Abstract
Vision begins with photoisomerization of 11-cis retinal to the all-trans conformation within the chromophore-binding pocket of opsin, leading to activation of a biochemical cascade. Release of all-trans retinal from the binding pocket curtails but does not fully quench the ability of opsin to activate transducin. All-trans retinal and some other analogs, such as β-ionone, enhance opsin’s activity, presumably on binding the empty chromophore-binding pocket. By recording from isolated salamander photoreceptors and from patches of rod outer segment membrane, we now show that high concentrations of β-ionone suppressed circulating current in dark-adapted green-sensitive rods by inhibiting the cyclic nucleotide-gated channels. There were also decreases in circulating current and flash sensitivity, and accelerated flash response kinetics in dark-adapted blue-sensitive (BS) rods and cones, and in ultraviolet-sensitive cones, at concentrations too low to inhibit the channels. These effects persisted in BS rods even after incubation with 9-cis retinal to ensure complete regeneration of their visual pigment. After long exposures to high concentrations of β-ionone, recovery was incomplete unless 9-cis retinal was given, indicating that visual pigment had been bleached. Therefore, we propose that β-ionone activates and bleaches some types of visual pigments, mimicking the effects of light.
Keywords: Retinoids, Phototransduction, Rhodopsin, Cyclic nucleotide-gated channel, Neurophysiology
Introduction
In vertebrate visual pigments, photoisomerization of 11-cis retinal to all-trans retinal changes the structure of the surrounding opsin protein, forming a catalytically active state (reviewed in Makino et al., 2003; Fu & Yau, 2007). The chromophore is released eventually, but unbound “free” opsin retains a low constitutive activity. An abundance of free opsin therefore results in an “equivalent light” that desensitizes photoreceptors to an extent far beyond that caused by the decrement in photon capture (Cornwall et al., 1990; Jones et al., 1993). The condition is referred to as pigment or bleaching adaptation. Photoreceptors recover from pigment adaptation after opsin’s inherent activity is shut off by the binding of another 11-cis retinal.
Opsin’s activity can be altered by retinal and analogs of varying length, but the direction of the effect is dependent on opsin type. For example, the truncated analog, β-ionone, quenches opsin’s activity in partially bleached salamander red-sensitive (RS) cones (Jin et al., 1993) but has the opposite effect in all other photoreceptor types: green-sensitive (GS) rods (Kefalov et al., 1999), blue-sensitive (BS) rods and cones, and ultraviolet (UV)-sensitive (UVS) cones (Isayama et al., 2006). Since β-ionone also slows the rate at which GS rod opsin binds 11-cis retinal (Matsumoto & Yoshizawa, 1975; Daemen, 1978), its effect on catalytic activity was presumed to target the empty chromophore-binding pocket of bleached visual pigments. On the other hand, all-trans retinal stimulates GS rod opsin (Jäger et al., 1996; Sachs et al., 2000; Kono et al., 2008), but it cannot access the chromophore-binding pocket (Jäger et al., 1996). It must act at another site on opsin. The question arose as to whether opsin could be activated pharmacologically when its chromophore-binding pocket is occupied by 11-cis retinal. To find out, we applied β-ionone to dark-adapted cells to see if it would induce pigment adaptation.
Materials and methods
Animals
Care and use of all animals conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and institutional guidelines. Larval tiger salamanders (Ambystoma tigrinum; Charles D. Sullivan, Inc., Nashville, TN) were maintained in Holtfretter’s solution with a twofold higher salinity on a 14-h light/10-h dark cycle at 16–20°C.
Nomenclature
We specify salamander rod and cone types according to the wavelength of light to which the cells are most sensitive; for example, RS cones absorb and are most sensitive to red light. In this nomenclature, GS rods correspond to “red rods” and BS rods to “green rods.”
Single-cell recording
Salamanders were dark adapted overnight and decapitated, and retinal tissue was prepared under infrared illumination (Makino et al., 1999). A piece of retina was mechanically dissociated, placed in the recording chamber, and perfused continuously with Ringer’s solution consisting of (mM): 108 NaCl, 2.5 KCl, 1.0 MgCl2, 10 HEPES, 1.5 CaCl2, 0.02 ethylene diamine tetraacetic acid (EDTA), 10 glucose, and 7.5 × 10−4 bovine serum albumin (BSA; Fraction V, c-globulin-free; Sigma, St. Louis, MO), pH 7.6, at 20–22°C. Photo-currents were recorded from the inner segment of a single rod or cone with a suction electrode and a patch clamp amplifier (Axopatch 200A; Axon Instruments, Foster City, CA), low pass filtered with an 8-pole Bessel (30 Hz, −3 dB), and digitized at 400 Hz. No corrections were made for the delay introduced by filtering. Cells were stimulated with light from a 75-W xenon arc lamp that passed through a six-cavity interference filter (10-nm bandwidth at half-maximal transmission; Omega Optical, Brattleboro, VT) and anelectronic shutter. A nominal duration of 22 ms was used for test flashes. Cells were identified by their morphology and by their responses to flashes at 377, 440, 500, and 620 nm. The light was calibrated with a photometer (UDT 350; Graseby, Orlando, FL) through a 200-μm-diameter pinhole (Melles Griot, Carlsbad, CA) placed at the level of the recording chamber.
β-Ionone was administered through one channel of a two-channel laminar flow perfusion system (Isayama et al., 2006). The other channel contained Ringer’s solution. β-Ionone, redistilled from a commercial source (Sigma), was dissolved in absolute ethanol and then diluted into Ringer’s solution. The actual concentration of β-ionone reaching the cells was determined by spectrophotometric analysis of perfusate collected after each experiment. Perfusions of RS cones with β-ionone were kept to durations less than 10 min to minimize pigment bleaching (Isayama et al., 2006). To address the possibility that β-ionone exerted an effect on large pools of free opsin present in dark-adapted cells, cells were subjected to Ringer’s solution through bulk flow in the bath and then perfused with 13 ± 2 μM 9-cis retinal (Sigma) for .20 min to fully regenerate the cell’s pigment followed by β-ionone before being returned to Ringer’s solution in the bath. The final ethanol concentration of all solutions never exceeded 0.1%. In control experiments, 0.1% ethanol had no effect on the flash responses of any cells either before or after partial bleaching.
