Abstract
A major impediment to novel drug development has been the paucity of animal models that accurately reflect symptoms of affective disorders. In animal models, prolonged social stress has proven to be useful in understanding the molecular mechanisms underlying affective-like disorders. When considering experimental approaches for studying depression, social defeat stress, in particular, has been shown to have excellent etiological, predictive, discriminative and face validity. Described here is a protocol whereby C57BL/6J mice that are repeatedly subjected to bouts of social defeat by a larger and aggressive CD-1 mouse results in the development of a clear depressive-like syndrome, characterized by enduring deficits in social interactions. Specifically, the protocol consists of three important stages, beginning with the selection of aggressive CD-1 mice, followed by agonistic social confrontations between the CD-1 and C57BL/6J mice, and concluding with the confirmation of social avoidance in subordinate C57BL/6J mice. The automated detection of social avoidance allows a marked increase in throughput, reproducibility and quantitative analysis. This protocol is highly adaptable, but in its most common form it requires 3–4 weeks for completion.
INTRODUCTION
Mood and anxiety disorders are prevalent conditions affecting one out of every six people in their lifetime, and costs the United States economy an excess of $50 billion per year1. However, the development of animal models to study depression has been a major challenge in addressing these problems in psychiatric research. Historically, researchers have used various forms of chronic stress to induce behavioral adaptations relevant to depression2–4. These include chronic unpredictable stress, restraint stress or foot-shock stress, followed by behavioral measures of anhedonia (e.g., sucrose preference) or behavioral despair (e.g., forced swim test and tail suspension test), which are often responsive to acute antidepressant treatment and do not address the multiple validities necessary for an effective animal model of depression4. It should be noted that some behavioral measures, such as learned helplessness and novelty-suppressed feeding, address some of these validities and are useful models in studying aspects of depression-like behavior5. However, many have argued that the limited availability of animal models may explain the relative paucity of novel therapeutic interventions, based on rational design, to act upon putative molecular mechanisms of major depressive disorders4. This is further hampered by the dynamic range of reactions that an individual can show in response to stressors, whether it is the development of a major depressive disorder, post-traumatic stress disorder or resiliency to such disease states6. Individual responses to social stressors within experimental preparations have thus become increasingly useful in modeling aspects of depression-like behavior with high construct, face, discriminative and predictive validity3,7–11.
Social stressors are known to control affective-like behavioral responses across a wide variety of mammalian species12,13. Repeated exposures to social defeat stress in rodents, for example, cause a robust depression-like phenotype marked by anhedonia, anxiety and social-avoidance behaviors10,14–17. A unique aspect of social defeat stress that distinguishes it from other environmental stressors is its ability to continuously activate the pituitary-adrenal axis over repeated social confrontations9,18,19. Experimental control over the intensity of social defeat stress exposure makes it possible to examine a wide range of individual responses across molecular, cellular and behavioral endpoints20,21. A modified and abbreviated version of the sensory contact model of social defeat stress in mice, initially described by Kudryavtseva and co-workers10,22, was introduced in 2006 by Berton et al.17. In essence, this protocol (as described here) includes a shorter duration of stress exposure and automation of the simple behavioral output of social avoidance, thereby increasing throughput while maintaining reliability and reproducibility of results. With this protocol, it is possible to run 60–80 defeats concurrently. It is worth briefly noting that potential pitfalls of utilizing this standardized social defeat model, as well as of any model based on social interaction, include a moderate financial investment, larger individual space requirements in the vivarium, inherent variability introduced by factors outside of experimenter control (i.e., aggression levels of the CD-1 mice) and a moderate time investment for each run.
In this standardized repeated social defeat model, C57BL/6J mice are repeatedly subjected to bouts of social defeat by a larger CD-1 mouse screened for aggressive behavior. Although each individual defeat lasts only 5–10 min, the defeated mouse is subjected to continuous psychological stress from sensory interaction with the aggressor for the duration of the experiment through a clear perforated divider in a shared home cage. This treatment leads, in a majority of animals (termed `susceptible'), to the development of marked social avoidance associated with a constellation of overlapping behavioral and physiological changes reminiscent of depressive and anxiety symptoms. Consistently, social defeat produces a subgroup of animals (termed `resilient') corresponding to approximately one-third of the entire population21 that fails to develop social avoidance. In addition, only susceptible mice display reduced sucrose preference and significant changes in weight and metabolic disturbances indicative of a depression-like phenotype. Interestingly, the metabolic disturbances associated with susceptibility have been linked to alterations in orexin, ghrelin and lipid regulation23–26. Conversely, both susceptible and resilient mice show increased anxiety-like behavior, as measured by the elevated plus maze, stress-induced polydipsia and increased corticosterone release in response to a swim stress. Taken together, these data suggest that social avoidance showed by susceptible mice is associated with a constellation of hedonic changes and weight gain, whereas both susceptible and resilient mice show increased anxiety and corticosterone reactivity21. The behavioral syndrome induced by social defeat makes this model useful in studying individual differences in depression- and anxiety-associated behavior, and has the discriminative ability to distinguish animals on depression- and anxiety-like behavioral domains.
