Abstract
Here I show that a “pure” research project, seemingly totally lacking in practical application when it was first published, can years later spark a whole new scientific field with the potential to revolutionize clinical practice. A 1961 publication describing adducts of nitric oxide (NO) with certain nucleophiles attracted little notice at the time, but later work showing that the adducts could be hydrolyzed to regenerate the NO in bioactive form has provided the foundation for a host of biomedical applications. Crucial to the discovery of widely used tools for studying NO’s chemical biology as well as for the design of a variety of promising therapeutic advances has been the increasingly detailed understanding of the physicochemical properties of these “diazeniumdiolates” (also known as NONOates).
Basic Research: Seeking Knowledge for the Sake of Knowledge
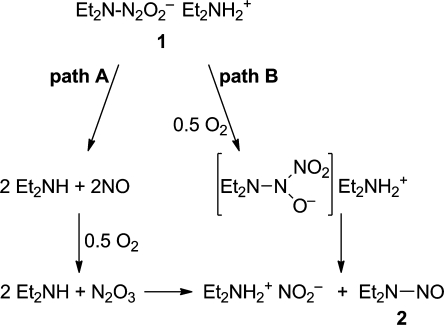
In 1961, a report emerged from the laboratory of inorganic chemist Russell Drago describing the reaction of nitric oxide (NO) with selected nucleophiles.(1) One of these was diethylamine, whose reaction with NO produced a white powder of formula 1 that, on standing exposed to air overnight, was converted to the potent carcinogen N-nitrosodiethylamine (2) (eq 1).(2) The authors speculated that the reaction proceeded by the mechanism shown in Figure 1, path A, which begins by reversing the synthesis reaction to regenerate NO from the solid material. On reading these reports, I hypothesized that this reaction might instead proceed by novel pathway B of Figure 1, which begins with oxygen directly abstracting the elements of NO– from 1 to produce 2 plus diethylammonium nitrite.
Figure 1.
Two theoretically possible mechanisms by which diazeniumdiolate salt 1 can be converted to nitrosamine 2.
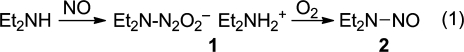
Confirmation that path B was operative would have been an interesting theoretical advance in the area of nitrosamine formation, a research theme I was pursuing at the time. Unfortunately for my hypothesis, however, this was not the case. NMR experiments supported path A, revealing that 1 simply regenerated the NO and diethylamine in solution, with the NO then being autoxidized to the well-known nitrosating agent N2O3, which recombined with the free amine to form 2. This was not the newsworthy mechanism of nitrosamine formation I had hoped for, because everybody already knew that aerobic NO could nitrosate amines, so we published a short account of the study in a 1982 meeting proceedings(3) and pursued other, at the time seemingly more relevant, facets of nitrosamine chemistry.
The picture changed dramatically in 1987 and 1988, when publications from the biomedical sector claiming NO to be a key effector of blood vessel dilation,(4) neurotransmission in the brain,(5) immune response,6,7 and anticoagulant activity(8) began to appear. Initially, I did not believe these reports, having learned a lot about the nefarious face of NO through its involvement as a metabolite and precursor of the carcinogenic N-nitroso compounds I had been studying. I realized, however, that if any part of that work was credible, something really important was breaking onto the biomedical scene.
Probing the Chemistry of 1 and Analogous Ions As a Starting Point for Developing Novel Tools for Chemical Biology Research
If reports of NO’s bioeffector roles were true, and if 1 and its analogues indeed hydrolyze to produce molecular NO in solution, then one might expect these ions to be pharmacologically active. As a platform for beginning tests of this hypothesis, my colleagues and I started by preparing a selection of salts of structure X-N(O)=NO– M+ and systematically cataloguing their physicochemical properties. Many were found to precipitate in pure form from solutions of the X-H precursor on exposure to gaseous NO under basic conditions, although success sometimes proved to be quite dependent on empirically choosing the correct solvent. Exchanging the dialkyammonium ion of 1 and homologues for sodium tended to improve the salts’ shelf life, with many examples proving indefinitely stable as solids at 4 °C. Structural studies confirmed the substantial double bond character of the N–N linkage of many X-N(O)=NO– ions, with the oxygens being cis to one another; this and other features of their structures led to their formal designation as “diazeniumdiolates”,(9) although earlier nomenclature proposals referring to them as “NOC compounds” or “NONOates” continue in common use. These salts proved to be UV-active, with a strong chromophore at ∼250 nm and εmax of ∼7 mM–1 cm–1 in the absence of electronic interaction with X, allowing for convenient characterization of their solution chemistry. They react with copper(II) to produce coordination complexes in which the metal binds the ligand’s two oxygens without oxidizing it,(10) although reductive nitrosylation has been implicated in the reactions of diazeniumdiolate ions with other metal centers.11,12
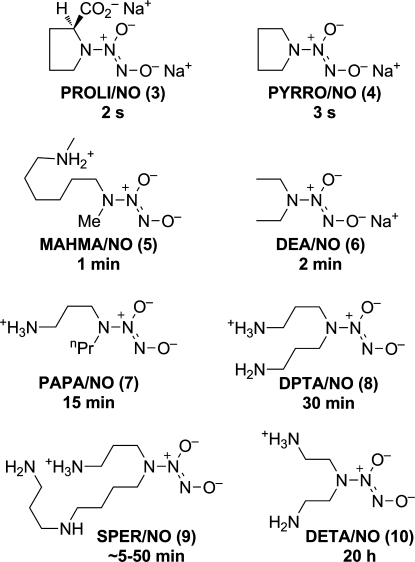
Examples for which X is a secondary amine residue were found to generate NO in yields approaching the theoretical 2 mol/mol at physiological temperature and pH, with reproducible half-lives ranging from 2 s to 20 h, depending on the structure of the amine moiety (Figure 2).(13) The mechanism of the cleanly first-order (at constant pH) hydrolysis was elucidated, showing that the favored equilibrium protonation site is the terminal oxygen, while the kinetically significant tautomer has the proton at the amino nitrogen of the diazeniumdiolated secondary amines.(14) From the standpoint of stoichiometry, the acid-catalyzed hydrolysis reaction was confirmed to consume acid and slow itself down as the pH climbs in the absence of proper buffering capacity. Medium effects, such as micellar encapsulation and liposome formation, have been reported to affect hydrolysis rates, sometimes dramatically.(15) Examples of X-N(O)=NO– ions for which X is carbon, oxygen, sulfur, or a primary amino group were also studied; they showed very similar structural and ultraviolet spectral characteristics,(9) though their reactivity patterns were much more diverse than those of the well-behaved secondary amine analogues, often generating little to no NO on hydrolysis, for example.
