Abstract
DNA minicircles found within the kinetoplast of the trypanosomatid Crithidia fasciculata, like those of most other kinetoplastid species, are heterogeneous in sequence. The pattern of minicircle DNA fragments generated by cleavage of kinetoplast DNA with various restriction enzymes has been used to demonstrate this heterogeneity. Here we describe a strain of Crithidia fasciculata in which more than 90% of the DNA minicircles exhibit a common pattern of restriction enzyme cleavage sites. A map of cleavage sites within this major minicircle DNA class is presented for seven restriction enzymes with hexanucleotide recognition sequences. Sequence homogeneity at an even finer level is reflected in minicircle DNA digestion patterns generated by restriction enzymes with tetranucleotide recognition sites. Partial DNA sequence analysis of multiple clones from the major minicircle class shows nearly complete homogeneity at the nucleotide level. The existence of a near homogeneous complement of DNA minicircles in Crithidia should facilitate the study of their replication in this organism.
Full text
PDF

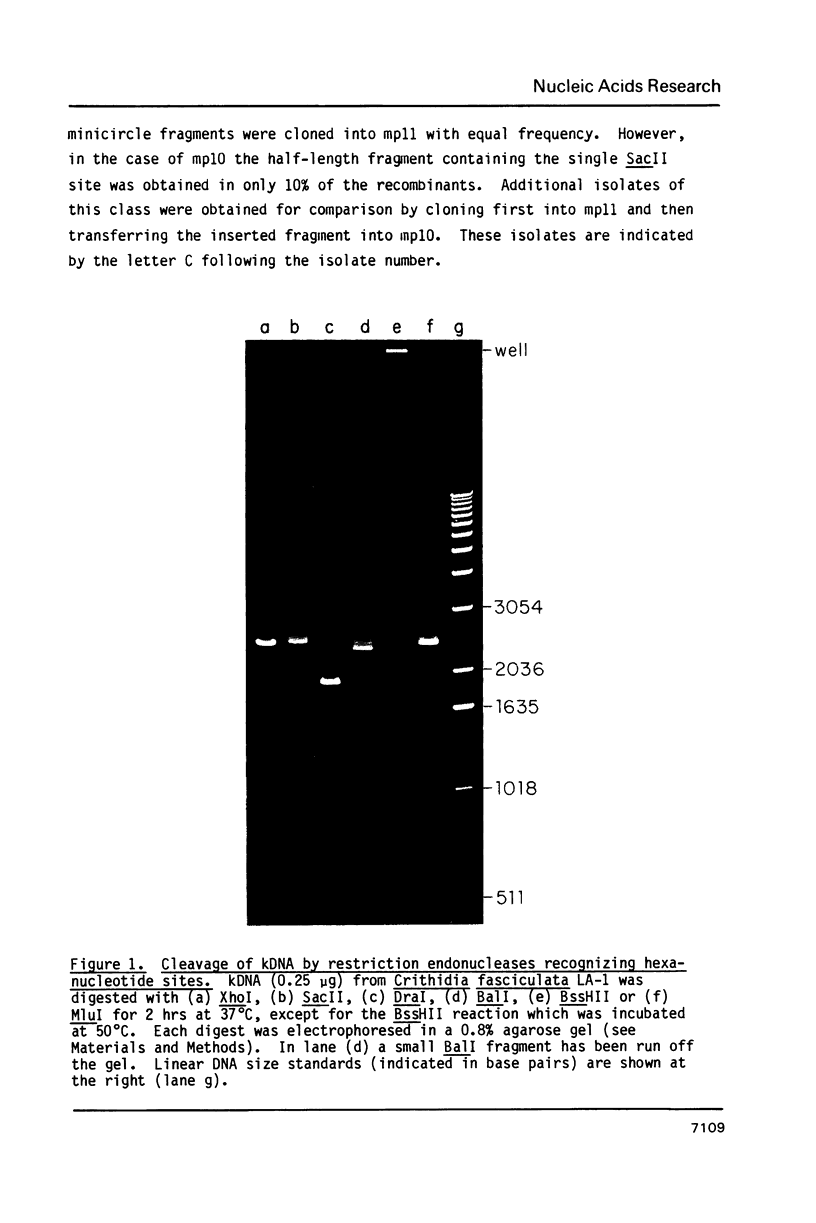
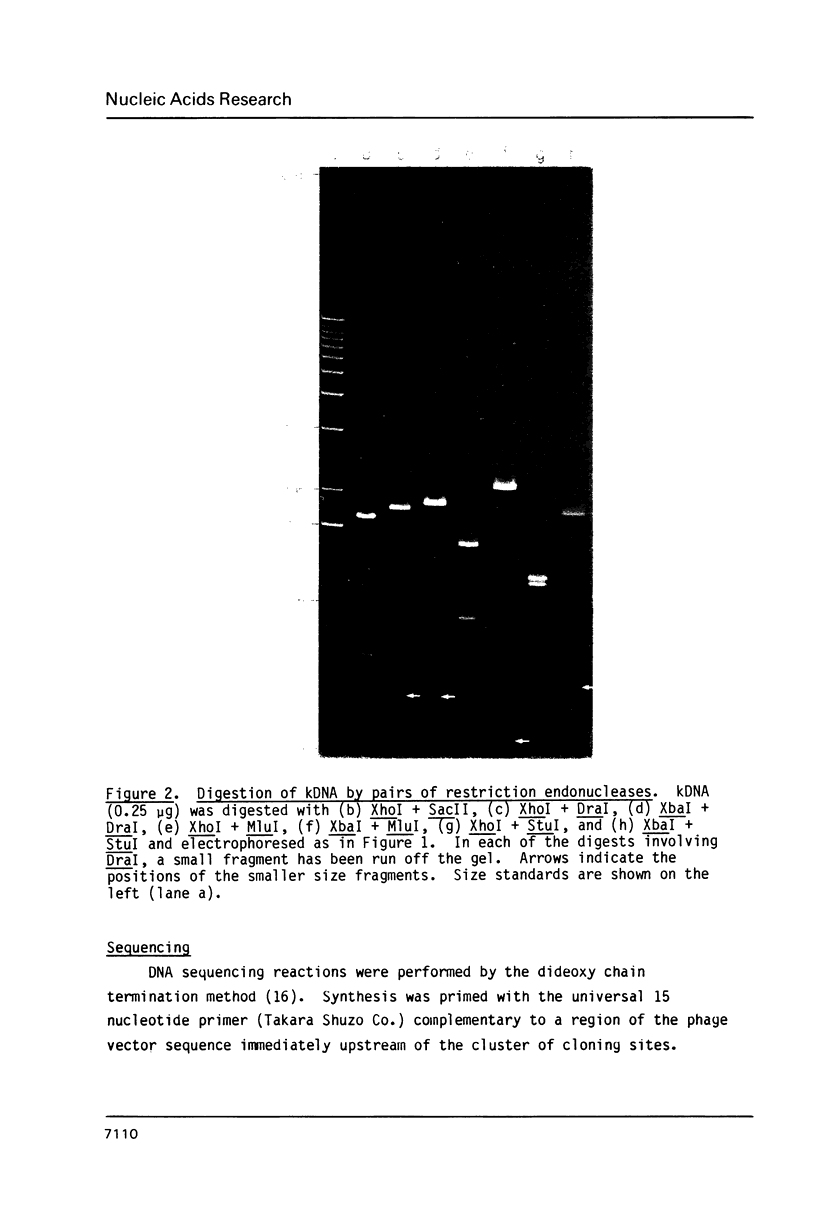



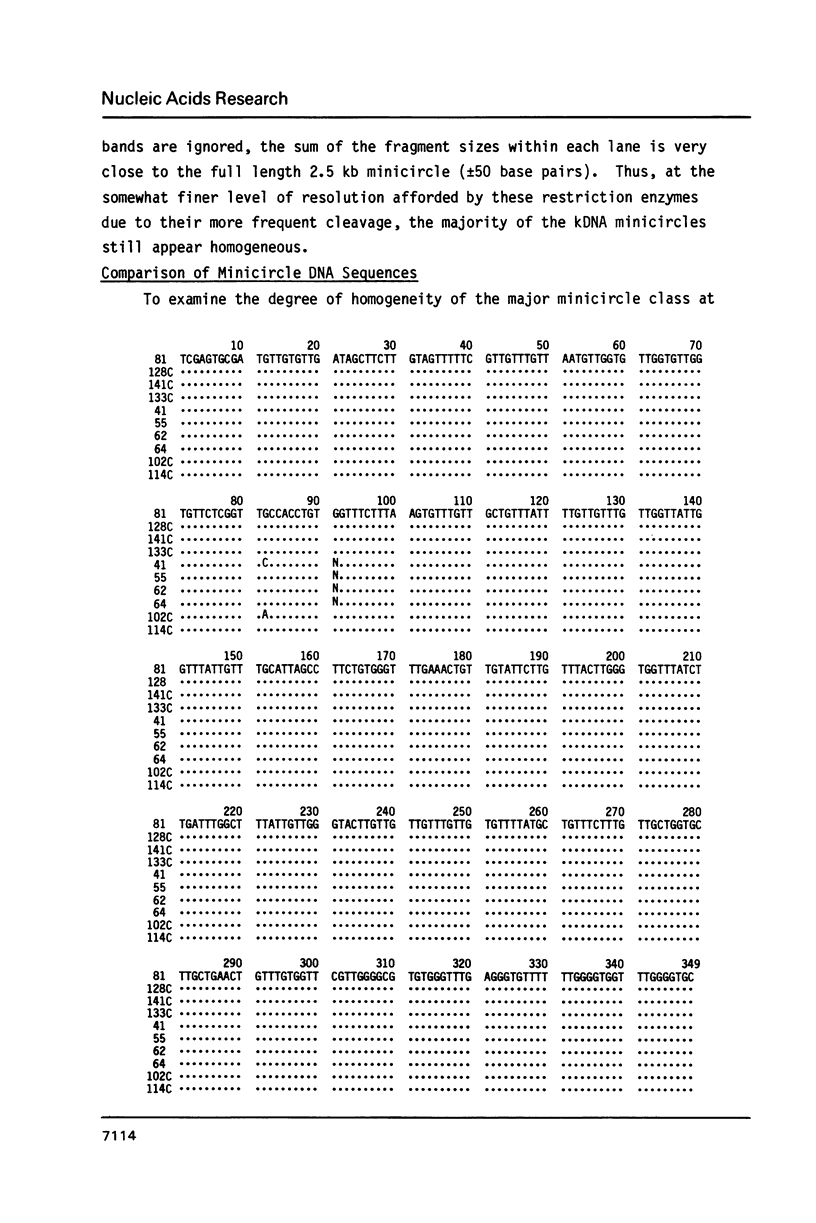



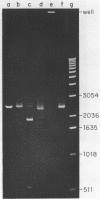
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P., Fase-Fowler F., Steinert M., Van Assel S. Maxi-circles in the kinetoplast DNA of Trypanosoma mega. Exp Cell Res. 1977 Nov;110(1):167–173. doi: 10.1016/0014-4827(77)90283-x. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Hajduk S. L., Hoeijmakers J. H., Borst P., Brunel E., Davison J. The kinetoplast DNA of Trypanosoma equiperdum. Biochim Biophys Acta. 1980 May 30;607(3):397–410. doi: 10.1016/0005-2787(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. II. Sequence evolution of the minicircles. Plasmid. 1982 May;7(3):210–220. doi: 10.1016/0147-619x(82)90002-6. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Schoutsen B., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. I. Sequence evolution and transcription of the maxicircle. Plasmid. 1982 May;7(3):199–209. doi: 10.1016/0147-619x(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Weijers P. J., Brakenhoff G. J., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. III. Heteroduplex analysis of the C. luciliae minicircles. Plasmid. 1982 May;7(3):221–229. doi: 10.1016/0147-619x(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Fein B. I., Englund P. T. Gapped Minicircles. A novel replication intermediate of kinetoplast DNA. J Biol Chem. 1984 Dec 25;259(24):15532–15539. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Isolation and characterization of kinetoplast DNA networks and minicircles from Crithidia fasciculata. J Protozool. 1974 Nov;21(5):774–781. doi: 10.1111/j.1550-7408.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Steinert M., Van Assel S. Sequence heterogeneity in kinetoplast DNA: reassociation kinetics. Plasmid. 1980 Jan;3(1):7–17. doi: 10.1016/s0147-619x(80)90030-x. [DOI] [PubMed] [Google Scholar]
- Sugden B., De Troy B., Roberts R. J., Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975 Sep;68(1):36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]