Data were analyzed using Igor Pro 5.03 (Wavemetrics, Lake Oswego, OR). Analysis of variance statistics and post hoc tests were performed using Stata (release 6; Stata Corporation, College Station, TX) or an MS Excel spreadsheet provided by the online Handbook of Biological Statistics Web site (http://udel.edu/~mcdonald/statintro.html; John McDonald, University of Delaware).
Patch clamp recording of excised membrane patches
Standard patch clamp methods were used to test for a direct effect of β-ionone on rod and cone cyclic nucleotide-gated channels. Oocytes were surgically removed from anesthetized Xenopus laevis frogs and injected with complementary RNA (cRNA) for either the bovine rod alpha (CNGA1) subunit or the human cone alpha (CNGA3) subunit in the pGEMHE plasmid (Liman et al., 1992). cRNA was made by in vitro transcription using the mMessage mMachine Kit™ (Ambion, Austin, TX). Injected oocytes were incubated at 16°C for 4–12 days prior to use (McCabe et al., 2004). Native channels were studied in membrane patches excised from the outer segments of GS salamander rods.
Ionic currents were recorded from excised inside–out patches bathed on both sides by a low-divalent saline solution consisting of (mM): 130 NaCl, 0.2 EDTA, and 2 HEPES, pH 7.2, with 2 mM 3′, 5′-cyclic guanosine monophosphate (cGMP) (Sigma) added to the solution bathing the “intracellular” side of the membrane. Macroscopic currents were recorded at voltages ranging from −100 to +100 mV, stepped from a holding potential of 0 mV, with a patch clamp amplifier (Axopatch 200A), low pass filtered with an 8-pole Bessel (2 kHz, −3 dB), and digitized at 10 kHz. Leak currents measured in the saline solution in the absence of cGMP were subtracted from each record.
β-Ionone stocks in absolute ethanol were stored at −80 or −20°C until use. For homomeric channel experiments, stocks were diluted in 2 mM cGMP in the saline solution with a final concentration of ethanol in the bath that was less than 0.1%. The addition of up to 0.24% ethanol alone to saline had no effect on channel behavior or seal resistance; however, higher concentrations of ethanol (around 1%) did elicit a reduction in cGMP-activated current. All-trans retinal was prepared in a similar manner. To apply β-ionone to patches, half of the bath volume was removed, vigorously mixed with the retinoid stock, and then returned to the bath. All experiments were performed in glass petri dishes to minimize retinoid adsorption.
For experiments on native channels, retinas were prepared as for single-cell recording. Patches were excised from isolated rods and perfused through the laminar flow apparatus with either the low-divalent saline solution or the same solution supplemented with 2 mM cGMP. β-Ionone was applied by bulk perfusion of the bath. The flow to the bath was switched from Ringer’s solution to low divalent saline containing 2mM cGMP and β-ionone. After 5 min for the concentration of β-ionone to equilibrate in the chamber, the patch pipette was moved from the laminar flow into the bath.
Results
All-trans retinal activates bovine (Jäger et al., 1996; Sachs et al., 2000; Kono et al., 2008), as well as salamander GS rod apo-opsin (Kefalov et al., 1999, 2001). In our initial experiments, all-trans retinal decreased flash sensitivity and circulating current and accelerated flash response kinetics in dark-adapted GS and BS rods (results not shown), suggesting that it might also activate some visual pigments. But the interpretation of these results was complicated by a number of issues: (1) all-trans retinal forms random Schiff’s bases (van Breugel et al., 1979), (2) it is reduced to retinol (e.g., Tsina et al., 2004), and (3) all-trans retinal and retinol decrease circulating current by direct inhibition of cyclic nucleotide-gated channels (Dean et al., 2002). So to simplify matters, we switched to the truncated retinal analog, β-ionone. β-Ionone activates free opsin, but is not a substrate for the dehydrogenase (Palczewski et al., 1994), and does not inhibit homomeric rod cyclic nucleotide-gated channels at concentrations as high as 10 μM (Horrigan et al., 2005). Furthermore, β-ionone has the added benefit that it can be removed from cells with relative ease by washing with Ringer’s solution containing BSA (Kefalov et al., 1999).
At 9–16 μM, β-ionone reduced the circulating current in dark-adapted BS rods, BS cones, and UVS cones (Figs. 1C and 2C–2E). Onset of the effect was rapid, and a new steady state was reached within the first few minutes of continuous exposure. Circulating current stabilized at a level 53 ± 2% (n = 3) of the starting value for BS rods, 62 ± 7% (n = 4) for BS cones, and 69 ± 1% (n = 3) for UVS cones. Flash responses had faster kinetics (Fig. 2M–2O and 2R–2T), and sensitivity declined 5- to 10-fold (Fig. 2H–2J). The relative magnitudes of these changes in circulating current and sensitivity were similar to those in the same type of partially bleached cell after exposure to β-ionone (Fig. 2; see also Isayama et al., 2006), as if β-ionone’s effects were independent of pigment bleaching. The effects reversed after washing off the β-ionone with Ringer’s solution containing BSA. In contrast, β-ionone had negligible immediate effects on the circulating current (Figs. 1A and 1B and 2A and 2B) or sensitivity to flashes (Figs. 1D and 1E and 2F and 2G) of dark-adapted RS cones or GS rods, as observed previously (Jin et al., 1993; Kefalov et al., 1999).