Figure 2.
Structures of selected diazeniumdiolate salts and zwitterions, along with their half-lives in 0.1 M phosphate at pH 7.4 and 37 °C. Each of these generates close to the theoretical 2 mols of NO per mole of anionic diazeniumdiolate. The ∼5- to 50-min half-life for 9 is attributable to a contribution from a substrate-squared term to the rate equation, i.e., to formation of a less reactive dimer as the concentration is increased.(14)
Two features of these fundamental chemistry studies set the diazeniumdiolate ions apart as advantageous tools for investigating the chemical biology of NO:
-
•
In contrast to other NO donors, they require no redox activation, releasing NO at repeatable first-order rates on simple dissolution in buffered aqueous fluids, cell culture media, or organ baths.
-
•
The broad array of reproducible NO generation rates allows researchers to probe the effects of exposure to short puffs of the bioeffector, to near-steady state fluxes, and to many half-lives of NO release in between.
Predicting Biological Effects Based on a Knowledge of the Diazeniumdiolates’ Physicochemical Properties
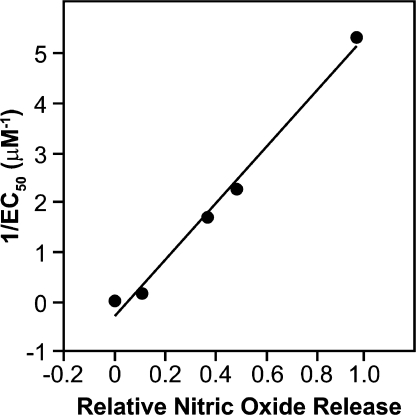
Of special note was the finding that data on the rate and extent of NO release in simple buffers could be used for quantitatively predicting biological outcomes. Figure 3 illustrates an early example. Knowing that NO had been identified as the endothelium-derived relaxing factor,(4) we chose a set of five physicochemically diverse ionic diazeniumdiolates, determined their hydrolysis rate constants, measured the number of moles of NO that were produced on hydrolyzing one mole of each, studied their effects on blood vessel dilation in an organ bath, and published a paper in 1991 showing how the physicochemical parameters could be plugged into an equation that quantitatively correlated with vasodilatory activities in this series of compounds. (16)
Figure 3.
Observed maximal in vitro vasodilatory potencies (1/EC50) of five ionic diazeniumdiolates correlated highly (r = 0.995) with theoretical values computed from the compounds’ rates and extents of NO release. (For details of the calculation, see ref (16)).
As this increasingly refined understanding of the basic chemistry and its pharmacological implications was developing, colleagues in the biomedical realm became interested in studying their physiological effects in vivo. Cardiologist colleagues showed that the 2-min half-life for DEA/NO (6) in physiological buffer translated to a profound but brief drop in blood pressure on bolus intravenous administration in rabbits, while 10-fold greater doses of the longer-lived analogue SPER/NO (9) had a blunted immediate effect but one that was correspondingly much more prolonged.(17) Similarly, the extent and time course of these agents’ anticoagulant action in vivo closely matched predictions based on their solution chemistry.(18)
These studies showing the surprising extent to which the biological properties quantitatively matched expectation based on the fundamental chemistry in turn elicited interest among clinicians in possible therapeutic strategies. Neurosurgeons studying models of cerebral vasospasm following subarachnoid hemorrhage showed that intracarotid infusions of the 2-min compound DEA/NO (6) just upstream from the affected vessel induced the desired vasodilation.(19) Alternatively, polymer blends of the 20-h NO donor DETA/NO (10) were shown to prevent vasospasm in a rat femoral artery model.(20) In a vascular surgery application, ionically diazeniumdiolated materials applied to the outside of rat carotid arteries undergoing balloon angioplasty dramatically improved healing at the inner surface of the vessel, relative to untreated controls.(21)
Protecting Group Strategies and the Design of Prodrugs for Internal Use
Spontaneous generation of NO can be beneficial for the kinds of therapeutic applications mentioned above, but in most cases it is imperative to target the NO selectively to the given tissue or cell type where it is needed without exposing the myriad other NO-sensitive bodily compartments. One convenient means of doing this is to take advantage of the diazeniumdiolate ions’ inherent nucleophilicity to generate neutral prodrug forms.(22) Initial studies with simple methylating and ethylating agents showed that alkylation occurred at the terminal oxygen (O2) of the X-N(O)=NO– ion, in contrast to an earlier structural assignment that erroneously placed the alkyl group on the other oxygen; the N–N linkage consistently had even more double bond character than was seen in the corresponding ionic starting material, with the oxygens again being cis to one another.(22) The alkylation products proved remarkably stable toward oxidation. With a functional group consisting of three sequential nitrogens, two of which are oxygenated, one might have expected otherwise; nevertheless, no problems were seen in subjecting them to copper-mediated Click chemistry(23) or sodium periodate/ruthenium trichloride-induced oxidation of a methylene group to a carbonyl.(24) Diazeniumdiolate ions can be sulfonated at the O2-position,(25) but the corresponding acylated analogues have so far defied attempts to isolate them.