Another attractive feature of this model is that social avoidance induced by 10 d of social defeat can be reversed by chronic, but not acute, antidepressant treatments17,27. There has been growing interest in identifying compounds that produce a more rapid antidepressant effect than classic selective serotonin reuptake inhibitor (SSRI) treatments5,28. Social defeat, unlike many other models that respond to acute antidepressants, is an attractive model to make efficacy comparisons between novel compounds and traditional SSRI antidepressants. In addition, molecular studies have identified similarities in chromatin remodeling29,30 and transcriptional activation31 between resilient and antidepressant-treated mice, providing new information about therapeutic targets. Similarly, unique synaptic reorganization has been shown to occur in susceptible populations; it is completely absent in resilient mice32. Thus, social defeat stress provides a potential means to investigate components of the basic neuropathology underlying affective illnesses8,33–36, which is providing fundamentally new information about the molecular basis of affective disorders to aid in the development of novel antidepressant treatment strategies20.
Because of the increasing popularity of social defeat stress as a model of affective distress in mice (e.g., a basic search for `Social Defeat Stress In Mice' in PubMed returns 61 instances spanning the 20 years between 1984 and 2005 and 40 + references from 2006 to present alone), we present here, in detail, a protocol that reliably induces prolonged social avoidance in a subset of susceptible mice, which is only reversed by chronic treatment with antidepressants. Utilization of this experimental protocol will help to establish continuity for future investigations.
Experimental design
Validity of the social defeat model
A major obstacle in the assessment and clinical treatment of stress-related disorders is the limited availability of validated preclinical animal models to determine their underlying biological mechanisms37. The social defeat stress model provides a system for investigating the molecular underpinnings of these variable behavioral outcomes in a preclinical setting while maintaining four important forms of the validity of a model. (i) Construct (etiologic) validity refers to the methods used to reproduce, or construct, the disease state. There is a rich psychology literature exploring the roles of stressors in developing depressive disorders that are accurately mimicked by repeated social subordinations38. (ii) Face validity assesses how effectively the neuropathological and behavioral phenotypes observed in the human condition are reproduced. As mentioned previously, there are several well-documented depression-like phenotypes derived from social defeat21. (iii) Discriminative validity suggests that a model can discriminate between behavioral domains such as anxiety and depression. Social defeat produces a population of susceptible animals that are distinct in their depression-like behavior but similar on anxiety measures21. (iv) Finally, and of perhaps most importance, is the fact that the model shows predictive validity to treatments in a manner similar to that observed in human patients. Social defeat responds only to chronic, but not acute, administration of antidepressants, suggesting a pharmacological validity not often seen in other stress models that tend to respond equally to both acute and chronic antidepressant treatments17,27. Below are some suggested considerations for designing social defeat experiments.
Timing and organization of social defeat experiments
Experimenter experience is a key factor in the reliability and throughput of our repeated social defeat protocol. Experiments are generally run in cohorts ranging from 20 to 80 mice, with defeats occurring once daily for each mouse in the cohort. We suggest that experimenters new to this model start with cohorts of no more than 20–30 mice. We also suggest that defeat sessions include no more than ten animals at a time. Although all the defeats in the batch of ten will have started within the first few minutes, wait for their completion before moving on to the next batch of ten defeats within the cohort. It is reasonable to expect that a single researcher, new to this model, will spend ~30 to 45 min on a full round of defeats per day per cohort of 30 mice. Experienced researchers can anticipate running larger cohorts of up to 80 mice within a similar timespan.
Social avoidance testing takes considerably longer, and researchers new to this component should anticipate spending a full day on testing (this is further dependent on the number of test arenas being used concurrently). An inexperienced researcher should anticipate spending ~10 min per mouse per test, whereas an experienced researcher can easily perform a single test within 6 min. Finally, we recommend that inexperienced researchers start with a single test arena until they are fully confident. However, with more experience and the proper Ethovision tracking software (as described here), it is possible to measure social interaction in up to four arenas concurrently.
Use of C57BL/6J mice
Although other strains may prove suitable, it should be noted that varying strains of mice show differences in stress and anxiety-like behaviors. Of note, a series of recent papers suggests that the C57BL/6J line appears to be less susceptible to stress when compared with other strains39–41. The established guidelines for social defeat in this protocol are based on data obtained from mice with C57BL/6J backgrounds starting defeat at 7–8 weeks of age. Establishing baseline behaviors for other strains will be an important next step in further increasing the utility of this model.
