Abstract
OBJECTIVES
The aim of this study was to determine the 2-year prognostic value of cardiac computed tomography (CT) for predicting major adverse cardiac events (MACE) in patients presenting to the emergency department (ED) with acute chest pain.
BACKGROUND
CT has high potential for early triage of acute chest pain patients. However, there is a paucity of data regarding the prognostic value of CT in this ED cohort.
METHODS
We followed 368 patients from the ROMICAT (Rule Out Myocardial Infarction Using Computer Assisted Tomography) trial (age 53 ± 12 years; 61% male) who presented to the ED with acute chest pain, negative initial troponin, and a nonischemic electrocardiogram for 2 years. Contrast-enhanced 64-slice CT was obtained during index hospitalization, and caregivers and patients remained blinded to the results. CT was assessed for the presence of plaque, stenosis (>50% luminal narrowing), and left ventricular regional wall motion abnormalities (RWMA). The primary endpoint was MACE, defined as composite cardiac death, nonfatal myocardial infarction, or coronary revascularization.
RESULTS
Follow-up was completed in 333 patients (90.5%) with a median follow-up period of 23 months. At the end of the follow-up period, 25 patients (6.8%) experienced 35 MACE (no cardiac deaths, 12 myocardial infarctions, and 23 revascularizations). Cumulative probability of 2-year MACE increased across CT strata for coronary artery disease (CAD) (no CAD 0%; nonobstructive CAD 4.6%; obstructive CAD 30.3%; log-rank p < 0.0001) and across combined CT strata for CAD and RWMA (no stenosis or RWMA 0.9%; 1 feature—either RWMA [15.0%] or stenosis [10.1%], both stenosis and RWMA 62.4%; log-rank p < 0.0001). The c statistic for predicting MACE was 0.61 for clinical Thrombolysis In Myocardial Infarction risk score and improved to 0.84 by adding CT CAD data and improved further to 0.91 by adding RWMA (both p < 0.0001).
CONCLUSIONS
CT coronary and functional features predict MACE and have incremental prognostic value beyond clinical risk score in ED patients with acute chest pain. The absence of CAD on CT provides a 2-year MACE-free warranty period, whereas coronary stenosis with RWMA is associated with the highest risk of MACE.
Keywords: computed tomography angiography, coronary artery disease, emergency department, long-term outcome, major adverse cardiac events
Coronary artery disease (CAD) is a leading cause of morbidity and mortality in the developed world (1). Multidetector cardiac computed tomography (CT) angiography has been well established as an accurate noninvasive modality to evaluate for CAD (2–6). Recent studies have examined the prognostic utility of CT in patients with known or suspected CAD for predicting major adverse cardiac events (MACE) and mortality (7–13). Not surprisingly, these CT studies showed that patients with severe CAD, such as significant stenosis or multivessel disease, had a higher risk of a worse outcome. These long-term studies, however, were from retrospective observational cohorts in which the CT results were used to guide management.
Acute chest pain is a common symptom among emergency department (ED) patients and remains a diagnostic challenge (14), especially when the findings on the initial electrocardiograms and cardiac bio-markers are normal. The ROMICAT (Rule Out Myocardial Infarction Using Computer Assisted Tomography) trial was a prospective, double-blind observational study that included 368 ED patients with acute chest pain and a low to intermediate risk of acute coronary syndrome (ACS) (15). In the ROMICAT trial, 50% of ED patients had no evidence of coronary atherosclerosis on CT and did not have ACS during the index hospitalization or MACE at 6 months (15). Thus, a normal finding on CT without evidence of any coronary atherosclerosis may allow earlier triage and change the future disposition decision of ED physicians in this patient population (16). Furthermore, CT could newly identify a subgroup of patients with coronary artery stenosis in the absence of ACS who may be restratified as at high risk of future cardiovascular events.
Although CT has great potential for use to triage ED patients, the prognostic outcome in this patient cohort is not known. Thus, we sought to assess the prognostic utility of cardiac CT coronary and functional features for predicting MACE in ED patients with acute chest pain from the ROMICAT trial.
METHODS
Patient selection
We followed the 368 enrolled patients from the ROMICAT trial for 2 years (±6 months) after the index hospitalization. These patients were enrolled from May 2005 to May 2007 and had an initial chief symptom of acute chest pain lasting >5 min during the 24-h period before the index hospitalization as well as normal initial troponin levels and electrocardiographic findings. Details regarding the inclusion and exclusion criteria were previously reported (15). Briefly, during the index hospitalization, all subjects underwent a standard contrast-enhanced coronary CT study before admission to the hospital. Both caregivers and patients remained blinded to the results of cardiac CT. The institutional review board approved the study protocol, and all patients provided written informed consent.
Coronary CT angiography image acquisition
CT imaging was performed using a 64-slice CT scanner (Sensation 64, Siemens Medical Solutions, Forchheim, Germany). Unless contraindications were present, patients received 0.6 mg of sublingual nitroglycerin and if the heart rate was >60 beats/min intravenous beta-blocker (5 to 20 mg metoprolol) in preparation for the scan. Per standard protocol, a test bolus of 20 ml of contrast agent was administered at a flow rate of 5 ml/s to determine the optimal timing of contrast injection. Coronary CT datasets were acquired with 64 × 0.6-mm slice collimation, a gantry rotation time of 330 ms, tube voltage of 120 kV, and an effective tube current of 850 mA using electrocardiogram-correlated tube current modulation in 46% of cases. Contrast agent (80 to 100 ml, iodixanol 320 mg/cm3, Visipaque, GE Healthcare, Princeton, New Jersey) was injected intravenously at a rate of 5 ml/s to ensure homogeneous enhancement of the entire coronary artery tree. For coronary assessment, axial images were reconstructed with a slice thickness of 0.75 mm and increment of 0.4 mm using a retrospectively electrocardiogram-gated half-scan algorithm with a temporal resolution of 165 ms. For functional analysis, 10 datasets of axial images were reconstructed for every 10% of the R-R interval from 5% to 95% with a slice thickness of 1.5 mm and increment of 1.5 mm. All reconstructions were transferred to an offline workstation for analysis (Leonardo, Siemens Medical Solutions).