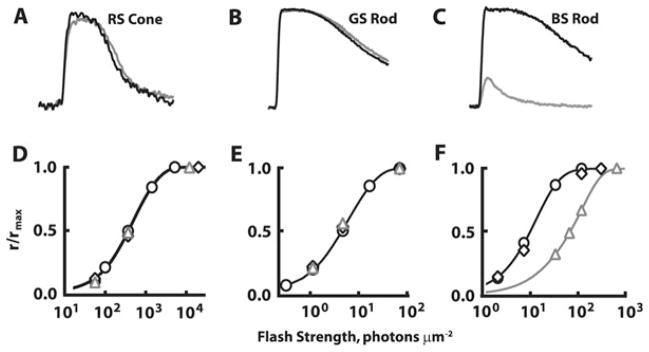
Fig. 1.
Cell type–specific effects of β-ionone on flash responses of a dark-adapted RS cone (A and D), GS rod (B and E), and BS rod (C and F). Flash strengths were 2 × 104 photons/μm2 at 625 nm, 70 photons/μm2 at 500 nm, and 122 photons/μm2 at 440 nm in (A–C), respectively. Black lines, responses in Ringer’s solution; Gray lines, responses in Ringer’s solution containing β-ionone. Stimulus–response relations in (D) (at 625 nm), (E) (at 500 nm), and (F) (at 440 nm) are for the cells in (A–C), respectively. ○, Ringer’s; △, β-ionone; ◇, washout. β-Ionone selectively reduced the circulating current and sensitivity of the BS rod (C and F).
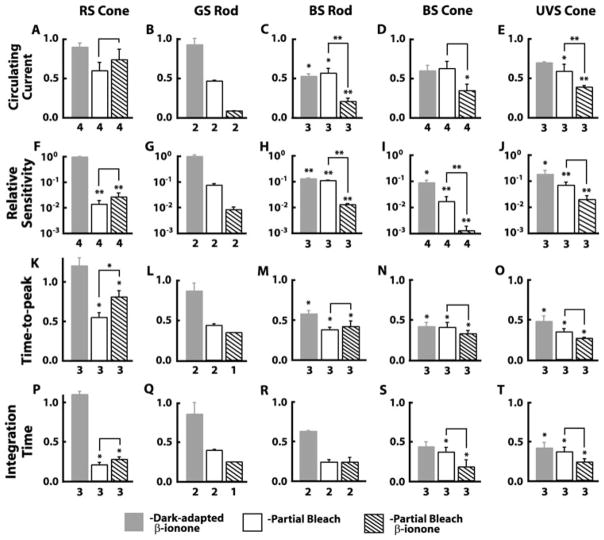
Fig. 2.
Comparison of β-ionone effects on dark-adapted and partially bleached salamander rods and cones. Responses under different experimental conditions were normalized to the dark-adapted untreated values. Dark-adapted cells were exposed to β-ionone (gray bars) (12.8 ± 0.3 μM; 6–19-min duration), returned to Ringer’s solution and partially bleached (open bars), and exposed to β-ionone again (hatched bars). (A–E) Circulating current, given by rmax, the saturating response amplitude under each condition, was suppressed in BS rods and cones and UVS cones in the presence of β-ionone (C–E) but not in RS cones (A) or GS rods (B). (F–J) RS cones and GS rods exhibited no change in relative sensitivity (F and G), while all other photoreceptor types showed a decrease on exposure to β-ionone (H–J). Relative sensitivity varies as the reciprocal of i0.5, the flash strength at kmax giving rise to a half-maximal response, so relative sensitivity was expressed as i0.5dark/i0.5treated. (K–T) Dim flash response kinetics of BS rods, BS cones, and UVS cones were accelerated by β-ionone. Flashes giving rise to responses whose amplitudes were <0.15 rmax were considered to be dim. Time to peak was taken as the interval from midflash to the peak of the dim flash response and integration time as the response integral divided by response amplitude. Changes in normalized circulating current and sensitivity to flashes were compared with analysis of variance (ANOVA) and post hoc Bonferroni analyses using log values to minimize skewness introduced by the use of ratios. Changes in dim flash response time to peak and integration time were compared with a one-way ANOVA followed by a post hoc Tukey–Kramer analysis using raw data but were plotted as ratios for standardization across cell types. Values for dark-adapted β-ionone, partial bleach, and β-ionone after partial bleach were compared to dark adapted/untreated, with additional comparisons between values for partial bleach and β-ionone after partial bleach (lines over bars): *P < 0.05; **P < 0.01. Comparisons were not made for data sets where n = 2 or lower. Error bars represent SEM, and numbers of observations are listed beneath the bars. SEM, standard error of mean.