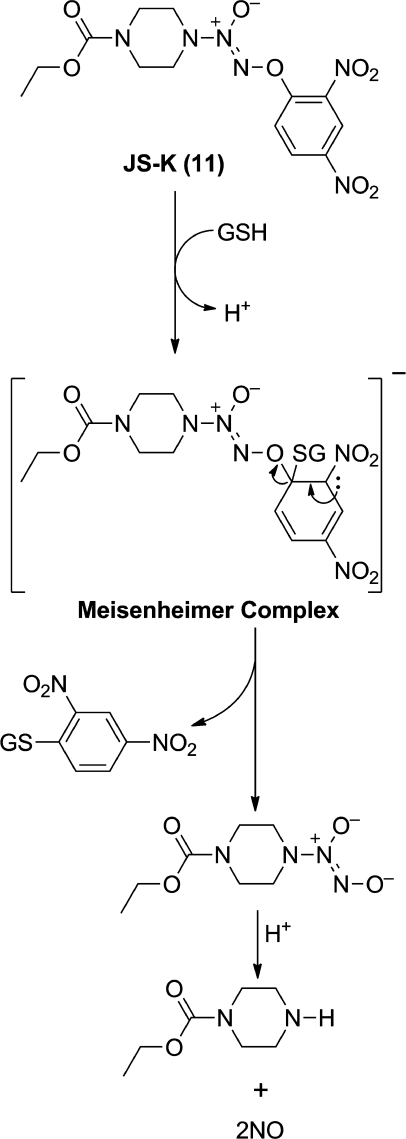
SNAr studies showed anionic diazeniumdiolate DEA/NO (6) to be intermediate between chloride and fluoride in its reactivity with 1-halo-2,4-dinitrobenzenes.(26) The resulting O2-arylated diazeniumdiolates hydrolyzed smoothly in base.(26) These aryl derivatives also generate NO on reaction with the nucleophilic thiol group of glutathione (exemplified in Figure 4) and have been widely studied for their potential use as anticancer agents.(27) Initial hypotheses in this area focused on their potential to selectively remove highly NO-sensitive leukemia cells from the bloodstream without inflicting collateral toxicity in their normal hematopoietic counterparts,(28) but their potential anticancer activity has, for reasons that remain to be fully elucidated, proven far more general than that. In studies that will be reviewed in detail elsewhere, our lead O2-arylated diazeniumdiolate JS-K (11) has moved into the late preclinical stage of evaluation as a broad-spectrum anticancer drug candidate, active in numerous in vivo rodent models of cancer including leukemia,(28) multiple myeloma,(29) and tumors of the prostate,(28) liver,(30) and lung.(27)
Figure 4.
Activation of O2-arylated diazeniumdiolate JS-K (11) as an NO donor by reaction with the thiol-containing peptide glutathione (GSH).(27)
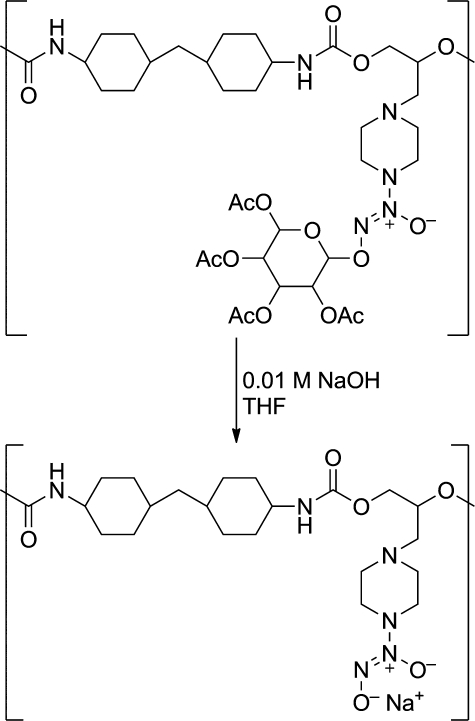
O2-Glycosylation has also proven advantageous as a diazeniumdiolate protecting group strategy. O2-Glucosylated DEA/NO was found to resist hydrolysis in neutral and acidic conditions but to hydrolyze remarkably rapidly in base.(31) This property proved useful during preparation of poly(urethane)s bearing ionic diazeniumdiolate groups. By O2-glucosylating monodiazeniumdiolated piperazine and attaching its other nitrogen via a linker to the diol segment of the poly(urethane) backbone, the polymer could be processed through various heating and other steps that the ionic form could not survive, then treated with base to remove the saccharide residues and regenerate the capacity for NO release (Figure 5).(31) Additionally, preliminary work with small-molecule O2-glycosylated diazeniumdiolates has revealed their possible utility in antimicrobial applications.(32)
Figure 5.