Coronary atherosclerotic plaque and stenosis assessment
The presence of nonobstructive coronary atherosclerotic plaque per segment, either calcified or noncalcified, was determined as described previously, using a modified 17-segment model of the coronary artery tree (17,18). Patients were stratified based on the presence and severity of CAD into no CAD, nonobstructive CAD, and obstructive CAD. Obstructive CAD was defined either as coronary artery stenosis with >50% luminal diameter obstruction or if coronary stenosis could not be excluded (n = 34) and deemed inconclusive.
Regional left ventricular function assessment
Regional wall motion abnormalities (RWMA) were assessed qualitatively based on the American Heart Association/American College of Cardiology 17-segment model, as previously described in the functional dataset (19,20). Briefly, functional analysis was assessed by viewing the left ventricle throughout the entire cardiac cycle in a cine mode and in standard cardiac orientations used in echocardiography (4-chamber, 2-chamber, and left ventricular [LV] short-axis). RWMA had to be present in at least 2 contiguous myocardial segments or in 1 segment visualized in 2 different views to be considered a true positive finding. Each LV segment was classified as having either normal or abnormal (hypokinetic, akinetic, dyskinetic, or aneurysmal) function (21). Patients in whom RWMA could not be assessed due to an incomplete LV function study (n = 12) were classified as having no RWMA.
Endpoints
The primary endpoint was MACE during the 2-year follow-up period. MACE was defined as cardiac death, ST-segment elevation myocardial infarction (STEMI), non–ST-segment elevation myocardial infarction (NSTEMI), or coronary revascularization. Coronary revascularization included percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery. An adjudication outcome panel of 2 physicians reviewed the patient data forms and verified by review of medical records to determine whether a patient had MACE during the follow-up period. The outcome panel was blinded to the findings of the cardiac CT. Disagreement was resolved by consensus, which included an additional senior cardiologist (22).
Follow-up
A follow-up telephone call was made to administer a standardized questionnaire at 6 months and 2 years (±6 months) after enrollment to determine the occurrence of MACE. The telephone numbers were collected from the patients at the time of the index hospitalization. If the telephone numbers were invalid, attempts were made to acquire current patient contact details from our electronic medical records and online telephone registries. Five attempts were made using the patient’s direct phone number, after which 2 attempts were made to reach the emergency contact person listed. If we were still unsuccessful, the primary care physician was contacted. If direct contact was not possible with either the patient or primary care physician, we reviewed the electronic medical records for information regarding the defined endpoints within the 2-year follow-up period. As a final step, we searched the Social Security Death Index for all patients 3 years after their enrollment in the trial. This also included those individuals for whom the aforementioned methods were not successful and whose status remained unknown.
Statistical analysis
Continuous variables were expressed as mean ± SD. Nominal variables were expressed as frequency and percentages. For continuous variables, differences in means were assessed with Student t tests and analysis of variance, as appropriate. For categorical variables, differences in proportions were compared using the Fisher exact or chi-square test and the Mantel-Haenszel trend test, as appropriate. Cumulative event rates as stratified by CT features were estimated using the product limit (Kaplan-Meier) methods and log-rank test. Patients were censored after the first event, if not otherwise specified. Unadjusted and adjusted Cox proportional hazards models controlling for medication use at baseline and 2-year follow-up were used to evaluate the association of cardiac CT findings for predicting MACE. In addition, Cox proportional hazards models were used to evaluate the prognostic value of the clinical Thrombolysis In Myocardial Infarction (TIMI) unstable angina/NSTEMI risk score (23) and the addition of cardiac CT findings for predicting MACE. The c-statistic, which is equivalent to the area under the receiver-operator characteristic curve (24), was determined to evaluate the prognostic discriminatory capacity for predicting MACE for each Cox model. The incremental prognostic value of adding each cardiac CT finding beyond that of clinical TIMI risk score was determined by comparing the global chi-square value of the models. We tested the proportional hazards assumption using time-varying covariates in all Cox regression models; no violations were observed and all Cox regression models included only non–time-dependent covariates. A 2-sided p value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Patient characteristics
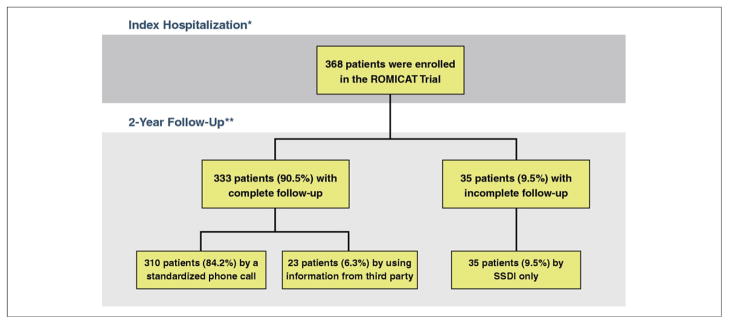
Figure 1 depicts the flow-chart of the ROMICAT trial patients and their follow-up. Of the 368 patients, there was complete follow-up for 333 patients (90.5%). The median follow-up period was 23 months. The majority of the patients (n = 310, 84.2%) were followed up by a standardized telephone call. In 23 patients (6.3%), the 2-year follow-up was completed by using information from a third party (emergency contact person, n = 2; primary care physician, n = 2; and medical records, n = 19). In the remaining 35 patients (9.5%), we used the Social Security Death Index. Overall, there was only 1 death, which was due to noncardiac etiology (lymphoma) during the follow-up period.