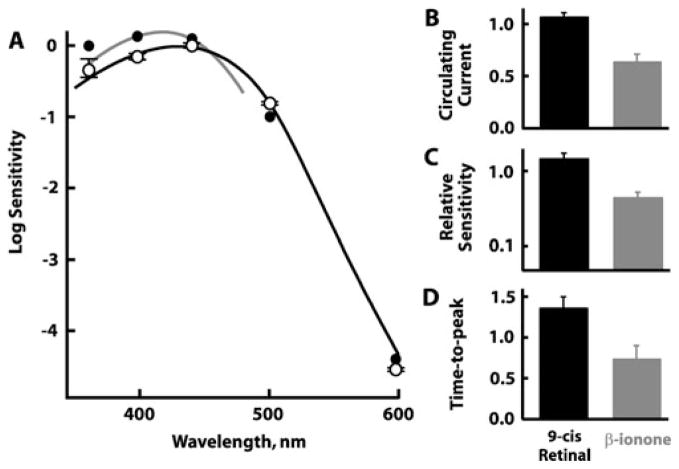
Could β-ionone’s effects on dark-adapted BS rods, BS cones, and UVS cones have arisen from residual free opsin that persisted even after 15 h of dark adaptation? As a test, seven dark-adapted BS rods were provided with 9-cis retinal to regenerate their full complement of visual pigment before being challenged with β-ionone. Had 9-cis retinal regenerated visual pigment from large pools of free opsin, we would have observed a shift in spectral sensitivity to shorter wavelengths (e.g., Makino et al., 1999; Kefalov et al., 2005), as well as relief of pigment adaptation, with increased circulating current and flash sensitivity and slowed flash response kinetics. These effects would have persisted after washing with Ringer’s solution because 9-cis retinal binds opsin covalently and requires photoisomerization for removal. Sensitivity to shorter wavelengths did increase substantially in one BS rod of seven (Fig. 3A, filled symbols). This cell exhibited the greatest increase in sensitivity to flashes, about fivefold. 9-cis Retinal produced inconsistent changes in the circulating current and sensitivity of the other cells; two of seven cells even lost current and sensitivity. One BS rod, whose circulating current increased with 9-cis retinal treatment, was returned to Ringer’s, whereupon it exhibited a further increase in current (results not shown). These results reflected multiple opposing actions of the retinoid: quench of opsin by pigment regeneration, inhibition of the cyclic nucleotide-gated channel (He et al., 2006), and activation of opsin (Kefalov et al., 2001) and visual pigment. On average, perfusion with 9-cis retinal increased slightly circulating current and sensitivity to flashes and invariably slowed the dim flash response time to peak (Fig. 3B–3D). Evidently, a number of “dark-adapted” BS rods did contain free opsin similar to previous observations on amphibian photoreceptors (Xiong & Yau, 2002; Kefalov et al., 2005).
Fig. 3.
(A) Spectral sensitivities of seven dark-adapted BS rods after incubation with 12.7 ± 4.5 μM 9-cis retinal for 20 min. Results for six cells are presented as mean ±SEM (open circles), while the cell with the greatest shift in spectral sensitivity is presented separately (filled circles). For reference, curves are shown for BS rods with native chromophore (black line; Ma et al., 2001) or BS cones bleached and pretreated with 9-cis retinal (gray line; Makino et al., 1999). The latter curve was shifted vertically to overlay the results of the cell with the greatest sensitivity to short wavelengths. (B–D) Effects of 9-cis retinal on the circulating current (n = 5) (B), relative sensitivity (n = 5) (C), and response kinetics (n = 3) (D) normalized to dark-adapted preexposure values. On average, the circulating current, relative sensitivity to flashes, and dim flash response time to peak increased after treatment with 9-cis retinal (black bars), but all three parameters were reduced during subsequent perfusion with β-ionone (gray bars). Error bars represent SEM. SEM, standard error of mean.
Nevertheless, 9-cis retinal did not prevent β-ionone from reducing circulating current, sensitivity to flashes, and time to peak of the dim flash response in any of the dark-adapted BS rods (Fig. 3B–3D; cf. Fig. 2C, 2H, and 2M). All effects disappeared once the β-ionone was removed. One dark-adapted UVS cone pretreated with 9-cis retinal was also activated by subsequent incubation in β-ionone (results not shown). Therefore, the lingering presence of free opsin cannot account for the full effect of β-ionone on dark-adapted rods and cones. We conclude that in some cell types, the chromophore-binding pocket in the opsin need not be vacant in order for β-ionone to produce pigment adaptation.
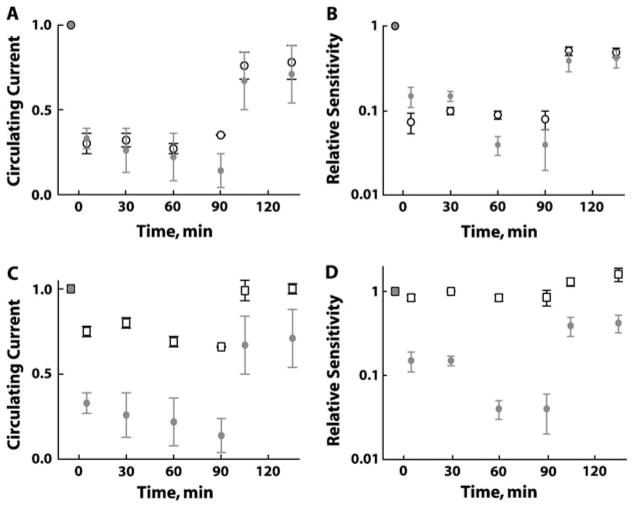
BS rods recovered completely after washout of β-ionone as long as the treatment was brief. Extended treatments introduced permanent deficits; after a 15-min treatment and subsequent washing, circulating current remained 3 ± 5% (n = 3) lower than pre-treatment levels, with an accompanied 1.4 ± 0.1-fold loss (n = 3) of sensitivity to flashes. To explore whether these residual changes resulted from visual pigment bleaching, β-ionone treatments were compared to light exposures. One set of BS rods illuminated with steady light for about 100 min to bleach ~1% of the cell’s visual pigment sustained a reduction in circulating current and sensitivity after the light was extinguished (Fig. 4A and 4B, open black symbols). Other BS rods were exposed to high concentrations of ~60 μM β-ionone to initially suppress 70% of the circulating current (Fig. 4A) and lower relative sensitivity by 5- to 18-fold (Fig. 4B). The latter change was slightly less than that produced by the steady light. Circulating current and sensitivity declined slightly over time during exposure to β-ionone so that eventually the deficits exceeded those caused by steady light. Following 100 min of exposure to β-ionone and a 30-min wash, BS rod recovery was incomplete (Fig. 4A and 4B). Circulating current returned to 71 ±17% (n = 3), and relative sensitivity climbed to a level threefold lower than at the start—similar to the 78% of circulating current and twofold loss in sensitivity found after bleaching by light. Full restoration of current and sensitivity was achieved in one β-ionone-treated BS rod after 9-cis retinal was added to the bath to regenerate any bleached pigment (data not shown). The similarity of the effects elicited by light and by β-ionone indicated that the catalytic activity in the latter treatment was associated with pigment bleaching and that the extent of bleaching was matched.