Base-induced removal of glucose protecting groups for preparation of NO-releasing diazeniumdiolated poly(urethane) (from ref (31)).
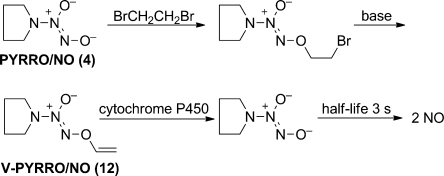
Diazeniumdiolate ions can also be O2-vinylated, as illustrated in Figure 6. The prototype in this series is V-PYRRO/NO (12, Figure 6), designed and shown to be oxidatively cleaved by cytochrome P450 enzymes concentrated in the liver to release NO selectively in that organ, with minimal effects elsewhere.(33) This strategy has shown considerable promise for treating fulminant liver failure,(33) acetaminophen toxicity,(34) and the ischemia/reperfusion injury that accompanies organ transplantation.(35)
Figure 6.
Synthesis and metabolism of V-PYRRO/NO (12), an O2-vinylated diazeniumdiolate that is activated for NO release by cytochromes P450.
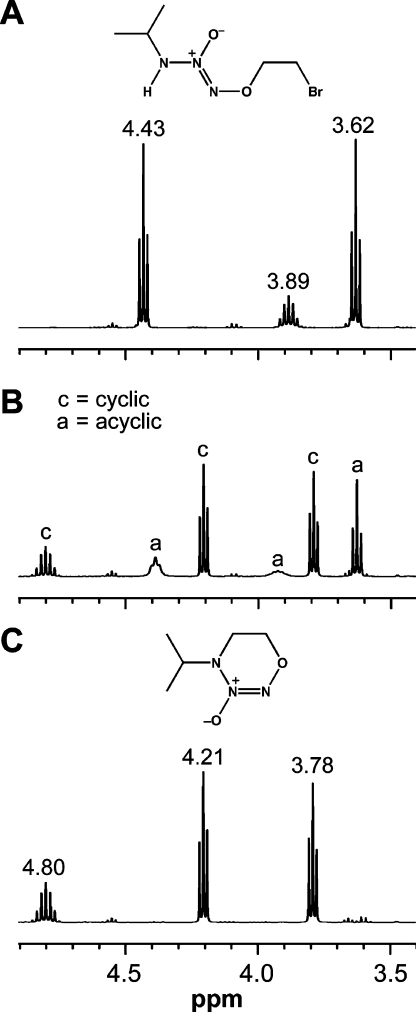
Interestingly, the synthesis of O2-vinylated derivatives as outlined in Figure 6 failed on attempted application to a primary amine diazeniumdiolate. In this case, the main product on treating the O2-(2-bromoethyl)ated intermediate with base resulted from a cyclization reaction instead of dehydrohalogenation, as documented in the NMR spectra of Figure 7. This reaction provided the first clear evidence that cis-to-trans isomerization about the N(O)=NO bond was possible. Theoretical and experimental studies revealed that the barrier to such isomerization dropped from a prohibitive ∼40 kcal/mol for the neutral molecule to an easily accessible value of ∼20 kcal/mol on ionization of the N–H bond.(36) The pKa for this process was estimated to be 12.4, lower than that needed to ionize the C–H bond en route to dehydrohalogenation.
Figure 7.
Proton spectra showing the conversion of the Z diazeniumdiolate structure in panel A at time zero to the cyclic E derivative of panel C after 2 h at 0 °C in basic D2O/methanol-d4 solution. Panel B shows the spectrum of the reaction mixture at the 15-min time point; note the line-broadening associated with the Z to E interconversion barrier (from ref (36)).
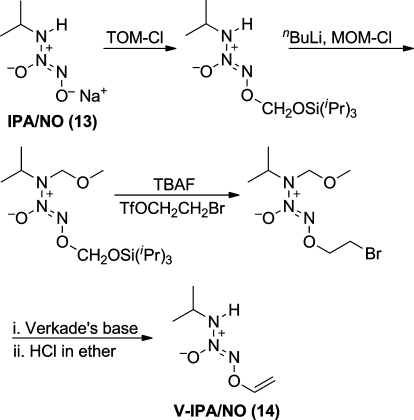
To accomplish the conversion of primary amine diazeniumdiolate 13 to the desired vinyl derivative 14, two new diazeniumdiolate protecting groups were developed and applied as shown in Figure 8.(37) Reaction of IPA/NO anion with (triisopropylsilyl)oxymethyl chloride (TOM-ylation) protected the O2-position, stabilizing and solubilizing IPA/NO while the methoxymethyl (MOM) group was installed at the amino nitrogen to render it non-nucleophilic and prevent ring closure. Fluoride-induced removal of the TOM group in the presence of 2-bromoethyl triflate led to an O2-bromoethylated diazeniumdiolate that on treatment with Verkade’s base followed by acid-induced removal of the MOM group produced the desired O2-vinylation product 14.(37)
Figure 8.