Screening of aggressor CD-1 mice
Successful application of chronic social defeat stress to C57BL/6J mice is dependent on appropriate selection of CD-1 mice with consistent levels of aggressive behaviors, as determined from the 3-d screening process detailed below. It is critical to note that although many sexually experienced male CD-1 mice will show aggression, the degree, quantity and quality of aggressive behavior across CD-1 mice can vary greatly. Roughly half of all screened CD-1 mice will not reach the criterion for inclusion, a fact that needs to be considered when designing experiments. Those aggressors that do meet the criteria can be used in multiple social defeat experiments for up to 3 months following their initial screening. As it is possible for aggressors to habituate to the presence of C57BL/6J mice over time, and decrease their antagonistic interactions, all aggressors are rescreened in a single screening session prior to use in consecutive social defeat experiments. For the purpose of screening the aggressors, C57BL/6J mice between 8 and 20 weeks of age are used. Use of older C57BL/6J mice that are substantially larger will result in decreased aggression from the CD-1 mice and lead to the inaccurate establishment of aggression criteria.
Adaptability of the defeat protocol
An increasingly beneficial aspect of the social defeat protocol is the inherent flexibility with which it can be modified. Three methods that have proven extremely valuable in the exploration of the molecular basis of depression are viral-mediated gene transfer, screening of genetically modified mice and optogentic manipulation of neuronal populations.
Genetically modified mice strains bred on C57BL/6J background are extremely useful in understanding the genetic basis of depression-like behavioral traits26,42,43. However, the protocol is not standardized for use in mice of other genetic backgrounds and care must be taken to validate responses in other mouse lines. In the case of viral-mediated gene transfer, several adaptations on the standard social defeat protocol have been implemented to account for the transient expression of herpes simplex virus-driven gene transfer (i.e., loss of optimal protein expression within 4–5 d of infection), such as development of the `microdefeat'. The microdefeat allows the investigation of the prosusceptibility effects of a molecular manipulation21,32. Specifically, in the microdefeat protocol, a C57BL/6J mouse is subjected to subthreshold levels of social defeat that consist of three 5-min defeat sessions given consecutively on a single day with 15 min of rest between each session. Social interaction is then tested 24 h later and does not produce any significant avoidance in wild-type C57BL/6J mice. Similarly, if the manipulation being evaluated is prodepressant, it produces levels of social avoidance comparable to that observed after a 10-d social defeat. It is worth noting that lentiviral and adeno-associated virus (AAV)-driven viral transduction can be used with no changes to the standard social defeat protocol, assuming that the duration of the experiment falls within the peak of protein expression.
A recent publication by Covington et al.44 has extended the exciting advances in optogenetic manipulation of neuronal populations to the social defeat protocol. Following the standard 10-d social defeat protocol and social avoidance testing, susceptible and control mice were surgically transduced with AAV-channel rhodopsin 2 (a light-activated cation channel) in the mouse medial prefrontal cortex to optogenetically stimulate cortical activation in vivo and examine the behavioral consequences. After this manipulation in the medial prefrontal cortex, susceptible mice show a reduction in social avoidance similar to what has been previously observed after antidepressant treatments.
The above-mentioned variations and uses of social defeat are simply given as examples of what has been done, and what can be achieved, through creative use and adaptation of social defeat to new technologies. The development of newer techniques for molecular manipulation of depression targets shows great appeal and promise for translation application, aided through the use of easily adaptable and validated animal models such as social defeat.
Physical wounding of defeated mice
A concern posed by social defeat is the consequences of physical aggression, such as wounding, on defeated C57BL/6J mice. In cases in which repeated defeats lead to the development of open wounds exceeding 1 cm, removal of the mouse from the study and immediate euthanasia is indicated. Further, defeats should be run under constant veterinary evaluation and with full approval of all necessary institutional review boards and standards. If wounding consistently exceeds the criterion, a reduction in the duration of individual defeat sessions may be called for (i.e., a reduction from 10 min defeats to 5 min defeats), and it may also be necessary to remove the offending aggressor CD-1 from the experiment. In and of itself, wounding is not a required component of the social defeat model, as it has been shown to have no correlation with social avoidance behavior21.
It is worth mentioning that social defeat rarely, if ever, leads directly to death in the mice undergoing defeat. However, death may occur in the hours following cessation of daily defeats, and therefore it is important to monitor the health of mice throughout the study. Veterinary assistance is suggested at first when new investigators are learning the procedure. Further, death can occur if either the CD-1 or C57BL/6J mice escape across the perforated divider during the 10 d of repeated social defeat. To prevent this, it is imperative to check that the divider is always flush against both the bottom of the defeat cage and the bottom of the cage cover. This may require weighting of the lid (i.e., with excess rodent chow) or removing excess bedding, along with daily assessments of divider placement.
Controls
Proper control groups are included within the design of each repeated social defeat stress experiment. At the onset of each experiment, control C57BL/6J mice are pair housed in defeat boxes with one mouse per side of the perforated divider. All control mice are rotated on a daily basis in a manner similar to that of mice undergoing defeat, but they are never actually allowed physical contact with their cage mate. Preventing physical contact is important for multiple reasons. First, similarly sized C57BL/6J mice will occasionally show aggression, which may artificially elevate stress levels in the control mice. Moreover, nonaggressive social interaction can be rewarding and, again, can artificially alter subsequent social interaction. Therefore, it is imperative that control mice be kept physically separated throughout their 10 d in the control condition.