Figure 1. Flow Chart of the ROMICAT Trial Follow-Up.
*All patients underwent cardiac computed tomography during index hospitalization and the caregiver and patients remained blinded to the results throughout the study. **Patients were followed by standardized telephone call, a third party, and/or the Social Security Death Index (SSDI). The SSDI search was performed in all patients 3 years after enrollment. ROMICAT = Rule Out Myocardial Infarction Using Computer Assisted Tomography.
Table 1 shows the patient characteristics of the entire ROMICAT trial cohort and comparison among patients with complete follow-up and those lost to follow-up. Patients in this trial were predominantly low risk, with 94.3% having a TIMI risk score of 0 to 2 points. Although patients lost to follow-up were all alive according to the Social Security Death Index, they were younger and more frequently male and having diabetes mellitus, although less likely to have hyperlipidemia or taking baseline cardiac medications than patients with complete follow-up. When comparing CT features, there was a trend for patients with more severe CAD to have complete follow-up (p = 0.06), whereas no difference was seen regarding RWMA (p = 0.28). Table 2 shows the patient characteristics as stratified by CT coronary categories of no CAD, nonobstructive CAD, and obstructive CAD. With increasing severity of CT-based CAD categories, patients were older and more likely to be men, had more cardiac risk factors and higher TIMI risk scores, and taking cardiac medications.
Table 1.
Baseline Patient Characteristics of the Entire ROMICAT Trial Cohort, Those With Complete Follow-Up, and Those Lost From Follow-Up
| Total Cohort (N = 368) | Follow-up (n = 333) | Lost to Follow-up (n = 35) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs | 52.8 ±11.8 | 53.3 ±11.8 | 47.6 ± 10.7 | 0.006 |
| Male | 226 (61.4) | 199 (59.8) | 27 (77.1) | 0.05 |
| Body mass index, kg/m2 | 29.0 ±6.0 | 29.4 ±5.9 | 29.0 ± 6.0 | 0.64 |
|
| ||||
| Risk factors | ||||
| Diabetes mellitus | 40 (10.9) | 32 (9.6) | 8 (22.9) | 0.04 |
| Hypertension | 145 (39.4) | 135 (40.5) | 10 (28.6) | 0.20 |
| Hyperlipidemia | 135 (36.7) | 129 (38.7) | 6 (17.1) | 0.02 |
| Smoking | 180 (48.9) | 167 (50.2) | 13 (37.1) | 0.16 |
| Family history of CAD | 89 (24.2) | 78 (23.4) | 11 (31.4) | 0.30 |
|
| ||||
| TIMI risks for UA/NSTEMI | 0.74 | |||
| 0–2 points | 347 (94.3) | 313 (94.0) | 34 (97.1) | |
| 3–4 points | 20 (5.4) | 19 (5.7) | 1 (2.9) | |
| 5–7 points | 1 (0.3) | 1 (0.3) | 0 (0) | |
|
| ||||
| Medications at baseline | ||||
| Aspirin | 117 (31.8) | 112 (33.6) | 5 (14.3) | 0.02 |
| Beta-blockers | 84 (22.8) | 82 (24.6) | 2 (5.7) | 0.01 |
| Statins | 103 (28.0) | 98 (29.4) | 5 (14.3) | 0.07 |
| ACE-I | 56 (15.2) | 53 (15.9) | 3 (8.6) | 0.33 |
| Any of the above medications | 204 (55.4) | 192 (57.7) | 12 (34.3) | 0.01 |
|
| ||||
| Features of cardiac CT | ||||
| CAD categories | 0.06 | |||
| No CAD | 183 (49.7) | 159 (47.8) | 24 (68.6) | |
| Nonobstructive CAD | 117 (31.8) | 111 (33.3) | 6 (17.4) | |
| Obstructive CAD | 68 (18.9) | 63 (18.9) | 5 (14.3) | |
| RWMA | 46 (12.5) | 44 (13.2) | 2 (5.7) | 0.28 |
Values are mean ± SD or n (%).
ACE-I = angiotensin-converting enzyme inhibitor; CAD = coronary artery disease; CT = computed tomography; RWMA = regional wall motion abnormalities; TIMI = Thrombolysis In Myocardial Infarction; UA/NSTEMI = unstable angina/non–ST-segment elevation myocardial infarction.
Table 2.