Fig. 4.
Comparison of treatments with β-ionone to steady light at 500 nm (9–33 × 102 photons/μm2/s). (A) Steady light (open black circles; 30.7 ± 5% of control, n = 3) and perfusion with 58–67 μM β-ionone (gray circles; 31.5 ± 5% of control, n = 4) each suppressed initially ~70% of the circulating current in BS rods. When light or β-ionone was turned off following 100+ min, recovery of circulating current was incomplete, indicating bleaching of visual pigment in both cases. (B) β-Ionone (gray circles) and steady light (open black circles) each decreased sensitivity to flashes and caused a measurable loss of sensitivity after cessation of treatment. High concentrations of β-ionone (52–58 μM) suppressed less circulating current in GS rods (C, open squares, n = 5) and had no effect on relative sensitivity (D, open squares, n= 5); gray circles are the corresponding BS rod data from (A) and (B), respectively. Error bars represent SEM. SEM, standard error of mean.
GS rods also responded rapidly to high concentrations of β-ionone (52–58 μM) but with a different profile of effects. Approximately 25% of the circulating current was suppressed with little or no change in relative sensitivity (Fig. 4C and 4D, squares) or time to peak of the dim flash response (data not shown). Circulating current recovered completely on washout of β-ionone while unexpectedly, sensitivity to flashes actually climbed slightly higher than the baseline value. The basis for enhanced sensitivity is not known. In a control experiment in a GS rod, light exposures suppressing 25% of the circulating current for 100 min reduced sensitivity 1.6-fold and quickened the flash response kinetics while the light was on but did not elicit pigment adaptation after the light was turned off (data not shown).
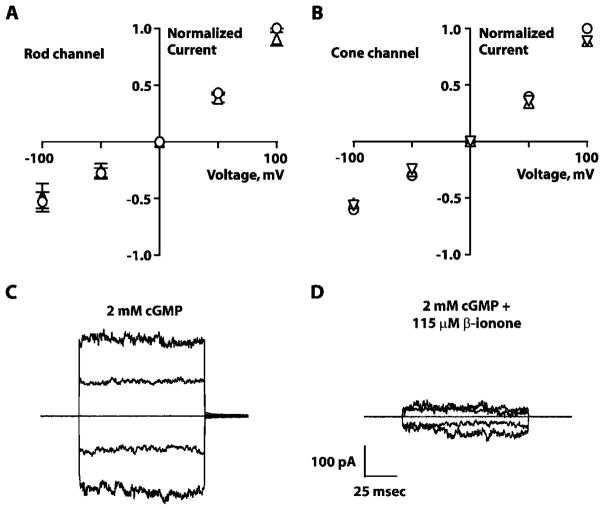
The effect of β-ionone on GS rods was reminiscent of cyclic nucleotide-gated channel inhibition by retinal (He et al., 2006). A previous study found that homomeric rod channels were not inhibited by 10 μM β-ionone (Horrigan et al., 2005). Except for a slightly reduced conductance at +100 mV, we confirmed that finding and extended it to homomeric cone cyclic nucleotide-gated channels with 20 μM β-ionone (Fig. 5A and 5B). The pharmacology of the native heteromeric cyclic nucleotide-gated channels, however, could differ from that of homomeric channels (reviewed in Brown et al., 2006), and it was important to test the higher concentrations of β-ionone applied to intact cells in this study.
Fig. 5.
Effects of β-ionone on rod and cone cyclic nucleotide-gated channels. Current–voltage relationships for homomeric rod (n = 3) (A) and cone cyclic nucleotide-gated channels (n = 1) (B). Xenopus oocytes expressing bovine rod (A) or human cone (B) homomeric channels. Voltage was stepped to ±100 mVin 50-mVincrements from a holding potential of 0 mV. Currents were normalized by the value obtained in saturating cGMP without β-ionone. ○, 2 mM cGMP; △, 2 mM cGMP + 10 μM β-ionone; ▽, 2 mM cGMP + 20 μM β-ionone. Error bars in (A and B) demarcate SEM. Low concentrations of β-ionone had little or no effect on the channels. Currents elicited through native cyclic nucleotide-gated channels from patches excised from tiger salamander GS rod outer segments in the presence of saturating cGMP without (C) or with 115 μM β-ionone (D). Current decreased when the patch was placed in concentrated β-ionone. Leak currents in the absence of cGMP were subtracted from all recordings. SEM, standard error of mean.
Indeed, three of three patches excised from salamander GS rod outer segments revealed that concentrations of β-ionone between 50 and 115 μM suppressed current through native channels (Fig. 5C and 5D). Inhibition by such high concentrations of β-ionone demonstrated a much faster time course (<1 min for onset and recovery) than that seen with channel inhibition by lower doses of retinal, which required tens of minutes for onset and over an hour for only partial recovery on washout (Dean et al., 2002; McCabe et al., 2004). Along with other evidence about the nature of the channel’s retinoid-binding site (Horrigan et al., 2005), the rapid time course of β-ionone inhibition suggests a different mechanism for inhibition by β-ionone than that by low concentrations of retinal.