MATERIALS
REAGENTS
-
C57BL/6J mice (Jackson Labs). Mice are ordered so as to arrive at 6–7 weeks of age and are group housed (no more than 5 mice per cage) in standard mice cages ▲ CRITICAL Although other strains may prove suitable, it should be noted that varying strains of mice show differences in stress and anxiety-like behaviors (see Experimental design for further details).
▲ CRITICAL Further, although social defeat has been run successfully on mice up to 20 weeks of age, the most reliable results are obtained from mice that are 7–8 weeks of age. ! CAUTION Experiments must follow all governmental and institutional guidelines for care and use of laboratory animals.
-
CD-1 mice (Charles River Laboratories). Orders are placed for mice that are retired breeders < 4 months of age and singly housed throughout.
▲ CRITICAL As with the C57BL/6J mice, there may be strain-specific differences in aggressive behavior between mice lines. If a change of strain is required, care should be taken to validate that the level of aggression showed across all defeat days is consistent and comparable to CD-1 levels. ! CAUTION Experiments must follow all governmental and institutional guidelines for care and use of laboratory animals.
EQUIPMENT
Clear rectangular hamster cages (26.7 cm (w) × 48.3 cm (d) × 15.2 cm (h); Allentown, cat. no. PC10196HT)
Paired steel-wire tops (Allentown, cat. no. WBL1019MMB)
Hard woodchip bedding (Quality Lab Products)
Clear perforated Plexiglas divider (0.6 cm (w) × 45.7 cm (d) × 15.2 cm (h); Nationwide Plastics, custom order)
Stopwatch for timing defeat sessions
Video tracking apparatus and software (EthovisionXT with Social Interaction Module; Noldus Information Technology)
Social interaction open-field arena custom-crafted from opaque Plexiglas (42 cm (w) × 42 cm (d)× 42 cm (h); Nationwide Plastics, custom order).
Removable wire-mesh enclosure (two per social interaction test arena) secured in Plexiglas (10 cm (w) × 6.5 cm (d) × 42 cm (h); Nationwide Plastics, custom order)
Cleaning solution (Virkon-S; VWR International) and wipes
EQUIPMENT SETUP
Hamster cage with divider and bedding for social defeat experiments
Defeats take place in clear rectangular hamster cages with paired steel-wire tops containing hard woodchip bedding. The hamster cage is divided in half by the clear perforated Plexiglas divider, which physically separates the mice following defeat sessions. Water and food are provided on both sides of the divider ad libitum. Figure 1 depicts a standard hamster cage defeat setup. ▲ CRITICAL We suggest woodchip-based bedding as it provides traction for the mice, without which the defeats are compromised as the C57BL/6J mice cannot show appropriate escape behavior.
Figure 1.

Picture of the standard hamster cage used in repeated social defeat stress experiments. The resident aggressor is permanently housed on one side of the perforated divider, and all defeats are performed within that compartment while rotating C57BL/6J intruders across defeat days so that these experimental animals do not habituate to a single aggressor. Note that care must be taken to ensure that the divider is firmly secure, thereby preventing mice from escaping their overnight compartments. Social defeat experiments must follow all governmental and institutional guidelines for care and use of laboratory animals.
Open-field arena and wire-mesh enclosures for social interaction testing
The social interaction open-field arena is custom-crafted from opaque Plexiglas, and may be easily constructed in-house with epoxy or purchased from a Plexiglas workshop. A wire-mesh enclosure secured in Plexiglas is centered against one wall of the arena during all social interaction sessions. The wire mesh should be large enough to clearly display a target CD-1, while preventing more than cursory physical contact between the target CD-1 and defeated C57BL/6J mouse. Specifically, the aggressor should be able to fit its snout and paws through the wire mesh but not actively pursue the C57BL/6J test mouse. The Plexiglas functions as a funnel, allowing easy placement of the CD-1 target mouse within the wire-mesh enclosure base; however, it should not extend over the wire mesh. The `interaction zone' of the test arena encompasses a 14 cm × 24 cm rectangular area projecting 8 cm around the wire-mesh enclosure. The `corner zones' encompass a 9 cm × 9 cm area projecting from both corner joints opposing the wire-mesh enclosure. Figure 2a is a schematic representation of the arena and its zones. Figure 2b depicts this device from both a front and side view. ▲ CRITICAL Two wire-mesh enclosures should be prepared for every open-field arena being used, and only one should be used in the presence of a target CD-1 to prevent the transmission of aggressor olfactory cues during sessions when an aggressor is absent.
Figure 2.