Use of TOM and MOM protecting groups in the synthesis of V-IPA/NO (14) (ref (37)).
MOM-ylation at the O2-position instead of N results in less hydrolytically sensitive diazeniumdiolates whose rates of spontaneous NO release are generally much slower than those of the corresponding ions. An example is diazeniumdiolated bovine serum albumin, which produces about 40 mols of NO per mole of protein with a half-life of about 3 weeks.(38) When placed in the pericardial sac surrounding the heart to suffuse the coronary arteries of pigs undergoing balloon angioplasty, this material not only reduced the cellular overgrowth that leads to narrowing of the arterial lumen but also beneficially remodeled the vessel by filling in the cracks in the wall that result from such balloon overstretch.(39)
Important synthetic opportunities were opened up when the sulfur analogue of the MOM group (MeSCH2– instead of MeOCH2–) was introduced at the O2-position. Treatment of the resulting thio-MOM derivative with sulfuryl chloride gives the corresponding O2-chloromethyl compound, whose chloride can be displaced by carboxylate ions to produce a variety of esterase-sensitive prodrugs.(40) Among the important mutual prodrugs made possible using this strategy is a hybrid molecule whose hydrolysis yields aspirin and NO concurrently; this NONO-aspirin was similar to aspirin itself in terms of anti-inflammatory activity but largely devoid of aspirin’s ulcerogenic side effects.(41)
Attaching esterase-sensitive protecting groups at the O2-position has proven especially illuminating. Acetoxymethylating PYRRO/NO (4) generates a prodrug that is relatively stable in cell culture media but that enzymatically hydrolyzes rapidly once inside the cell to generate a concentrated intracellular flux of diazeniumdiolate ions whose antiproliferative effects on the cells are 2 to 3 orders of magnitude more potent than is seen when the unprotected ionic diazeniumdiolate is dissolved directly in the medium.(42) A particularly interesting effect of acetoxymethylation is described in the next section.
From NO to HNO
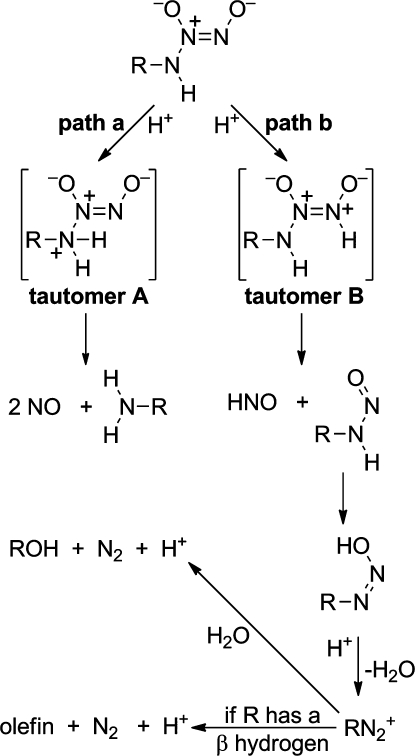
Our initial 1991 study of vasodilatory activity on the part of five structurally diverse diazeniumdiolates (Figure 3) included the primary amine derivative IPA/NO (13), but this compound was both relatively unstable and much less potent as an NO generator and vasodilator than its secondary amine analogues.(16) For this reason, it remained little studied until news of the biological activities of nitroxyl (HNO), the one-electron reduction product of NO, began to break some years later.43−45 Of special importance in the present connection was a 2005 paper by Miranda and colleagues addressing the following question: if IPA/NO (13) produced only 36% of the theoretical amount of NO on hydrolysis,(16) what happened to the other 64%? The answer: the missing nitrogenous product was HNO.(46)
To study the utility of diazeniumdiolated primary amines as HNO donors, the problem of their instability had to be overcome. The prototype in this series, IPA/NO (13), has a tendency to decompose on isolation and storage, sometimes producing a mixture of the corresponding diazoate plus other decomposition products that on visual inspection is indistinguishable from pure IPA/NO but at other times disintegrates explosively without warning or apparent provocation.
This problem can be largely controlled by exactingly avoiding excesses of acids and bases in the freshly synthesized material,(47) but conversion to stable and rigorously purifiable O2-substituted derivatives has provided a more general solution. Alkylation of the ionic diazeniumdiolates occurs preferentially at the O2-position,(48) providing such stable derivatives. The resulting RHN-N(O)=N-OR species proved to be an ambident nucleophile under basic conditions, undergoing alkylation at either end of the N–N–N system.(49) MOM-ylation of 13 led to an N,O bis-substituted product in the presence of excess derivatizing agent, with the O2-monosubstituted intermediate also being isolable; 13 could also be O2-glycosylated.(48)
Acetoxymethylation of IPA/NO unveiled some properties that were especially intriguing from the drug discovery point of view.(50) One was that the product hydrolyzed spontaneously in the absence of esterase with a half-life of 41 min at physiological temperature and pH, an order of magnitude longer than that of IPA/NO(50) and thus a considerable step toward the goal of providing an array of reliable half-lives for use as research tools in studying the chemical biology of HNO, much as has been the case with the NO donors discussed above.