Patient Characteristics as Stratified by CT CAD Categories
| No CAD (n = 183) | Nonobstructive CAD (n = 117) | Obstructive CAD (n = 68) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs | 47.5 ± 9.5 | 55.9 ± 10.5 | 61.6 ± 12.5 | <0.0001 |
| Male | 101 (55.2) | 77 (65.8) | 48 (70.6) | 0.01 |
| Body mass index, kg/m2 | 28.6 ± 6.0 | 29.2 ± 5.7 | 29.8 ± 6.5 | 0.33 |
|
| ||||
| Risk factors | ||||
| Diabetes mellitus | 13 (7.1) | 9 (7.7) | 18 (26.5) | 0.0001 |
| Hypertension | 47 (25.7) | 50 (42.7) | 48 (70.6) | <0.0001 |
| Hyperlipidemia | 41 (22.4) | 57 (48.7) | 37 (54.4) | <0.0001 |
| Smoking | 76 (41.5) | 58 (49.6) | 46 (67.7) | 0.0003 |
| Family history of CAD | 39 (21.3) | 26 (22.2) | 24 (35.3) | 0.04 |
|
| ||||
| TIMI risk score for UA/NSTEMI | <0.0001 | |||
| 0–2 points | 180 (98.4) | 115 (98.3) | 52 (76.5) | |
| 3–4 points | 3 (1.6) | 2 (1.7) | 15 (22.1) | |
| 5–7 points | 0 (0) | 0 (0) | 1 (1.5) | |
|
| ||||
| Medications at baseline | ||||
| Aspirin | 49 (26.8) | 37 (31.6) | 31 (45.6) | 0.007 |
| Beta-blockers | 30 (16.4) | 27 (23.1) | 27 (39.7) | 0.0002 |
| Statins | 31 (16.9) | 40 (34.2) | 32 (47.1) | <0.0001 |
| ACE-I | 17 (9.3) | 17 (14.5) | 22 (32.4) | <0.0001 |
| Any of the above medications | 79 (43.2) | 70 (59.8) | 55 (80.9) | <0.0001 |
|
| ||||
| Medications at 2-yr follow-up | ||||
| Aspirin | 42 (26.4) | 42 (39.3) | 32 (51.6) | 0.0003 |
| Beta-blockers | 34 (21.5) | 25 (23.4) | 34 (54.8) | <0.0001 |
| Statins | 29 (18.2) | 40 (37.0) | 33 (53.2) | <0.0001 |
| ACE-I | 24 (15.2) | 23 (21.5) | 24 (38.7) | 0.0003 |
| Any of the above medications | 77 (48.4) | 74 (68.5) | 48 (77.4) | <0.0001 |
Values are mean ± SD or n (%).
Abbreviations as in Table 1.
Cardiac CT findings
Table 1 shows CAD categories as determined by CT. When assessed by stenosis, there were 300 patients (81.5%) with no stenosis and 68 (18.5%) with stenosis. There were 46 patients (12.5%) with regional LV dysfunction. When stratified by CT coronary stenosis and functional categories, 280 patients (76.1%) had no stenosis or RWMA, 20 (5.4%) had no stenosis but had RWMA, 42 (11.4%) had stenosis without RWMA, and 26 (7.1%) had stenosis and RWMA. The effective radiation dose was lower in patients scanned with electrocardiogram tube current modulation (11.4 ± 2.2 mSv) than without (17.6 ± 2.2 mSv) (p < 0.0001).
Hard cardiac events
At the end of the follow-up period, 25 patients (6.8%) had a total of 35 MACE (no cardiac deaths, 12 myocardial infarctions, 23 coronary revascularizations). During the first 30 days (acute phase), MACE developed in 20 patients (1 STEMI, 7 NSTEMI, 16 PCI, and 2 coronary artery bypass grafts). After this acute phase, MACE developed in 5 more patients (1 STEMI, 3 NSTEMI, and 4 PCI). Of the 20 patients with MACE during the acute phase, 1 patient with initial NSTEMI treated with PCI had a recurrent MACE (second PCI at 1.8 years after enrollment for recurrent chest pain).
MACE as stratified by cardiac CT findings
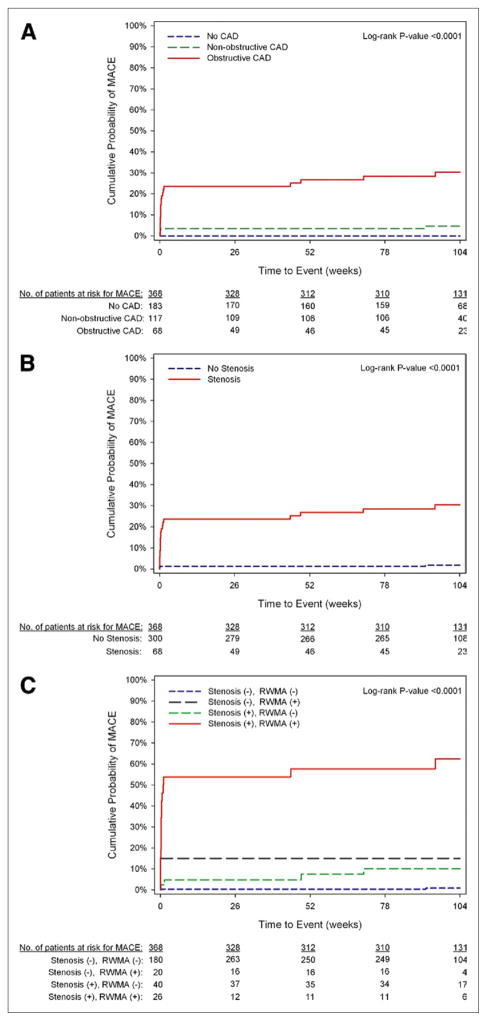
Figure 2 depicts the Kaplan-Meier estimates of the cumulative probability of MACE as stratified by CT findings; Table 3 provides the probability of events at various time points. The probability of 2-year MACE increased significantly across the strata of CT CAD categories (0% for patients without CAD, 4.6% for patients with nonobstructive CAD, and 30.3% for patients with obstructive CAD; log-rank p < 0.0001). Patients with stenosis had a nearly 20-fold increase in the risk of MACE than those without stenosis, with MACE of 30.3% versus 1.8%, respectively (hazard ratio: 19.84, p < 0.0001). As shown in Table 4, even after adjustment for being on cardiac medications, patients with CT stenosis had a 16- to 21-fold increase in risk of MACE than those without (both p < 0.0001). With functional analysis added to CT coronary categories (Table 3, Fig. 2), a gradient stepwise increase was seen in the 2-year probability for MACE in patients with no stenosis or RWMA (0.9%), followed by patients with only 1 feature (either stenosis [10.1%] or RWMA [15.0%]), highest in patients with both stenosis and RWMA (62.4%, log-rank p < 0.0001). This confers to a >92-fold increase adjusted risk in MACE for patients with both CT features of stenosis and RWMA and a >12-fold increase in adjusted -risk for patients with either stenosis or RWMA compared with patients without stenosis or RWMA as the reference standard (Table 4).