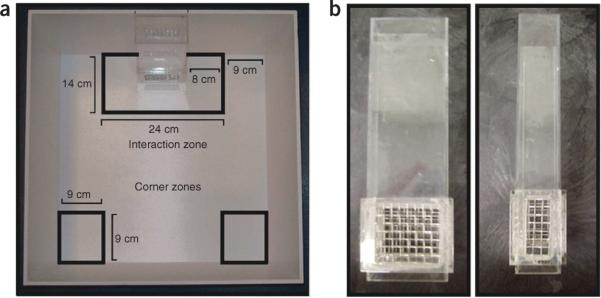
Schematic of the social interaction arena and representative front and side pictures of a wire-mesh enclosure. (a) Top-down view of the social interaction arena, delineated with zones and dimensions. (b) Picture of wire-mesh enclosure used to secure the target CD-1 during the `target present' portion of the social interaction test. Note that this is custom designed, and, assuming that dimensions are held constant, it can be fabricated with some flexibility in construction materials. Of importance is the ability for visual and olfactory cue transmission, with physical separation of the target aggressor and defeated mouse. On the left is a front view, and on the right a side view, of the same wire-mesh enclosure.
Video-tracking apparatus and software
A video-tracking system is used to monitor approach and avoidance behavior in C57BL/6J mice during social interaction testing. Other video-tracking packages can be substituted, assuming that they can accurately track a C57BL/6J in the presence and absence of a CD-1 mouse, and breakdown these movements by regions within the social interaction open-field arena. Within the software suite selected, the social interaction open-field arena should be topographically divided into two distinct areas (interaction zone and rear corners) as depicted in Figure 2a and described above. We recommend selection of a software suite that permits automated analysis of multiple arenas concurrently. Suggested variables for automated scoring include duration of time spent in the interaction zone, duration of time spent in the corner zones and total movement in the arena. All social interaction testing is conducted under red-light conditions in a soundproof room.
Cleaning and sterilization
Between all completed 10-d rounds of social defeats, the hamster cages and steel-wire tops should be disinfected and sterilized via autoclave. This is especially important because of the vigorous nature of the defeats. The Plexiglas dividers should be handwashed, and not autoclaved, as in our lab facility they have shown a common tendency to warp and lose shape when autoclaved. After every social interaction test, and especially between social defeat runs, the open-field arena and wire-mesh enclosures should be thoroughly hand cleaned and disinfected with a odorless cleaning solution, and wiped dry.
PROCEDURE
Screening for aggressive CD-1 mice • TIMING 1 week acclimation, 3 d for screening
-
1|
Purchase male CD-1 retired breeder mice at 4–6 months of age. House CD-1 mice (known as aggressor mice) singly, with free access to food and water, and allow mice to habituate to their new colony facility for a minimum of 7 d prior to screening.
-
2|
Use `screener' C57BL/6J mice during the screening process. These mice are only used for CD-1 screening and may range in age from 8 to 20 weeks. They may be used repeatedly for subsequent screenings. ▲ CRITICAL STEP Mice older than 20 weeks of age are less likely to show normal escape behavior and may lead to incorrect selection of aggressors.
-
3|
Perform screening in the home cage of the CD-1 mouse. This is done by placing the screener C57BL/6J mouse directly into the home cage of the aggressor for 180 s with the aggressor present. There should be no items within the home cage that can be used to aid the screener in escaping from the aggressor. Regardless of latency to aggression, do not remove the screener C57BL/6J until the full 180-s duration has elapsed. Once the screening session has elapsed, note the latency to aggression and remove the screener. The aggressor is not removed from its home cage at anytime during the screening process. Perform three screening sessions, once daily, using different screeners on each subsequent day for each aggressor, such that no aggressor defeats the same screener twice.
-
4|
Select CD-1 mice for use as aggressors in subsequent social defeat experiments based upon two criteria: During three 180-s screening sessions, once daily, the CD-1 mouse must attack in at least two consecutive sessions; and the latency to initial aggression, which is recorded during each session, must be less than 60 s.
-
5|
Exclude those CD-1 mice that do not meet this criterion for use in social defeat experiments. ▲ CRITICAL STEP Anticipate that roughly half of screened CD-1 mice will not reach the criteria for inclusion when planning experiments.
? TROUBLESHOOTING
-
6|
Following screening, house experimental aggressors singly with free access to food and water. Aggressors can be used for up to 3 months after their initial screen, and may be used for three bouts of social defeat. However, they should be rescreened prior to the start of each new social defeat experiment.
? TROUBLESHOOTING
Chronic social defeat stress • TIMING ~10 d
-
7|
Assemble defeat hamster cages, as described in EQUIPMENT SETUP, 24 h before initiating the first social defeat stress. Place an aggressor mouse from Step 6, termed the resident, on one side of the divided hamster cage, known as the home cage, overnight prior to the start of defeat sessions.
-
8|
Expose intruder C57BL/6J mice to social defeat stress for 5–10 min on 10 consecutive days. On the first day, place an intruder C57BL/6J mouse directly within the resident aggressor's home cage compartment. After 5–10 min of social defeat, transfer the intruder across the perforated divider to the opposite compartment and house within this compartment for the remainder of the 24-h period.