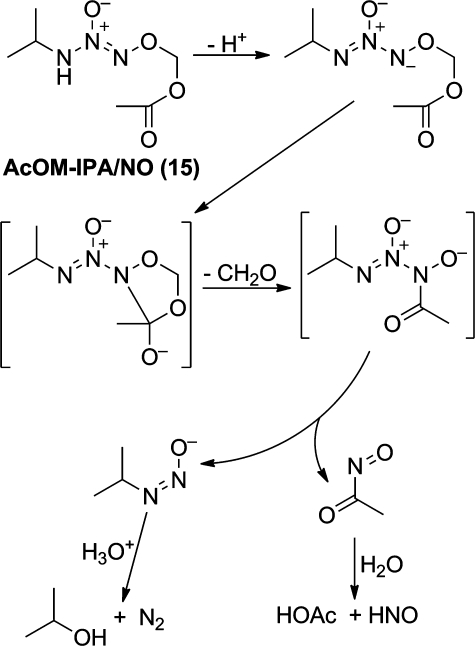
The other major surprise was that hydrolysis of acetoxymethylated IPA/NO in the absence of esterase produced HNO without cogenerating NO.(50) We had expected the reaction to proceed by straightforward ester hydrolysis to produce 13, which would then hydrolyze according to the mechanism outlined in Figure 9, which involves generation of similar fluxes of NO and HNO according to the indicated competing pathways.(51) Prodrug 15’s behavior as a “pure” HNO donor has been ascribed to the mechanism shown in Figure 10.(50) Future work will seek to embellish on this chemistry as a platform for producing improved HNO donor research tools and drugs.
Figure 9.
Partitioning of a diazeniumdiolated primary amine anion between its NO- and HNO-generating hydrolysis pathways (adapted from ref (51)).
Figure 10.
Spontaneous hydrolysis of the “pure” HNO donor AcOM-IPA/NO (from ref (50)).
Tackling the Nitrosamine Toxicity Issue
Having begun researching diazeniumdiolates by studying them as precursors for carcinogenic nitrosamines (Figure 1),(3) we realized from the outset that this possible source of toxicity must be dealt with head-on during any effort to reap practical benefits from their chemistry. One way to do this is to employ diazeniumdiolates whose corresponding N-nitroso derivatives are considered noncarcinogenic; an example is PROLI/NO (3, Figure 2),(52) whose potential metabolite N-nitrosoproline is a normal constituent of human urine that has failed to induce tumors in any of the long-term toxicity studies published so far.53−59 Another strategy is to attach the diazeniumdiolate functional group to primary amines or carbon-based nucleophiles, though care must still be taken to identify the byproducts of NO release and avoid their toxicity.
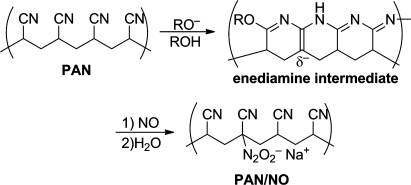
Yet another approach is to tie the NO-releasing moiety covalently to insoluble materials that limit NO exposure only to the cells and tissues with which they are in immediate physical contact while at the same time preventing release of any byproduct including nitrosated species into the surrounding medium. An example that combines the latter two approaches is diazeniumdolated poly(acrylonitrile) (“PAN/NO”), whose synthesis and structure are shown in Figure 11.(60) In addition to poly(urethane) (Figure 5) and poly(acrylonitrile), other insoluble materials whose surfaces can be beneficially converted to NO-releasing form for a variety of antiplatelet, antimicrobial, and wound healing applications by covalently diazeniumdiolating them include poly(ethyleneimine),(61) silicone rubber,(62) and silicon-based hydrogels.(63)
Figure 11.
Diazeniumdiolation of poly(acrylonitrile) (PAN), a water-insoluble polymer that generates NO only at its surface while retaining all byproducts of its spontaneous hydrolysis within the solid polymer matrix (from ref (60)).
Studies of an O-alkylated diazeniumdiolate’s photochemistry led to the surprising finding that its primary photoproducts resulted from cleavage of the formal N–N double bond to form the corresponding nitrosamine plus an O-nitrene.(64) Although photochemical nitrosamine formation can be largely overcome by judicious selection of O2-substituents that channel the reaction toward NO generation,(65) the findings reinforce the importance of excluding light as well as other potentially destructive stimuli during synthesis and storage so as to avoid unwanted contamination by impurities in general and carcinogenic nitrosamines in particular.
Some Remaining Questions
Many questions about diazeniumdiolate chemistry remain to be answered if we are to realize the full inherent potential of these compounds as tools for researching the chemical biology of NO and HNO as well as for rationally designing biomedical innovations. Below is a selection for your consideration and, hopefully, input:
-
•
How can it be explained that amines generally react smoothly with NO, whereas water and hydroxide apparently do not? The product of a water/hydroxide diazeniumdiolation reaction would be Angeli’s salt [NaO-N(O)=NONa], an HNO donor that is synthesized by nitrating hydroxylamine. Like most other ionic diazeniumdiolates, Angeli’s salt is increasingly stabilized as the pH is raised, such that if it were to be formed in 1 M solutions of sodium hydroxide in contact with 3 atm of NO it would be spectrophotometrically detectable. But this does not happen. Why not?
