Abstract
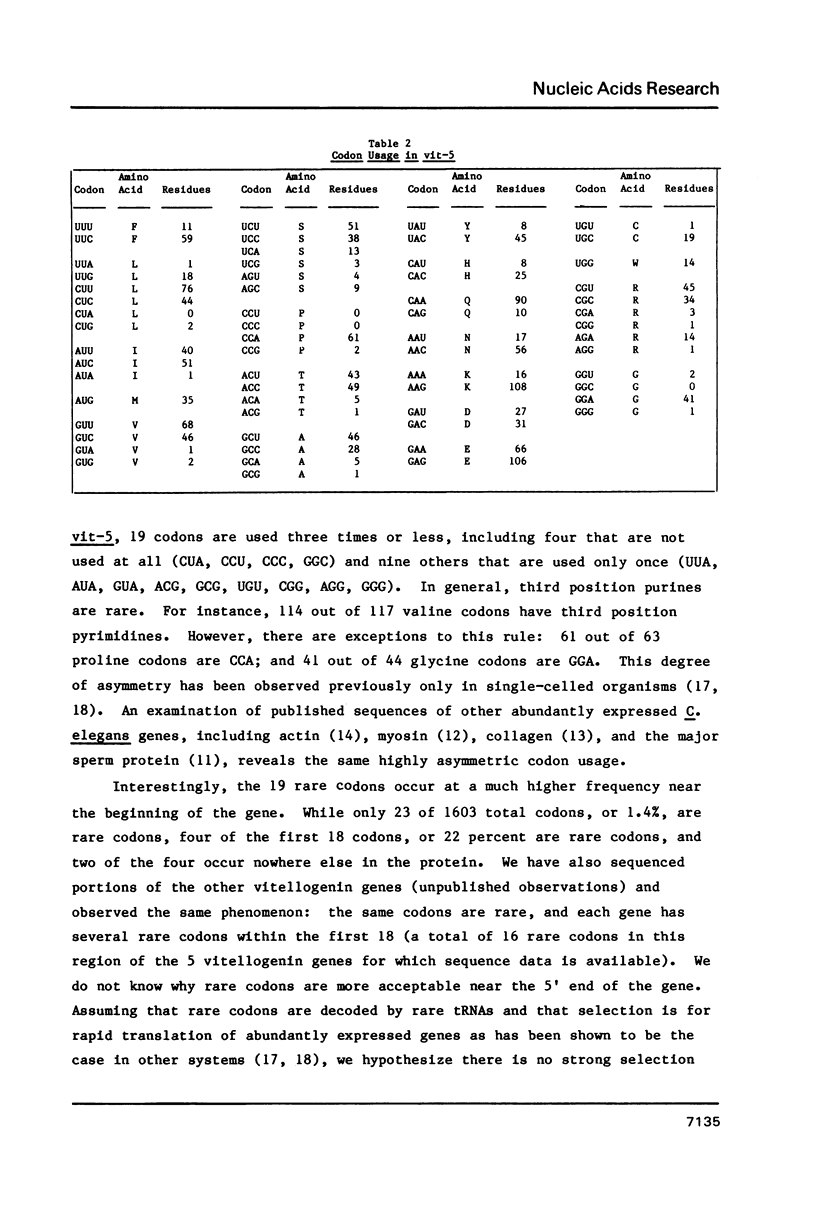
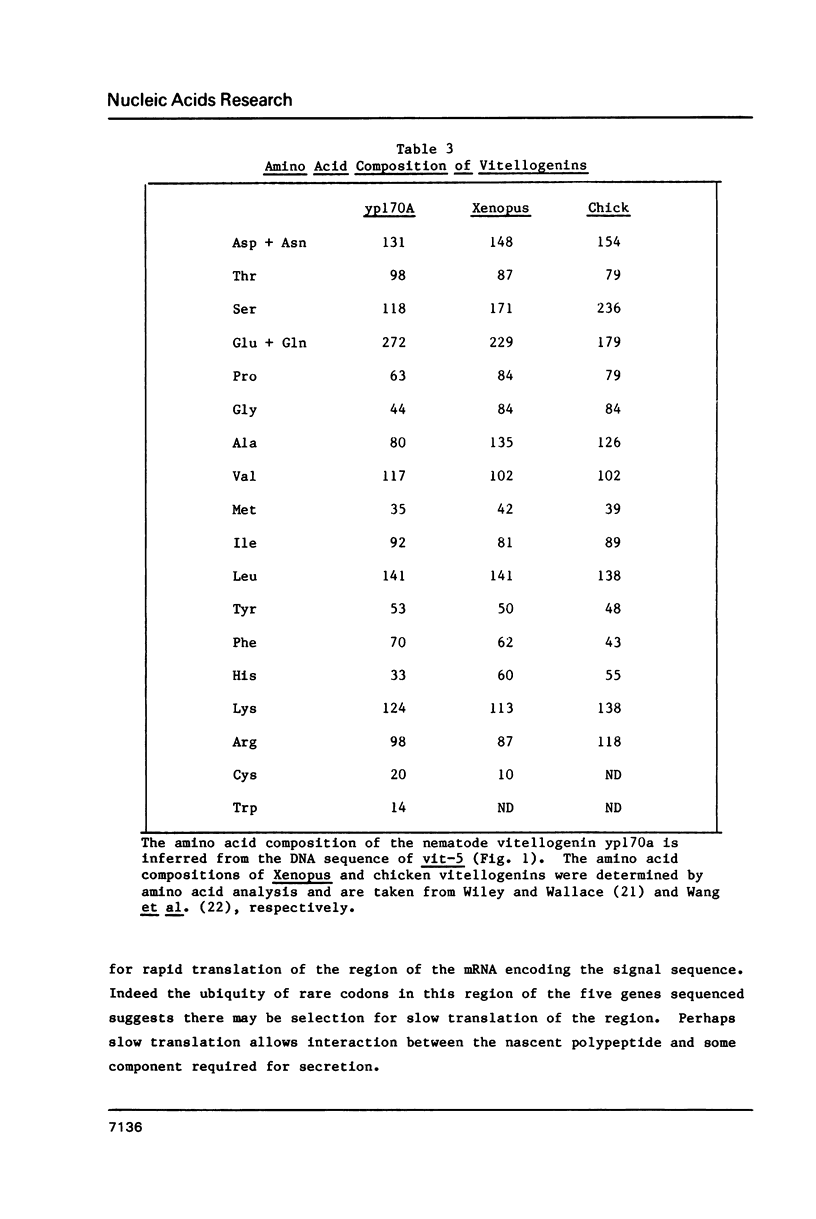
The nematode, Caenorhabditis elegans, contains a family of six genes that code for vitellogenins. Here we report the complete nucleotide sequence of one of these genes, vit-5. The gene specifies a mRNA of 4869 nucleotides, including untranslated regions of 9 bases at the 5' end and 51 bases at the 3' end. Vit-5 contains four short introns totalling 218 bp. The predicted vitellogenin, yp170A, has a molecular weight of 186,430. At its N terminus it is clearly related to the vitellogenins of vertebrates. However, the vit-5-encoded protein does not contain a serine-rich sequence related to the vertebrate vitellin, phosvitin. In fact, the amino acid composition of the nematode protein is very similar to that of the vertebrate protein without phosvitin. Vit-5 has a highly asymmetric codon choice dictionary. The favored codons are different from those favored in other organisms, but are characteristic of highly expressed C. elegans genes. The strong selection against rare codons is not as great near the 5' end of the gene; rare codons are 15 times more frequent within the first 54 bp than in the next 4.8 kb.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnberg A. C., Meijlink F. C., Mulder J., van Bruggen E. F., Gruber M., Geert A. B. Isolation and characterization of genomic clones covering the chicken vitellogenin gene. Nucleic Acids Res. 1981 Jul 24;9(14):3271–3286. doi: 10.1093/nar/9.14.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Blumenthal T., Squire M., Kirtland S., Cane J., Donegan M., Spieth J., Sharrock W. Cloning of a yolk protein gene family from Caenorhabditis elegans. J Mol Biol. 1984 Mar 25;174(1):1–18. doi: 10.1016/0022-2836(84)90361-9. [DOI] [PubMed] [Google Scholar]
- Byrne B. M., van het Schip A. D., van de Klundert J. A., Arnberg A. C., Gruber M., Ab G. Amino acid sequence of phosvitin derived from the nucleotide sequence of part of the chicken vitellogenin gene. Biochemistry. 1984 Sep 11;23(19):4275–4279. doi: 10.1021/bi00314a003. [DOI] [PubMed] [Google Scholar]