? TROUBLESHOOTING
-
9|
For each subsequent daily defeat of 5–10 min, expose the intruder C57BL/6J to a novel resident's home cage compartment, in order to prevent any habituation to the resident aggressor. After 5–10 min of social defeat, again transfer the intruder across the perforated divider to the opposite compartment and house within this compartment for the remainder of the 24-h period. ▲ CRITICAL STEP During the 10-d defeat period, resident aggressors are not removed from their home cage; rather, the C57BL/6J intruders are alternated daily. We have found that this helps to maintain internal consistency and reproducibility. We suggest that basic observation be made throughout the defeat sessions to ensure that high-quality aggressive bouts occur. It is not necessary to qualitatively score defeat behavior. High-quality aggressive bouts are defined as repetitive antagonistic interactions (no less than 1 bout per minute; each lasting approximately 5–10 s) lasting throughout the 5–10 min defeat session. These interactions are not required to be continuous, but must occur periodically from start to finish. The clearest sign of an underachieving aggressor CD-1 is observation of grooming between the CD-1 and C57BL/6J. If grooming is observed, replace the CD-1 for subsequent defeats.
? TROUBLESHOOTING
-
10|
Place control animals in pairs within an identical home cage setup, one control animal per side divided by a perforated Plexiglas divider, for the duration of the defeat sessions (see Experimental design for further details). ▲ CRITICAL STEP Rotate control C57BL/6J mice to a new cage on a daily basis, but never allow them physical contact with their cage mates.
-
11|
Immediately following the last defeat session, house all intruder C57BL/6J mice singly in standard mouse cages with ad libitum access to food and water. Carry out social interaction testing ~24 h later, or as required by experimental design.
Social interaction testing • TIMING 1 d
-
12|
For social interaction testing, the CD-1 aggressor mouse should be completely novel to the defeated C57BL/6J mouse from Step 11 (i.e., not used during the 10 defeats in Steps 8–9). Screen the target CD-1 mouse, as performed in Steps 1–3 and using criteria from Step 4 (for only one session rather than three), prior to use in testing to ensure that aggressive characteristics are present.
-
13|
Assemble social interaction open-field arenas and set up the video-tracking apparatus and software as described in EQUIPMENT SETUP.
▲ CRITICAL STEP All data will be collected in an automated manner by the video-tracking apparatus and software for all test phases. There is no human experimenter-recorded data acquisition. The software is set up to automatically record time in interaction zone, time in corner zone and total movement as indicated in EQUIPMENT SETUP.
-
14|
Habituate the C57BL/6J mice from Step 11 to the testing suite for ~1 h before testing.
▲ CRITICAL STEP Testing conditions always occur under red-light conditions in a room isolated from external sound sources.
-
15|
Each social interaction test is composed of two 150-s phases, separated by a duration of 30 s, either with or without the target CD-1 mouse present in the interaction zone. All data during these phases are automatically recorded as described in Step 13. During the first phase, when the target CD-1 aggressor is absent, take the C57BL/6J mouse from its home cage and place it directly into the rear center of the open field opposite the empty wire-mesh enclosure, allowing for exploration of the open-field arena.
-
16|
Immediately after terminating phase 1, remove the C57BL/6J mouse from the arena and return the mouse to its home cage until phase 2. Also remove the `no target' wire-mesh cage.
-
17|
In the 30-s break between phases, place the target CD-1 mouse within a `target-only' designated wire-mesh enclosure and place this within the arena.
-
18|
Again, remove the C57BL/6J mouse from its home cage and place it into the rear center of the open field opposite the wire-mesh enclosure as in Step 15.
-
19|
At the end of this test session, remove both the target and intruder and clean the arena as described in the EQUIPMENT SETUP. After completing each test, verify that the software acquired time in the interaction zone, time in the corner zone and total movement before moving on to the next mouse.
▲ CRITICAL STEP The wire-mesh enclosure used in conjunction with the target should now only be used with the target for future tests to avoid any odor confounds that remain on the wire mesh.
-
20|
After completion of the social interaction test, both CD-1 and C57BL/6J mice are returned to their singly housed standard mice cages, and may be used for further behavioral or molecular analysis.
-
21|
After completing all test sessions for the cohort, data may be analyzed as explained in ANTICIPATED RESULTS.
? TROUBLESHOOTING
Troubleshooting advice can be found in table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 5 | A majority of CD-1 mice fail to pass criteria for inclusion | Old/large screener mice; insufficient acclimation period | Change the screener mice and extend the number of days of screening |
| 6 | CD-1 mice show aggressive behavior but do not induce susceptible populations of defeated mice | CD-1 mice have been used for more than three consecutive social defeat batches | Replace CD-1 mice |
| 8,9 | Defeated mice show excessive wounding | Duration of daily defeats are too long; specific aggressors repeatedly overachieve | Switch from 10- to 5-min daily defeats; remove specific aggressors from the experiment if they are consistently wounding mice |
| Mice escape their compartment between defeats | Too much bedding; too little food/water on top of cage lid; warped divider | Remove some bedding and add weight to lid; check divider for proper fit | |
| Aggressors appear uninterested in defeat session | Habituation to defeat protocol | Remove aggressor from experiment |
• TIMING
Steps 1–6, Screening for aggressive CD-1 mice: ~7 d acclimation to new colony, ~3 d of screening for inclusion criteria
Steps 7–11, Chronic social defeat stress: 10 d of defeats, ~45 min per cohort of 30 mice per day per experimenter
Steps 12–21, Social interaction testing: 1 d, ~10 min per test
The duration of an entire chronic social defeat experiment, including acclimation and screening of aggressors, is ~21 d. If aggressors are already screened from a previous defeat experiment, the duration is 13 d including a single day of aggressor rescreening.