-
•
What are the structural and stereoelectronic determinants of the partition between the NO and HNO generation pathways in the primary amine diazeniumdiolate series? Are the results for IPA/NO (13), which generates substantial amounts of both NO and HNO on hydrolysis at physiological pH (Figure 9), typical for this series of ions? Perhaps future structure variation studies will reveal analogues with NO- or HNO-only buffer hydrolysis profiles among the diazeniumdiolated primary amine anions.
-
•
What are the structural and stereoelectronic determinants of hydrolysis half-life? I find this to be an enduring mystery. Some generalizations are possible, with for example diazeniumdiolated pyrrolidines having relatively shorter lifetimes in solution, but how can the tremendous spread seen among the diazeniumdiolated di- and polyamines [e.g., 1 min for MAHMA/NO (5) and 1 day for DETA/NO(10)] be explained? My answer so far has been to rely completely on the empirical approach—synthesize a new compound and determine its hydrolysis rate experimentally—but I’m hopeful that someone will someday establish a means of rationally predicting such properties in advance.
Conclusion
I hope that what I have written has piqued your imagination. Much remains to be done if the full potential of the science presented here is to be realized, both in further probing the basic chemistry of the diazeniumdiolates, in using the knowledge thus gained to design additionally improved tools for chemical biology research, and in engineering important advances in medical practice. I am convinced that there are lives to be saved in the technology described here and would warmly welcome your participation in the effort to exploit its full promise.
Acknowledgments
This work was supported by the Intramural Program of the NIH, National Cancer Institute, Center for Cancer Research.
Glossary
Key Words
- Nitric oxide
a multifaceted diatomic bioeffector molecule
- nitroxyl
the one-electron reduction product of NO, itself an important bioeffector molecule
- NO
the formula for nitric oxide
- HNO
the formula for nitroxyl
- diazeniumdiolates
the formal name for compounds of structure XN( O)=NOR, where R represents an electrophile and X is a nucleophile residue
- NONOate
a commonly used synonym for diazeniumdiolate
- prodrug
(Wikipedia definition) “A ... pharmacological substance (drug) administered in an inactive (or significantly less active) form. Once administered, the prodrug is metabolized in vivo into an active metabolite, a process termed bioactivation.”
Funding Statement
National Institutes of Health, United States
References
- Drago R. S.; Karstetter B. R. (1961) The reaction of nitrogen(II) oxide with various primary and secondary amines. J. Am. Chem. Soc. 83, 1819–1822. [Google Scholar]
- Ragsdale R. O.; Karstetter B. R.; Drago R. S. (1965) Decomposition of the adducts of diethylamine and isopropylamine with nitrogen(II) oxide. Inorg. Chem. 4, 420–422. [Google Scholar]
- Hansen T. J.; Croisy A. F.; Keefer L. K. (1982) N-Nitrosation of secondary amines by nitric oxide via the 'Drago complex'. IARC Sci. Publ. 41, 21–29. [PubMed] [Google Scholar]
- Palmer R. M. J.; Ferrige A. G.; Moncada S. (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526. [DOI] [PubMed] [Google Scholar]
- Garthwaite J.; Charles S. L.; Chess-Williams R. (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336, 385–388. [DOI] [PubMed] [Google Scholar]
- Marletta M. A.; Yoon P. S.; Iyengar R.; Leaf C. D.; Wishnok J. S. (1988) Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 27, 8706–8711. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B. Jr.; Taintor R. R.; Vavrin Z.; Rachlin E. M. (1988) Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157, 87–94. [DOI] [PubMed] [Google Scholar]
- Moncada S.; Radomski M. W.; Palmer R. M. J. (1988) Endothelium-derived relaxing factor: identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 37, 2495–2501. [DOI] [PubMed] [Google Scholar]
- Keefer L. K.; Flippen-Anderson J. L.; George C.; Shanklin A. P.; Dunams T. M.; Christodoulou D.; Saavedra J. E.; Sagan E. S.; Bohle D. S. (2001) Chemistry of the diazeniumdiolates. 1. Structural and spectral characteristics of the [N(O)NO]− functional group. Nitric Oxide 5, 377–394. [DOI] [PubMed] [Google Scholar]
- Christodoulou D.; George C.; Keefer L. K. (1993) An unusual bi-tri-binuclear sandwich complex formed in the reaction of CuCl2 with the Et2N-N2O2– ion. J. Chem. Soc., Chem. Commun. 937–939. [Google Scholar]
- Piciulo P. L.; Scheidt W. R. (1975) Synthesis of nitrosyl manganese porphyrins: use of the novel reagent, N2O2 amine adducts. Inorg. Nucl. Chem. Lett. 11, 309–311. [Google Scholar]