Discussion
Here, we show that β-ionone suppressed circulating current, decreased sensitivity to flashes, and accelerated dim flash response kinetics reversibly in dark-adapted BS rods, BS cones, and UVS cones in the absence of photon absorption but not in GS rods or RS cones. The changes in affected cells are characteristics of light adaptation and pigment adaptation caused by ongoing pigment isomerization or the constitutive activity of apo-opsins, respectively. In principle, β-ionone could produce a state of adaptation by activating the visual pigment, transducin or phosphodiesterase (PDE); however, four lines of evidence argue against direct action on the latter two targets. First, there was a selectivity for certain cell types despite the expression of the same rod-specific complement of downstream phototransduction cascade components in rods and the expression of the same cone-specific counterparts in all cones (e.g., Ma et al., 2001). Second, in a reconstituted system, β-ionone increased GTP exchange in rod transducin in the presence of salamander GS rod and BS cone opsin but decreased it in the presence of RS cone opsin (Isayama et al., 2006). Third, retinoids alone failed to activate either transducin (Cohen et al., 1993; Jäger et al., 1996) or PDE (Keirns et al., 1975; Fukada & Yoshizawa, 1981; He et al., 2006) in biochemical assays. Fourth, activation of either transducin or PDE does not explain the bleaching of visual pigment caused by β-ionone. We therefore attribute the different effects of β-ionone to the visual pigment, which varied between the different rod and cone types.
Since β-ionone activates apo-opsin in all photoreceptor types except RS cones (Isayama et al., 2006), we tested the trivial explanation that bleached pigment was present in BS rods even after extensive dark adaptation (Kefalov et al., 2005). Although some cells did contain bleached pigment, pretreatment with 9-cis retinal to fully regenerate their pigment failed to prevent the pigment adaptation produced by β-ionone.
When the duration of treatment with β-ionone was extended, the BS rods did not recover completely after washing. Our suspicion that pigment had been bleached by β-ionone was con-firmed when 9-cis retinal was shown to support full recovery. We conclude that β-ionone activates and bleaches BS rod and cone and UVS cone visual pigment but not GS rod visual pigment.
Concentrations of β-ionone greater than 50 μM did affect GS rods, but the target was the cyclic nucleotide-gated channels rather than the visual pigment. Part of the current suppression in BS rods treated with high concentrations of β-ionone almost certainly had the same origin. Assuming that the fractions of current suppressed by pigment activation and by channel inhibition were additive, the component due to pigment activation was 0.71 – 0.25 = 0.46, that is, less than the fractional suppression by light of 0.7. Applying the relation between current suppression and light intensity for BS rods from Ma et al. (2001), the activity produced by β-ionone was comparable to a 1.5-fold lower light intensity, that is, the integral of the response to β-ionone was 1.5-fold less than that of the response to a photon. Consistent with this interpretation, sensitivity to flashes was reduced to a greater extent by light initially (Kefalov et al., 2001). But over time, the effect of β-ionone eventually dominated as the combined effect of β-ionone activation of both pigment and opsin released by bleaching surpassed that of photoactivated pigment and opsin alone.
Interestingly, high concentrations of β-ionone produced very nearly the same state of pigment adaptation as low concentrations: ~50% current suppression (once channel inhibition was taken into account), ninefold loss in sensitivity to flashes, and twofold acceleration of dim flash response time to peak. Apparently, 9–16 μM was already saturating, so the k1/2 of BS rod visual pigment for β-ionone must have been less than a few micromolar. In contrast, GS rod visual pigment appeared to be completely resistant to β-ionone at concentrations up to 60 μM. The cyclic nucleotide-gated channel was unaffected by β-ionone concentrations less than 20 μM over the physiological voltage range, and ~25% of the channels in intact GS rods were closed by ~50 μM β-ionone. Thus, compared to BS rod visual pigment, the cyclic nucleotide-gated channel had a k1/2 for β-ionone that was one to two orders of magnitude higher, while GS rod pigment had a k1/2 that was more than two orders of magnitude higher.
Although β-ionone activated and bleached visual pigment in BS rods, the mechanism remains unclear. One possibility is that β-ionone simply competes with the native 11-cis retinal for the chromophore-binding pocket (Kefalov et al., 2005). In the X-ray crystal structure of GS rod pigment, the β-ionone ring of 11-cis retinal wedges tightly between helices 3 and 6 (reviewed in Sakmar et al., 2002; Filipek et al., 2003), precluding multiple occupancy of the pocket. A restrictive pocket would keep β-ionone from entering the chromophore-binding pocket of dark-adapted GS rod pigment and could explain the inability of β-ionone to activate it. Yet, evidence from mutagenesis studies indicates that the binding pocket of BS opsin resembles that of GS rod opsin (Fasick et al., 1999). Perhaps the ring portion of 11-cis retinal occasionally vacates the chromophore-binding pocket, affording β-ionone an opportunity to take its place. RS cone pigment may undergo thermal bleaching at a high rate (Rieke & Baylor, 2000; Kefalov et al., 2003) at least in part because the ring portion of 11-cis retinal pops in and out of the pocket at a high frequency, which might explain why RS cone pigment is especially susceptible to bleaching by β-ionone at low concentrations (Isayama et al., 2006). β-Ionone is an inverse agonist for RS cone opsin (Jin et al., 1993), so the opsin freed from 11-cis retinal is not activated. But by extending the lifetime of the activated state (Bartl et al., 2005), the β-ionone ring is a partial agonist for all other opsins (Kefalov et al., 1999, 2001; Isayama et al., 2006). Then, the substantially greater stability of the GS rod pigment may protect it against chromophore exchange (Defoe & Bok, 1983), while BS rod pigment stability falls somewhere in between (Rieke & Baylor, 2000; Kefalov et al., 2005).