ANTICIPATED RESULTS
Behavioral results from social defeat stress are reported in two ways: (i) as a comparison of total time spent by the C57BL/6J mouse in the interaction zone during each social interaction test session when the target is absent or present, or (ii) as a ratio of these two times. The social interaction ratio (SI ratio) is obtained by dividing the time spent in the interaction zone when the target is present by the time spent in the interaction zone when the target is absent. Historically, a SI ratio equal to 1, in which equal time is spent in the presence versus absence of a social target, has been used as the threshold for dividing defeated mice into the susceptible and resilient categories17,21. Control C57BL/6J mice show a strong tendency to spend greater than or equal amounts of time in the interaction zone in each session. Mice below this criterion are grouped as susceptible, whereas mice above are grouped as resilient. Across a large population of defeated mice, approximately 30–40% show a resilient phenotype. It is worth noting that other variables, such as time spent in the corner zone or total distance traveled, can also be recorded.
When analyzing social interaction ratios, one-way analysis of variance (ANOVA) is appropriate for comparisons between control, resilient and susceptible groups. However, we also include analysis of the total time in the interaction zone when the target is absent or present for each session. For this analysis, a 2 × 3 ANOVA is used to compare the interaction zone times between target absent and target present in susceptible, resilient and control mice, followed by post hoc tests when the interaction is significant at P < 0.05.
The results described here and in Figure 3 are unpublished representative data collected and combined from three social defeat experiments (S.A.G. and S.J.R., unpublished data). As indicated in Figure 3a, 41 (37.6%) out of a total of 109 defeated mice failed to show social avoidance behavior as indicated by their social interaction ratio. A total of 68 mice (62.4%) showed significant social avoidance behavior (36.19 ± 2.48 s s.e.m.) in the presence of a target compared with control (70.94 ± 3.31 s s.e.m.) and resilient (72.86 ± 2.48 s s.e.m.) mice. Multivariate ANOVAs reveal a significant interaction between phenotype and time spent in the interaction zone, in which susceptible mice spend less time when the target is present than control and resilient mice (F(2,266) = 53.51, P < 0.05, Bonferroni post hoc P < 0.05, Fig. 3b). One-way ANOVA reveals a main effect of phenotype on social interaction ratio, in which susceptible mice have lower SI ratios than control and resilient mice (F(2,161) = 75.61, P < 0.05 and Bonferroni post hoc P < 0.05, Fig. 3d). Similar results are observed if corner zone times are assessed, with susceptible mice spending significantly more time in the corners (51.47 ± 3.33 s s.e.m.) compared with both control (19.86 ± 1.81 s s.e.m.) and resilient mice (21.43 ± 1.70 s s.e.m.). Multiple comparison ANOVAs reveal a significant interaction between phenotype and time spent in the corner zone, in which susceptible mice spend more time in the corner when the target is present compared with control and resilient mice (F(2,266) = 28.90, P < 0.05, Bonferroni post hoc P < 0.05, Fig. 3c). One-way ANOVA reveals a main effect of phenotype on corner time ratio, in which susceptible mice have higher corner time ratios compared with control and resilient mice (F(2,161) = 32.21, P < 0.05 and Bonferroni post hoc P < 0.05, Fig. 3e). The numbers presented here are highly indicative of a standard social defeat experiment.
Figure 3.