Figure 2. Kaplan-Meier Curves of MACE as Stratified by Cardiac Computed Tomography Features.
(A) Coronary artery disease (CAD) categories stratified into no CAD, nonobstruc-tive CAD, and obstructive CAD. (B) Coronary stenosis was stratified into no stenosis and stenosis. (C) Coronary and functional computed tomography categories stratified into no stenosis or regional wall motion abnormalities, no stenosis with regional wall motion abnormalities (RWMA), stenosis without RWMA, and stenosis with RWMA. MACE = major adverse cardiac event.
Table 3.
Kaplan-Meier Cumulative Probability of MACE at 30 Days, 1 Year, and 2 Years as Stratified by CT Findings
| n (%) | MACE (Probability)
|
|||
|---|---|---|---|---|
| 30 Days | 1 Yr | 2 Yrs | ||
| CAD categories | ||||
| No CAD | 183 (49.7) | 0 (0) | 0 (0) | 0 (0) |
| Nonobstructive CAD | 117 (31.8) | 4 (3.4) | 4 (3.4) | 5 (4.6) |
| Obstructive CAD | 68 (18.5) | 14 (20.6) | 18 (26.7) | 20 (30.3) |
|
| ||||
| CT stenosis | ||||
| Stenosis (−) | 300 (81.5) | 4 (1.3) | 4 (1.3) | 5 (1.8) |
| Stenosis (+) | 68 (18.5) | 14 (20.6) | 18 (26.7) | 20 (30.3) |
|
| ||||
| CT stenosis + regional LV function | ||||
| Stenosis (−), RWMA (−) | 280 (76.1) | 1 (0.4) | 1 (0.4) | 2 (0.9) |
| Stenosis (−), RWMA (+) | 20 (5.4) | 3 (15.0) | 3 (15.0) | 3 (15.0) |
| Stenosis (+), RWMA (−) | 42 (11.4) | 2 (4.8) | 3 (7.4) | 4 (10.1) |
| Stenosis (+), RWMA (+) | 26 (7.1) | 14 (53.9) | 15 (57.7) | 16 (62.4) |
Table 4.
Unadjusted and Adjusted HR of Cardiac CT Features for Predicting MACE
| Model 1
|
Model 2
|
Model 3
|
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| CT stenosis
| ||||||
| No stenosis | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Stenosis | 19.84 (7.44–52.89) | <0.0001 | 21.53 (7.68–60.38) | <0.0001 | 16.00 (5.92–43.22) | <0.0001 |
|
| ||||||
| CT stenosis + regional LV function
| ||||||
| Stenosis (−), RWMA (−) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Stenosis (−), RWMA (+) | 24.23 (4.05–145.06) | 0.0005 | 25.64 (4.25–154.68) | 0.0004 | 21.03 (3.50–126.33) | 0.0009 |
| Stenosis (+), RWMA (−) | 13.47 (2.47–73.54) | 0.003 | 15.04 (2.67–84.79) | 0.002 | 12.09 (2.20–66.37) | 0.004 |
| Stenosis (+), RWMA (+) | 124.92 (28.63–545.03) | <0.0001 | 138.81 (30.74–626.80) | <0.0001 | 92.49 (20.84–410.54) | <0.0001 |
Model 1 = unadjusted Cox proportional hazards model; Model 2 = model 1 adjusted for being on any baseline medications; Model 3 = model 1 adjusted for being on any medications at 2-year follow-up.
Incremental prognostic value of cardiac CT findings
Table 5 details the incremental value of adding both CT coronary stenosis and functional findings beyond that of the clinical TIMI risk score for predicting MACE. Both CT stenosis and RWMA remained independently predictive of MACE, whereas the clinical TIMI risk score was no longer significant after adding in stenosis and/or RWMA. The discriminatory capacity for predicting MACE improved from a c-statistic of 0.61 with the clinical TIMI risk score alone to 0.84 when CT stenosis was added (p < 0.0001), which further improved to 0.91 with the addition of RWMA (p < 0.0001).
Table 5.
