=0.0179). Two patients had three postoperative complications, including wound infection and intraabdominal fluid collection in one patient in the complete omentectomy group and atelectasis in one patient in the partial omentectomy group. All complications were successfully managed with conservative treatment.
Table 2.
Post-Operative Outcomes of Partial Omentectomy and Complete Omentectomy Group
Fisher's exact test for other variables.
*All values are the mean and standard deviation.
†Unpaired t-test.
All patients were alive without recurrence until December 30, 2009, although one patient in the partial omentectomy group underwent surgery for a metachronous colon cancer.
DISCUSSION
Comparison of partial with complete omentectomy
In the present study, patients who underwent a partial omentectomy had a significantly shorter operation time and a higher concentration of serum albumin on the first post-operative day. Operation time is one of the important parameters in assessing feasibility and learning curve for a specific procedure.26,27 Also, operation time can be associated with post-operative morbidity.28 Ultrasonic shears have contributed to a reduction in the operation time in procedures by cutting many omental branches given off from the gastroepiploic arcade. The serum albumin level on the first post-operative day is influenced by the extent of tissue dissection and manipulation during surgery and the amount of peri-operative fluid replacement. Ryan, et al.29 concluded that the serum albumin concentration on the first post-operative day following esophagectomy is a better predictor of surgical outcome than many other pre-operative risk factors. With the exception of two factors described above, there are no significant differences in surgical or oncologic parameters, such as the number of retrieved lymph nodes, post-operative complication rate, and recurrence between both groups.
Functions of the greater omentum and omentectomy for intraabdominal malignancies
The greater omentum hangs inferiorly from the greater curvature of the stomach. The greater omentum is a double sheet; each sheet consists of two layers of peritoneum separated by a scant amount of connective tissue. One of the important functions of the greater omentum is to contain the wide spread of infection by adhesions to inflamed bowels.30 In addition, the peritoneum, including the omentum, is a relatively common site of either recurrent disease or primary seeding in both gastrointestinal and ovarian cancers. Then, the standard treatment practice for epithelial ovarian cancer includes the removal of the omentum as part of the surgical treatment in more advanced stages and to allow adequate staging in perceptible early stage disease.1 Complete omentectomy and extensive lymphadenectomy have been recommended in Japan for improving the prognosis of gastric cancer patients with peritoneal metastases in the adjacent peritoneum.31
Partial omentectomy in LADG for early gastric cancer
The greater omentum during gastric cancer surgery has been routinely removed for two reasons: 1) complete dissection of second level lymph nodes without any disturbance of the lymphatic basin; and 2) removal of macro- or micro-metastatic omental lesions. In patients with serosal infiltration in which cancer is located in the anterior wall of the stomach, complete dissection of second level lymph nodes with a complete omentectomy and omental bursectomy is required.32 However, partial omentectomy during LADG for early gastric cancer is preferable to complete omentectomy for three reasons: 1) more challenging technique, especially in obese patients; 2) rarity of nodal or omental metastases in early gastric cancer; and 3) complete omentectomy can incur serious complications such as colon and mesocolon injuries. However, Lee and Kim33 has suggested for advanced gastric cancer that complete omentectomy and dissection of first and second level lymph nodes during LAG should be achieved.
Advantages of partial omentectomy
Based on our results, if surgeons have a thorough knowledge of surgical anatomy of the greater omentum, partial omentectomy can be offered as a favorable option for treating early gastric cancer with respect to the post-operative outcomes, such as operation time and post-operative albumin level. Theoretically, the residual omentum after a partial omentectomy might fill up some anastomotic microleakages by adhesions to the inflamed bowel. Although risks and benefits exist, it is anticipated that macrophages residing within the omentum might play an important role in clearing minimal residual disease or free peritoneal tumor cells, therefore, a complete omentectomy may impair the anit-tumor immune response.34,35 In contrast to these benefits, the residual omentum is able to affect post-operative intra-abdominal adhesions and omental infarction, however, we had no patients with clinical symptoms caused by intra-operative adhesions or omental infarction.
Further study
With respect to surgical or oncologic safety, there are some drawbacks in the present study, such as retrospective analysis, small number of patients, and short follow-up period. The number of cases in the present study was too small to detect a significant difference in both groups with respect to oncologic aspects. However, previous large-scale, long-term results of LADG with partial omentectomy can provide apparent surgical or oncologic evidence.14-18,22,23
In conclusion, partial omentectomy during open radical distal gastrectomy can be considered a more useful procedure than complete omentectomy for treating early gastric cancer. To document the long-term technical and oncologic safety of this procedure, a large-scale prospective randomized trial will be needed.