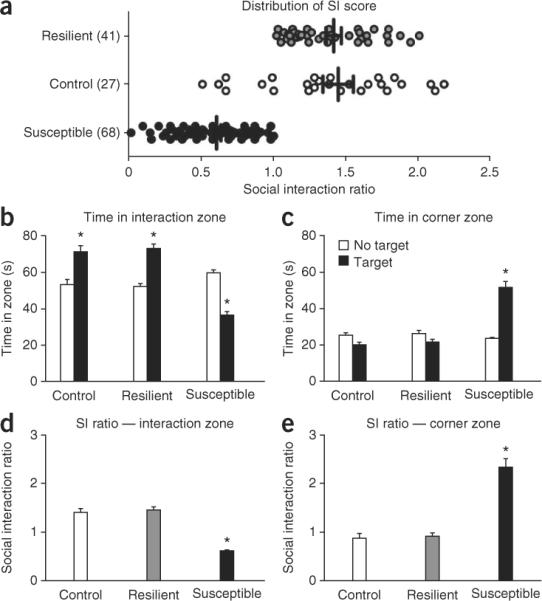
Repeated social defeat stress induces avoidance behavior in susceptible mice. (a) Repeated social defeat stress results in a spectrum of avoidance behavior, divided between susceptible and resilient phenotypes as a function of their social interaction (SI) ratio score. This is the ratio of time a mouse spends in the interaction zone in the presence of a target CD-1 compared with the absence of a target CD-1. (b,c) Susceptible mice spend significantly more time in the corner zone than in the interaction zone, whereas resilient mice spend comparable amounts of time in the interaction zone to control mice that have never undergone a defeat procedure. Both control and resilient mice spend significantly more time in the interaction zone when a target is present. (d,e) Social avoidance behavior can also be expressed as a social interaction ratio. In this panel, the same data are shown in both manners, for comparison. Error bars represent means ± s.e.m. *P < 0.05, multivariate ANOVA/ANOVA. All data shown were collected while conforming to governmental and institutional guidelines for care and use of laboratory animals.
ACKNOWLEDGMENTS
We thank D. Christoffel for his helpful review of this manuscript. We also acknowledge the intrepid efforts of the Mount Sinai School of Medicine animal facility personnel. This research was supported by US National Institute of Mental Health grant 1R01MH090264-01A1.
Footnotes
AUTHOR CONTRIBUTIONS S.A.G., H.E.C., O.B. and S.J.R. contributed to study design, data collection, analysis and writing.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Greenberg PE, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am. J. Psychiatry. 2008;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann. NY Acad. Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 7.Butterweck V, Winterhoff H, Herkenham M. St John's wort, hypericin, and imipramine: a comparative analysis of mRNA levels in brain areas involved in HPA axis control following short-term and long-term administration in normal and stressed rats. Mol. Psychiatry. 2001;6:547–564. doi: 10.1038/sj.mp.4000937. [DOI] [PubMed] [Google Scholar]
- 8.Rygula R, et al. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav. Pharmacol. 2008;19:183–196. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- 9.Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta. Physiol. Scand. Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- 10.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 11.Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brain PF. Mammalian behavior and the adrenal cortex. A review. Behav. Biol. 1972;7:453–477. doi: 10.1016/s0091-6773(72)80209-8. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs E, Flugge G. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol. Biochem. Behav. 2002;73:247–258. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- 14.Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav. Brain. Res. 2006;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Rygula R, et al. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav. Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- 16.Rygula R, et al. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain. Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 18.Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol. Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- 19.Covington HE, III, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- 20.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Avgustinovich DF, Gorbach OV, Kudryavtseva NN. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol. Behav. 1997;61:37–43. doi: 10.1016/s0031-9384(96)00303-4. [DOI] [PubMed] [Google Scholar]
- 23.Lutter M, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang JC, et al. Chronic social defeat stress disrupts regulation of lipid synthesis. J. Lipid Res. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang JC, et al. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol. Psychiatry. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutter M, et al. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 28.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covington HE, III, et al. Antidepressant actions of histone deacetylase inhibitors. J. Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson MB, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J. Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vialou V, et al. [Delta]FosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christoffel DJ, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johren O, Flugge G, Fuchs E. Regulation of hippocampal glucocorticoid receptor gene expression by psychosocial conflict. Ann. NY Acad. Sci. 1994;746:429–430. doi: 10.1111/j.1749-6632.1994.tb39276.x. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard DC, et al. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 35.Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B. Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex. Prog. Brain Res. 1998;119:437–448. doi: 10.1016/s0079-6123(08)61586-1. [DOI] [PubMed] [Google Scholar]
- 36.Vivian JA, Miczek KA. Interactions between social stress and morphine in the periaqueductal gray: effects on affective vocal and reflexive pain responses in rats. Psychopharmacology (Berl) 1999;146:153–161. doi: 10.1007/s002130051101. [DOI] [PubMed] [Google Scholar]
- 37.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 38.Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm. Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 39.Dadomo H, et al. Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010 November 17; doi: 10.1016/j.pnpbp.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Razzoli M, et al. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol. Biochem. Behav. 2010;97:566–576. doi: 10.1016/j.pbb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Razzoli M, Carboni L, Andreoli M, Ballottari A, Arban R. Different susceptibility to social defeat stress of BALB/c and C57BL6/J mice. Behav. Brain Res. 2010;216:100–108. doi: 10.1016/j.bbr.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Gimsa U, Kanitz E, Otten W, Ibrahim SM. Behavior and stress reactivity in mouse strains with mitochondrial DNA variations. Ann. NY Acad. Sci. 2009;1153:131–138. doi: 10.1111/j.1749-6632.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 43.Haenisch B, Bilkei-Gorzo A, Caron MG, Bonisch H. Knockout of the norepinephrine transporter and pharmacologically diverse antidepressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J. Neurochem. 2009;111:403–416. doi: 10.1111/j.1471-4159.2009.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covington HE, III, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