Incremental Predictive Value of Cardiac C Findings Beyond That of Clinical Risk Score
| Models | HR (95% CI) | p Value | C-Statistic | Global Chi-Square Value | Model Comparisons, p Value |
|---|---|---|---|---|---|
| Clinical risk score
| |||||
| TIMI | 7.29 (3.04–17.46) | <0.0001 | 0.61 | 13.97 | N/A |
|
| |||||
| Clinical risk score + CT findings
| |||||
| TIMI + stenosis | 0.84 | 49.56 | <0.0001 | ||
| TIMI | 1.77 (0.71–4.40) | 0.22 | |||
| Stenosis | 17.07 (6.14–47.45) | <0.0001 | |||
| TIMI + stenosis + RWMA | 0.91 | 78.90 | <0.0001 | ||
| TIMI | 1.03 (0.41–2.57) | 0.96 | |||
| Stenosis | 7.48 (2.53–22.13) | 0.0003 | |||
| RWMA | 12.30 (4.50–33.65) | <0.0001 | |||
Late MACE after 30 days
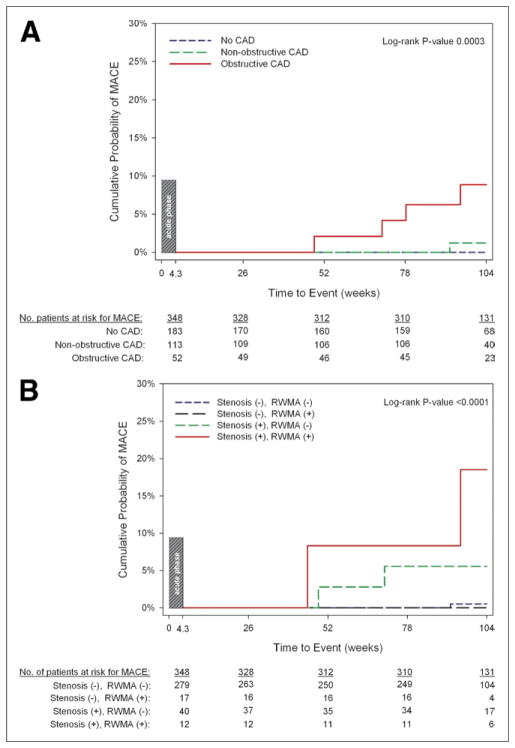
For patients without MACE during the acute phase (Fig. 3), the estimated 2-year event rate for late MACE (>30 days) increased across CAD categories as determined by CT (0% for no CAD, 1.2% for nonobstructive CAD, and 8.9% for obstructive CAD; log-rank p < 0.0003). In these patients, when functional analyses were added to CT coronary categories, the estimated 2-year late MACE rate was low for patients with no stenosis independent of RWMA (0.6% for no stenosis or RWMA and 0% for no stenosis with RWMA) and increased with the presence of stenosis (5.6% for stenosis and no RWMA), and greatest for patients with both features of stenosis and RWMA (18.5%, log-rank p = 0.0001).
Figure 3. Kaplan-Meier Curves of Late (>30 Days) MACE as Stratified by Cardiac CT Features.
(A) Coronary artery disease (CAD) categories stratified into no CAD, nonobstructive CAD, and obstructive CAD. (B) Coronary and functional computed tomography (CT) categories stratified into no stenosis or regional wall motion abnormalities (RWMA), no stenosis with RWMA, stenosis without RWMA, and stenosis with RWMA.
In patients who had MACE during the acute phase, the estimated recurrent event rate was 10% at 2 years. There was no difference between the rate of recurrence in these patients and the rate of late MACE in those with obstructive CAD but initially no MACE (8.9%, log-rank p = 0.75).
DISCUSSION
In this 2-year follow-up study of the ROMICAT trial, we evaluated the prognostic value of cardiac CT for MACE defined as the hard endpoint of cardiac death, nonfatal myocardial infarction, and coronary revascularization. The strength of our study is that it provides 2-year prognostic data, as stratified by CT coronary stenosis and functional results, in acute chest pain patients from a large prospective observational ED cohort. Unlike intention-to-treat studies, our study is particularly unique in that it has a double-blind, prospective, observational study design (CT results remained blinded to both caregivers and patients), allowing us to observe the natural course of this ED cohort without the influence of the CT results on clinical outcomes. More specifically, our endpoint, which includes coronary revascularization, is not driven by the cardiac CT findings. Given the acceptance of cardiac CT into clinical practice, such a study design is unlikely to be possible nowadays.
A promising clinical application of CT appears to be its use in the early triage of acute chest pain patients in the ED. Although CT offers results with a high sensitivity and negative predictive value for the detection of ACS in ED patients, as previously reported by our group and others (15,20,25), less is known about the prognostic value of CT in this patient population. Previous studies demonstrated in different patient cohorts that the absence of CAD by CT is associated with the absence of MACE in a follow-up period ≤60 months (7,10,11,26). However, these studies were not specifically designed for evaluation of ED patients with acute chest pain and the exclusion of ACS. Recently, Hollander et al. (27) reported 1-year outcome data in ED chest pain patients with a low to intermediate risk of ACS and concluded that those with no CAD on CT have a very low likelihood of cardiovascular events at 1 year. Our study expands on their results in that we provide 2-year outcome data, not just 1-year follow-up data. Importantly, in our patients without CAD on CT, the cumulative 2-year probability for MACE was 0%, which provides support for at least a 2-year MACE-free warranty period in patients with normal CT findings in the ED patient cohort. This represents 50% of our ED patients with acute chest pain, initial negative troponin, normal or inconclusive findings on electrocardiography, and low to intermediate risk of ACS and provides further reassurance to ED physicians that early triage using cardiac CT would be a safe strategy.