A more intriguing possibility is that opsin contains an alternate binding site(s) through which β-ionone exerts its effects. Many G protein–coupled receptors are subject to allosteric regulation by compounds that contain ring structures (reviewed in Soudijn et al., 2002; Jensen & Spalding, 2004). Opsins of fly photoreceptors are known to bind retinols or 3-hydroxyretinols at sites distinct from the chromophore-binding pocket (reviewed in Kirschfeld, 1986). Indeed, a growing body of evidence led Heck and colleagues to propose the existence of more than one retinoid-binding site on bovine GS rod rhodopsin (reviewed in Heck et al., 2003). First, all-trans retinal enhances transducin activation by rod opsin (Fukada et al., 1982; Cohen et al., 1992), yet in contrast to β-ionone, it does not slow pigment regeneration with 11-cis retinal by competing for the primary chromophore-binding pocket (Sachs et al., 2000). Second, the P500 product formed by photic stimulation of the meta-II photointermediate cannot be formed by a similar means from opsin and free all-trans retinal (Sachs et al., 2000), consistent with the location of an all-trans retinal-binding site away from the chromophore-binding pocket. Third, exogenous application of all-trans retinal quenches the fluorescence of opsin and that of metarhodopsin II that has not yet released its isomerized chromophore (Schädel et al., 2003). Since all-trans retinal cannot access the chromophore-binding pocket, it instead activates opsin by occupying an alternate site (Heck et al., 2003). In this model, our results show that either the binding affinity or the efficacy of β-ionone varies greatly across different types of visual pigments. Binding affinity and/or efficacy is very low for GS rod visual pigment; consequently, pigment adaptation is not induced in dark-adapted cells. β-Ionone is a better agonist for BS rods and cones that share the same opsin (Ma et al., 2001) and UVS cones, in which it binds, activates, and bleaches the visual pigment. Binding of β-ionone to an allosteric site and competition for the chromophore-binding pocket need not be mutually exclusive.
Our experiments with all-trans retinal and 9-cis retinal suggest that retinoids of longer chain length may bind the alternate site and activate certain visual pigments. In BS rods, the ability of β-ionone to produce pigment adaptation following treatment with 9-cis retinal suggests that of the two compounds, β-ionone has the greater efficacy. Additional studies are needed to address the impact of retinoid chain length, isomeric conformation, and functional group of the terminal carbon on the pharmacological properties of retinoids on each pigment type.
Acknowledgments
We thank William N. Zagotta for the CNGA1 clone, Michael Varnum for the CNGA3 clone, and V. Kefalov and M.C. Cornwall for helpful discussions. This work was supported by NEI EY11358, EY12944, EY07774, EY04939, EY014793, EY014104, and an unrestricted grant to Medical University of South Carolina (MUSC) from Research to Prevent Blindness, Inc. R.K.C. is an Research to Prevent Blindness Senior Scientific Investigator.
References
- Bartl FJ, Fritze O, Ritter E, Herrmann R, Kuksa V, Palczewski K, Hofmann KP, Ernst OP. Partial agonism in a G protein-coupled receptor: Role of the retinal ring structure in rhodopsin activation. The Journal of Biological Chemistry. 2005;280:34259–34267. doi: 10.1074/jbc.M505260200. [DOI] [PubMed] [Google Scholar]
- Brown RL, Strassmaier T, Brady JD, Karpen JW. The pharmacology of cyclic nucleotide-gated channels: Emerging from the darkness. Current Pharmaceutical Design. 2006;12:3597–3613. doi: 10.2174/138161206778522100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GB, Oprian DD, Robinson PR. Mechanism of activation and inactivation of opsin: Role of Glu113 and Lys296. Biochemistry. 1992;31:12592–12601. doi: 10.1021/bi00165a008. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Yang T, Robinson PR, Oprian DD. Constitutive activation of opsin: Influence of charge at position 134 and size at position 296. Biochemistry. 1993;32:6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- Cornwall MC, Fein A, MacNichol EF., Jr Cellular mechanisms that underlie bleaching and background adaptation. The Journal of General Physiology. 1990;96:345–372. doi: 10.1085/jgp.96.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen FJ. The chromophore binding space of opsin. Nature. 1978;276:847–848. doi: 10.1038/276847a0. [DOI] [PubMed] [Google Scholar]
- Dean DM, Nguitragool W, Miri A, McCabe SL, Zimmerman AL. All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: A novel role for photoreceptor retinoids in the response to bright light? Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8372–8377. doi: 10.1073/pnas.122681899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe DM, Bok D. Rhodopsin chromophore exchanges among opsin molecules in the dark. Investigative Ophthalmology & Visual Science. 1983;24:1211–1226. [PubMed] [Google Scholar]
- Fasick JI, Lee N, Oprian DD. Spectral tuning in the human blue cone pigment. Biochemistry. 1999;38:11593–11596. doi: 10.1021/bi991600h. [DOI] [PubMed] [Google Scholar]
- Filipek S, Teller DC, Palczewski K, Stenkamp R. The crystallographic model of rhodopsin and its use in studies of other G protein-coupled receptors. Annual Review of Biophysics and Bio-molecular Structure. 2003;32:375–397. doi: 10.1146/annurev.biophys.32.110601.142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflügers Archiv: European Journal of Physiology. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y, Yoshizawa T. Activation of phosphodiesterase in frog rod outer segment by an intermediate of rhodopsin photolysis. II. Biochimica et Biophysica Acta. 1981;675:195–200. doi: 10.1016/0304-4165(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Fukada Y, Yoshizawa T, Ito M, Tsukida K. Activation of phosphodiesterase in frog rod outer segment by rhodopsin analogues. Biochimica et Biophysica Acta. 1982;708:112–117. doi: 10.1016/0167-4838(82)90210-2. [DOI] [PubMed] [Google Scholar]