- Files J. G., Carr S., Hirsh D. Actin gene family of Caenorhabditis elegans. J Mol Biol. 1983 Mar 5;164(3):355–375. doi: 10.1016/0022-2836(83)90056-6. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. Protein structural domains in the Caenorhabditis elegans unc-54 myosin heavy chain gene are not separated by introns. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4253–4257. doi: 10.1073/pnas.80.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J., Sharrock W. J. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol. 1983 Mar;96(1):189–196. doi: 10.1016/0012-1606(83)90322-6. [DOI] [PubMed] [Google Scholar]
- Klass M. R., Kinsley S., Lopez L. C. Isolation and characterization of a sperm-specific gene family in the nematode Caenorhabditis elegans. Mol Cell Biol. 1984 Mar;4(3):529–537. doi: 10.1128/mcb.4.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M. R., Wolf N., Hirsh D. Further characterization of a temperature-sensitive transformation mutant in Caenorhabditis elegans. Dev Biol. 1979 Mar;69(1):329–335. doi: 10.1016/0012-1606(79)90295-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock W. J. Cleavage of two yolk proteins from a precursor in Caenorhabditis elegans. J Mol Biol. 1984 Apr 15;174(3):419–431. doi: 10.1016/0022-2836(84)90329-2. [DOI] [PubMed] [Google Scholar]
- Sharrock W. J. Yolk proteins of Caenorhabditis elegans. Dev Biol. 1983 Mar;96(1):182–188. doi: 10.1016/0012-1606(83)90321-4. [DOI] [PubMed] [Google Scholar]
- Spieth J., Denison K., Kirtland S., Cane J., Blumenthal T. The C. elegans vitellogenin genes: short sequence repeats in the promoter regions and homology to the vertebrate genes. Nucleic Acids Res. 1985 Jul 25;13(14):5283–5295. doi: 10.1093/nar/13.14.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Weber R., Ryffel G. U. Comparative analysis of the structural organization of two closely related vitellogenin genes in X. laevis. Cell. 1980 May;20(1):107–117. doi: 10.1016/0092-8674(80)90239-1. [DOI] [PubMed] [Google Scholar]
- Walker P., Brown-Luedi M., Germond J. E., Wahli W., Meijlink F. C., van het Schip A. D., Roelink H., Gruber M., Ab G. Sequence homologies within the 5' end region of the estrogen-controlled vitellogenin gene in Xenopus and chicken. EMBO J. 1983;2(12):2271–2279. doi: 10.1002/j.1460-2075.1983.tb01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., Wallace R. A. The structure of vitellogenin. Multiple vitellogenins in Xenopus laevis give rise to multiple forms of the yolk proteins. J Biol Chem. 1981 Aug 25;256(16):8626–8634. [PubMed] [Google Scholar]