The CT diagnosis of obstructive CAD and/or LV dysfunction have been previously shown to predict all-cause mortality and future cardiac events, but these studies included unselected patients with suspected CAD and were not from a pre-defined ED cohort (7,9–13,26,28–31). Our results confirm these other CT studies and provide prognostic values, which can be specifically applied to the ED acute chest pain cohort. Patients with nonobstructive CAD had a nontrivial 4.6% cumulative probability for 2-year MACE. Patients with coronary stenosis had a 30.3% cumulative probability of events at 2 years with a 16-fold increase risk in MACE after adjustment for medications. If CT functional data are available, the highest risk of MACE was in patients with the presence of both CT stenosis and RWMA with an adjusted 92-fold increase risk compared with those with neither feature. Although the presence of no CAD on CT may lead to an early and safe hospital discharge of patients (15,32), the detection of nonobstructive CAD, obstructive CAD, and RWMA should require a more conservative triage approach and aggressive management strategy by caregivers.
Another important finding is that MACE developed in the majority of patients (n = 20, 80%) during the first 30 days after presentation to the ED and perhaps the strength of cardiac CT is in its diagnostic ability during the acute phase. However, it is interesting to observe that once the acute phase has passed, there remains prognostic utility with a gradient effect despite the relatively low rate of late MACE that comprises less than one-third of the cumulative 2-year event rate in patients with nonobstructive CAD (1.2% of 4.6%) and obstructive CAD (8.9% of 30.3%). Patients with MACE during the acute phase had the highest 2-year rate of late MACE or recurrence rate of 10%, although this was not significantly different from those with obstructive CAD without initial MACE in the ED (p = 0.75) and must be interpreted cautiously as exploratory and may be underpowered due to the low event rate. Most importantly, patients with normal coronary arteries had an estimated 2-year event rate of 0% for late MACE, providing further support of at least a 2-year warranty period for MACE after CT was performed and showed no CAD.
For cardiac CT to be implemented in the ED, its use must be incremental to pre-existing readily available data for the clinicians. The TIMI score has been the most widely used clinical risk stratification score in the low-risk ED patients with acute chest pain, although it remains less than “perfect” (23,33,34). With the addition of CT stenosis (coronary anatomy alone) to TIMI risk score, there was significant improvement in predicting MACE from 0.61 to 0.84 (p < 0.0001). Our findings are in keeping with previous non–ED-based CT prognostic studies that found both coronary atherosclerotic plaque and stenosis to be predictive of MACE independent of traditional risk factors and risk scores (7,12,13,29,30). The addition of functional data to coronary anatomy and clinical TIMI risk score further improved the discriminatory capacity to 0.91 for predicting MACE and provides more support of the incremental prognostic value of functional data over coronary assessment alone (31).
Study limitations
First, this is a prospective, observational study from a single-center tertiary referral hospital, which may limit the generalizability of our results to similar care settings. Thus, our findings need to be further validated in larger multicenter, randomized diagnostic trials, although it may not be feasible anymore to obtain unbiased outcome data because blinding of CT results may not be realistic due to the advancement of cardiac CT in clinical practice. Second, although this study used a retrospectively gated CT protocol, the newest CT technology permits frequent use of low radiation dose, prospectively triggered protocols. In our study, the radiation dose using electrocardiogram tube current modulation was 11.4 mSv, which is still greater than the 2 to 4 mSv published for prospective triggered protocols (35). Thus, the benefit of the incremental prognostic value of LV functional data needs to be weighed against the risk of the additional radiation exposure given to the patient with retrospectively gated CT scanning algorithms (36). In contrast, the benefit of prospectively triggered CT scanning algorithms to offer a much reduced radiation dose needs to be weighed against the loss of not having LV functional data (35,37). Further, the assessment of CT wall motion is qualitative and may be prone to being more subjective. However, it has been validated in the past against cardiac magnetic resonance (38). The number of events was low as expected in this low to intermediate risk ED population, thus limiting our ability to control for all potential confounders. Last, although the cumulative data from the ROMICAT trial and other observational studies suggest that cardiac CT angiography is a potentially valuable diagnostic study for use in early triage of ED patients with chest pain (15,32,39), future multicenter, randomized diagnostic trials in these patients are needed to provide a definitive answer as to whether this modality is indeed a cost-effective strategy compared with the existing standard of care.
CONCLUSIONS
In acute chest pain ED patients, CT coronary and functional features predict MACE and have incremental prognostic value beyond clinical risk score. The absence of CAD on CT provides a 2-year MACE-free warranty period, whereas coronary stenosis with RWMA is associated with highest risk of MACE.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) R01 HL080053 and in part supported by Siemens Medical Solutions and GE Healthcare. Dr. Truong was supported by NIH grants K23HL098370 and L30HL093896.
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndrome(s)
- CT
computed tomography
- ED
emergency department
- LV
left ventricular
- MACE
major adverse cardiac event(s)
- NSTEMI
non–ST-segment elevation myocardial infarction
- RWMA
regional wall motion abnormalities
- TIMI
Thrombolysis In Myocardial Infarction
Footnotes
All other authors have reported that they have no relationships to disclose.