ACKNOWLEDGEMENTS
This work was supported by the Dong-A University Research Fund in 2007.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S FIGO Committee on Gynecologic Oncology. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. Int J Gynaecol Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 2.Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76. doi: 10.1007/s101200200012. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H. Surgical treatment for gastric cancer: the Japanese approach. Semin Oncol. 1996;23:360–368. [PubMed] [Google Scholar]
- 5.Siewert JR, Sendler A. The current management of gastric cancer. Adv Surg. 1999;33:69–93. [PubMed] [Google Scholar]
- 6.Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 years' experience at a single institute in Korea. Eur J Surg Oncol. 2008;34:36–41. doi: 10.1016/j.ejso.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Noh SH, Hyung WJ, Cheong JH. Minimally invasive treatment for gastric cancer: approaches and selection process. J Surg Oncol. 2005;90:188–193. doi: 10.1002/jso.20228. [DOI] [PubMed] [Google Scholar]
- 8.Jee YS, Hwang SH, Rao J, Park DJ, Kim HH, Lee HJ, et al. Safety of extended endoscopic mucosal resection and endoscopic submucosal dissection following the Japanese Gastric Cancer Association treatment guidelines. Br J Surg. 2009;96:1157–1161. doi: 10.1002/bjs.6686. [DOI] [PubMed] [Google Scholar]
- 9.Shimoyama S, Seto Y, Yasuda H, Kaminishi M. Wider indications for the local resection of gastric cancer by adjacent lymphadenectomy. J Surg Oncol. 2000;75:157–164. doi: 10.1002/1096-9098(200011)75:3<157::aid-jso2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Morii Y, Arita T, Shimoda K, Hagino Y, Yoshida T, Kitano S. Indications for pylorus-preserving gastrectomy for gastric cancer based on lymph node metastasis. Hepatogastroenterology. 2002;49:1477–1480. [PubMed] [Google Scholar]
- 11.Kitagawa Y, Saha S, Kubo A, Kitajima M. Sentinel node for gastrointestinal malignancies. Surg Oncol Clin N Am. 2007;16:71–80. doi: 10.1016/j.soc.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Strong VE, Devaud N, Karpeh M. The role of laparoscopy for gastric surgery in the West. Gastric Cancer. 2009;12:127–131. doi: 10.1007/s10120-008-0516-1. [DOI] [PubMed] [Google Scholar]
- 13.Yoon SS, Yang HK. Lymphadenectomy for gastric adenocarcinoma: should west meet east. Oncologist. 2009;14:871–882. doi: 10.1634/theoncologist.2009-0070. [DOI] [PubMed] [Google Scholar]
- 14.Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale Korean multicenter study. Ann Surg Oncol. 2008;15:2692–2700. doi: 10.1245/s10434-008-0075-z. [DOI] [PubMed] [Google Scholar]
- 15.Kim W, Song KY, Lee HJ, Han SU, Hyung WJ, Cho GS. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg. 2008;248:793–799. doi: 10.1097/SLA.0b013e3181887516. [DOI] [PubMed] [Google Scholar]
- 16.Cho GS, Kim W, Kim HH, Ryu SW, Kim MC, Ryu SY. Multicentre study of the safety of laparoscopic subtotal gastrectomy for gastric cancer in the elderly. Br J Surg. 2009;96:1437–1442. doi: 10.1002/bjs.6777. [DOI] [PubMed] [Google Scholar]
- 17.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009;16:1507–1513. doi: 10.1245/s10434-009-0386-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 21.Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007;33:700–705. doi: 10.1016/j.ejso.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Shiraishi N, Yasuda K, Kitano S. Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric Cancer. 2006;9:167–176. doi: 10.1007/s10120-006-0380-9. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SH, Park do J, Jee YS, Kim MC, Kim HH, Lee HJ, et al. Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg. 2009;144:559–564. doi: 10.1001/archsurg.2009.110. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138–1142. doi: 10.1001/archsurg.2009.223. [DOI] [PubMed] [Google Scholar]
- 25.Tinoco RC, Tinoco AC, El-Kadre LJ, Sueth DM, Conde LM. Laparoscopic gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech. 2009;19:384–387. doi: 10.1097/SLE.0b013e3181ba4701. [DOI] [PubMed] [Google Scholar]
- 26.Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009;146:1048–1055. doi: 10.1016/j.surg.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Sammon J, Perry A, Beaule L, Kinkead T, Clark D, Hansen M. Robot-assisted radical prostatectomy: learning rate analysis as an objective measure of the acquisition of surgical skill. BJU Int. 2010;106:855–860. doi: 10.1111/j.1464-410X.2009.09187.x. [DOI] [PubMed] [Google Scholar]
- 28.McGillicuddy EA, Schuster KM, Davis KA, Longo WE. Factors predicting morbidity and mortality in emergency colorectal procedures in elderly patients. Arch Surg. 2009;144:1157–1162. doi: 10.1001/archsurg.2009.203. [DOI] [PubMed] [Google Scholar]
- 29.Ryan AM, Hearty A, Prichard RS, Cunningham A, Rowley SP, Reynolds JV. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg. 2007;11:1355–1360. doi: 10.1007/s11605-007-0223-y. [DOI] [PubMed] [Google Scholar]
- 30.Uzunköy A, Ozbilge H, Horoz M. The influence of omentectomy on bacterial clearance: an experimental study. Ulus Travma Acil Cerrahi Derg. 2009;15:541–545. [PubMed] [Google Scholar]
- 31.Hagiwara A, Sawai K, Sakakura C, Shirasu M, Ohgaki M, Yamasaki J, et al. Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology. 1998;45:1922–1929. [PubMed] [Google Scholar]
- 32.Yokota T, Ishiyama S, Saito T, Teshima S, Shimotsuma M, Yamauchi H. Treatment strategy of limited surgery in the treatment guidelines for gastric cancer in Japan. Lancet Oncol. 2003;4:423–428. doi: 10.1016/s1470-2045(03)01140-9. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Kim W. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol. 2009;100:693–698. doi: 10.1002/jso.21400. [DOI] [PubMed] [Google Scholar]
- 34.Oosterling SJ, van der Bij GJ, Bögels M, van der Sijp JR, Beelen RH, Meijer S, et al. Insufficient ability of omental milky spots to prevent peritoneal tumor outgrowth supports omentectomy in minimal residual disease. Cancer Immunol Immunother. 2006;55:1043–1051. doi: 10.1007/s00262-005-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosch B, Guller U, Schnider A, Maurer R, Harder F, Metzger U, et al. Perioperative detection of disseminated tumour cells is an independent prognostic factor in patients with colorectal cancer. Br J Surg. 2003;90:882–888. doi: 10.1002/bjs.4129. [DOI] [PubMed] [Google Scholar]