- He Q, Alexeev D, Estevez ME, McCabe SL, Calvert PD, Ong DE, Cornwall MC, Zimmerman AL, Makino CL. Cyclic nucleotide-gated ion channels in rod photoreceptors are protected from retinoid inhibition. The Journal of General Physiology. 2006;128:473–485. doi: 10.1085/jgp.200609619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck M, Schädel SA, Maretzki D, Hofmann KP. Secondary binding sites of retinoids in opsin: Characterization and role in regeneration. Vision Research. 2003;43:3003–3010. doi: 10.1016/j.visres.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Horrigan DM, Tetreault ML, Tsomaia N, Vasileiou C, Borhan B, Mierke DF, Crouch RK, Zimmerman AL. Defining the retinoid binding site in the rod cyclic nucleotide-gated channel. The Journal of General Physiology. 2005;126:453–460. doi: 10.1085/jgp.200509387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayama T, Chen Y, Kono M, DeGrip WJ, Ma JX, Crouch RK, Makino CL. Differences in the pharmacological activation of visual opsins. Visual Neuroscience. 2006;23:899–908. doi: 10.1017/S0952523806230256. [DOI] [PubMed] [Google Scholar]
- Jäger S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Spalding TA. Allosteric modulation of G-protein coupled receptors. European Journal of Pharmaceutical Science. 2004;21:407–420. doi: 10.1016/j.ejps.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Jin J, Crouch RK, Corson DW, Katz BM, MacNichol EF, Cornwall MC. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron. 1993;11:513–522. doi: 10.1016/0896-6273(93)90155-k. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Fein A, MacNichol EF, Jr, Cornwall MC. Visual pigment bleaching in isolated salamander retinal cones. The Journal of General Physiology. 1993;102:483–502. doi: 10.1085/jgp.102.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Cornwall MC, Crouch RK. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. The Journal of General Physiology. 1999;113:491–503. doi: 10.1085/jgp.113.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Crouch RK, Cornwall MC. Role of non-covalent binding of 11-cis-retinal to opsin in dark adaptation of rod and cone photoreceptors. Neuron. 2001;29:749–755. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]
- Kefalov V, Fu Y, Marsh-Armstrong N, Yau KW. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. Breaking the covalent bond—A pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirns JJ, Miki N, Bitensky MW, Keirns M. A link between rhodopsin and disc membrane cyclic nucleotide phosphodiesterase. Action spectrum and sensitivity to illumination. Biochemistry. 1975;14:2760–2766. doi: 10.1021/bi00683a032. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K. Activation of visual pigment: Chromophore structure and function. In: Stieve H, editor. The Molecular Mechanism of Photoreception. Berlin, Germany: Springer-Verlag; 1986. pp. 31–49. [Google Scholar]
- Kono M, Goletz PW, Crouch RK. 11-cis- and all-trans-retinols can activate rod opsin: Rational design of the visual cycle. Biochemistry. 2008;47:7567–7571. doi: 10.1021/bi800357b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Ma JX, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Harosi FI, Makino CL, Crouch RK. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001;32:451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Makino CL, Groesbeek M, Lugtenburg J, Baylor DA. Spectral tuning in salamander visual pigments studied with dihydro-retinal chromophores. Biophysical Journal. 1999;77:1024–1035. doi: 10.1016/S0006-3495(99)76953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Wen XH, Lem J. Piecing together the timetable for visual transduction with transgenic animals. Current Opinion in Neurobiology. 2003;13:404–412. doi: 10.1016/s0959-4388(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Yoshizawa T. Existence of a β-ionone ring-binding site in the rhodopsin molecule. Nature. 1975;258:523–526. doi: 10.1038/258523a0. [DOI] [PubMed] [Google Scholar]
- McCabe SL, Pelosi DM, Tetreault M, Miri A, Nguitragool W, Kovithvathanaphong P, Mahajan R, Zimmerman AL. All-trans-retinal is a closed-state inhibitor of rod cyclic nucleotide-gated ion channels. The Journal of General Physiology. 2004;123:521–531. doi: 10.1085/jgp.200409011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Jäger S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinal dehydrogenase: Substrate specificity and role in photo-transduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron. 2000;26:181–186. doi: 10.1016/s0896-6273(00)81148-4. [DOI] [PubMed] [Google Scholar]
- Sachs K, Maretzki D, Meyer CK, Hofmann KP. Diffusible ligand all-trans-retinal activates opsin via a palmitoylation-dependent mechanism. The Journal of Biological Chemistry. 2000;275:6189–6194. doi: 10.1074/jbc.275.9.6189. [DOI] [PubMed] [Google Scholar]
- Sakmar TP, Menon ST, Marin EP, Awad ES. Rhodopsin: Insights from recent structural studies. Annual Review of Biophysics and Biomolecular Structure. 2002;31:443–484. doi: 10.1146/annurev.biophys.31.082901.134348. [DOI] [PubMed] [Google Scholar]
- Schädel SA, Heck M, Maretzki D, Filipek S, Teller DC, Palczewski K, Hofmann KP. Ligand channeling within a G-protein-coupled receptor: The entry and exit of retinals in native opsin. The Journal of Biological Chemistry. 2003;278:24896–24903. doi: 10.1074/jbc.M302115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudijn W, Van Wijngaarden I, Ijzerman AP. Allosteric modulation of G protein-coupled receptors. Current Opinions on Drug Discovery and Development. 2002;5:749–755. [PubMed] [Google Scholar]
- Tsina E, Chen C, Koutalos Y, Ala-Laurila P, Tsacopoulos M, Wiggert B, Crouch RK, Cornwall MC. Physiological and microfluorometric studies of reduction and clearance of retinal in bleached rod photoreceptors. The Journal of General Physiology. 2004;124:429–443. doi: 10.1085/jgp.200409078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel PJ, Bovee-Geurts PH, Bonting SL, Daemen FJ. Biochemical aspects of the visual process. XL. Spectral and chemical analysis of metarhodopsin III in photoreceptor membrane suspensions. Biochimica et Biophysica Acta. 1979;557:188–198. doi: 10.1016/0005-2736(79)90101-9. [DOI] [PubMed] [Google Scholar]
- Xiong WH, Yau KW. Rod sensitivity during Xenopus development. The Journal of General Physiology. 2002;120:817–827. doi: 10.1085/jgp.20028702. [DOI] [PMC free article] [PubMed] [Google Scholar]