References
- 1.Michaud CM, Murray CJ, Bloom BR. Burden of disease—implications for future research. JAMA. 2001;285:535–9. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28:3042–50. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 3.Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006;48:1896–910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Hamon M, Morello R, Riddell JW, Hamon M. Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography—meta-analysis. Radiology. 2007;245:720–31. doi: 10.1148/radiol.2453061899. [DOI] [PubMed] [Google Scholar]
- 5.Mowatt G, Cook JA, Hillis GS, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–93. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 6.Schuijf JD, Bax JJ, Shaw LJ, et al. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J. 2006;151:404–11. doi: 10.1016/j.ahj.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Carrigan TP, Nair D, Schoenhagen P, et al. Prognostic utility of 64-slice computed tomography in patients with suspected but no documented coronary artery disease. Eur Heart J. 2009;30:362–71. doi: 10.1093/eurheartj/ehn605. [DOI] [PubMed] [Google Scholar]
- 8.Gaemperli O, Valenta I, Schepis T, et al. Coronary 64-slice CT angiography predicts outcome in patients with known or suspected coronary artery disease. Eur Radiol. 2008;18:1162–73. doi: 10.1007/s00330-008-0871-7. [DOI] [PubMed] [Google Scholar]
- 9.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 10.Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49:62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 11.Aldrovandi A, Maffei E, Palumbo A, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol. 2009;19:1653– 60. doi: 10.1007/s00330-009-1344-3. [DOI] [PubMed] [Google Scholar]
- 12.Hadamitzky M, Freissmuth B, Meyer T, et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. J Am Coll Cardiol Img. 2009;2:404–11. doi: 10.1016/j.jcmg.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Van Werkhoven J, Gaemperli O, Schuijf JD, et al. Multi-slice computed tomography coronary angiography for risk stratification in patients with an intermediate pre-test likelihood. Heart. 2009;95:1607–11. doi: 10.1136/hrt.2009.167353. [DOI] [PubMed] [Google Scholar]
- 14.Goldman L, Kirtane AJ. Triage of patients with acute chest pain and possible cardiac ischemia: the elusive search for diagnostic perfection. Ann Intern Med. 2003;139:987–95. doi: 10.7326/0003-4819-139-12-200312160-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagurney JT, Bamberg F, Nichols JH, et al. The disposition decision on emergency department patients with chest pain is affected by the results of multi-detector computed axial tomography scan of the coronary arteries. J Emerg Med. 2010;39:57– 64. doi: 10.1016/j.jemermed.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 17.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 18.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 19.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 20.Seneviratne SK, Truong QA, Bam-berg F, et al. Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT Trial. Circ Cardiovasc Imaging. 2010;3:375–83. doi: 10.1161/CIRCIMAGING.109.892638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Nagurney JT, Brown DF, Chae C, et al. Disagreement between formal and medical record criteria for the diagnosis of acute coronary syndrome. Acad Emerg Med. 2005;12:446–52. doi: 10.1197/j.aem.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 25.Rubinshtein R, Halon DA, Gaspar T, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762–8. doi: 10.1161/CIRCULATIONAHA.106.618389. [DOI] [PubMed] [Google Scholar]
- 26.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–43. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Hollander JE, Chang AM, Shofer FS, et al. One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Acad Emerg Med. 2009;16:693–8. doi: 10.1111/j.1553-2712.2009.00459.x. [DOI] [PubMed] [Google Scholar]
- 28.van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53:623–32. doi: 10.1016/j.jacc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Russo V, Zavalloni A, Bacchi Reggiani ML, et al. Incremental prognostic value of coronary ct angiography in patients with suspected coronary artery disease. Circ Cardiovasc Imaging. 2010;3:351–9. doi: 10.1161/CIRCIMAGING.109.880625. [DOI] [PubMed] [Google Scholar]
- 30.Chow BJ, Wells GA, Chen L, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 55:1017–28. doi: 10.1016/j.jacc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Min JK, Lin FY, Dunning AM, et al. Incremental prognostic significance of left ventricular dysfunction to coronary artery disease detection by 64-detector row coronary computed tomographic angiography for the prediction of all-cause mortality: results from a two-centre study of 5330 patients. Eur Heart J. 2010;31:1212–9. doi: 10.1093/eurheartj/ehq020. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863–71. doi: 10.1016/j.jacc.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 33.Pollack CV, Jr, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13:13–8. doi: 10.1197/j.aem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 34.Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the Thrombolysis in Myocardial Infarction Risk Score in the emergency department chest pain population. Ann Emerg Med. 2006;48:252–9. doi: 10.1016/j.annemergmed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Husmann L, Valenta I, Gaemperli O, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–7. doi: 10.1093/eurheartj/ehm613. [DOI] [PubMed] [Google Scholar]
- 36.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–7. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 37.Lell M, Marwan M, Schepis T, et al. Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol. 2009;19:2576–83. doi: 10.1007/s00330-009-1558-4. [DOI] [PubMed] [Google Scholar]
- 38.Belge B, Coche E, Pasquet A, Vanoverschelde JL, Gerber BL. Accurate estimation of global and regional cardiac function by retrospectively gated multidetector row computed tomography: comparison with cine magnetic resonance imaging. Eur Radiol. 2006;16:1424–33. doi: 10.1007/s00330-006-0169-6. [DOI] [PubMed] [Google Scholar]
- 39.Ladapo JA, Hoffmann U, Bamberg F, et al. Cost-effectiveness of coronary MDCT in the triage of patients with acute chest pain. AJR Am J Roentgenol. 2008;191:455–63. doi: 10.2214/AJR.07.3611. [DOI] [PubMed] [Google Scholar]