- Hassanin H. A.; Hannibal L.; Jacobsen D. W.; El-Shahat M. F.; Hamza M. S. A.; Brasch N. E. (2009) Mechanistic studies on the reaction between R2N-NONOates and aquacobalamin: evidence for direct transfer of a nitroxyl group from R2N-NONOates to cobalt(III) centers. Angew. Chem., Int. Ed. 48, 8909–8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer L. K.; Nims R. W.; Davies K. M.; Wink D. A. (1996) “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 268, 281–293. [DOI] [PubMed] [Google Scholar]
- Davies K. M.; Wink D. A.; Saavedra J. E.; Keefer L. K. (2001) Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. J. Am. Chem. Soc. 123, 5473–5481. [DOI] [PubMed] [Google Scholar]
- Dinh B.; Dove K.; Jappar D.; Hrabie J. A.; Davies K. M. (2005) Effect of hydrophobic structure on the catalysis of nitric oxide release from zwitterionic diazeniumdiolates in surfactant and liposome media. Nitric Oxide: Biol. Chem. 13, 204–209. [DOI] [PubMed] [Google Scholar]
- Maragos C. M.; Morley D.; Wink D. A.; Dunams T. M.; Saavedra J. E.; Hoffman A.; Bove A. A.; Isaac L.; Hrabie J. A.; Keefer L. K. (1991) Complexes of ·NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 34, 3242–3247. [DOI] [PubMed] [Google Scholar]
- Diodati J. G.; Quyyumi A. A.; Keefer L. K. (1993) Complexes of nitric oxide with nucleophiles as agents for the controlled biological release of nitric oxide: hemodynamic effect in the rabbit. J. Cardiovasc. Pharmacol. 22, 287–292. [DOI] [PubMed] [Google Scholar]
- Diodati J. G.; Quyyumi A. A.; Hussain N.; Keefer L. K. (1993) Complexes of nitric oxide with nucleophiles as agents for the controlled biological release of nitric oxide: antiplatelet effect. Thromb. Haemostasis 70, 654–658. [PubMed] [Google Scholar]
- Pluta R. M.; Oldfield E. H.; Boock R. J. (1997) Reversal and prevention of cerebral vasospasm by intracarotid infusions of nitric oxide donors in a primate model of subarachnoid hemorrhage. J. Neurosurg. 87, 746–751. [DOI] [PubMed] [Google Scholar]
- Tierney T. S.; Clatterbuck R. E.; Lawson C.; Thai Q.-A.; Rhines L. D.; Tamargo R. J. (2001) Prevention and reversal of experimental posthemorrhagic vasospasm by the periadventitial administration of nitric oxide from a controlled-release polymer. Neurosurgery 49, 945–953. [DOI] [PubMed] [Google Scholar]
- Pearce C. G.; Najjar S. F.; Kapadia M. R.; Murar J.; Eng J.; Lyle B.; Aalami O. O.; Jiang Q.; Hrabie J. A.; Saavedra J. E.; Keefer L. K.; Kibbe M. R. (2008) Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radical Biol. Med. 44, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. E.; Dunams T. M.; Flippen-Anderson J. L.; Keefer L. K. (1992) Secondary amine/nitric oxide complex ions, R2N[N(O)NO]−. O-Functionalization chemistry. J. Org. Chem. 57, 6134–6138. [Google Scholar]
- Oladeinde O. A.; Hong S. Y.; Holland R. J.; Maciag A. E.; Keefer L. K.; Saavedra J. E.; Nandurdikar R. S. (2010) “Click” reaction in conjunction with diazeniumdiolate chemistry: developing high-load nitric oxide donors. Org. Lett. 12, 4256–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani H.; Showalter B. M.; Kong L.; Keefer L. K.; Saavedra J. E. (2007) V-PROLI/NO, a prodrug of the nitric oxide donor, PROLI/NO. Org. Lett. 9, 3409–3412. [DOI] [PubMed] [Google Scholar]
- D’Sa R. A.; Wang Y.; Ruane P. H.; Showalter B. M.; Saavedra J. E.; Davies K. M.; Citro M. L.; Booth M. N.; Keefer L. K.; Toscano J. P. (2003) Preparation and reactivity of O2-sulfonated diazeniumdiolates. J. Org. Chem. 68, 656–657. [DOI] [PubMed] [Google Scholar]
- Saavedra J. E.; Srinivasan A.; Bonifant C. L.; Chu J.; Shanklin A. P.; Flippen-Anderson J. L.; Rice W. G.; Turpin J. A.; Davies K. M.; Keefer L. K. (2001) The secondary amine/nitric oxide complex ion R2N[N(O)NO]− as nucleophile and leaving group in SNAr reactions. J. Org. Chem. 66, 3090–3098. [DOI] [PubMed] [Google Scholar]
- Maciag A. E.; Chakrapani H.; Saavedra J. E.; Morris N. L.; Holland R. J.; Kosak K. M.; Shami P. J.; Anderson L. M.; Keefer L. K. (2011) The nitric oxide prodrug JS-K is effective against non-small-cell lung cancer cells in vitro and in vivo: involvement of reactive oxygen species. J. Pharmacol. Exp. Ther. 336, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shami P. J.; Saavedra J. E.; Wang L. Y.; Bonifant C. L.; Diwan B. A.; Singh S. V.; Gu Y.; Fox S. D.; Buzard G. S.; Citro M. L.; Waterhouse D. J.; Davies K. M.; Ji X.; Keefer L. K. (2003) JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol. Cancer Ther. 2, 409–417. [PubMed] [Google Scholar]
- Kiziltepe T.; Hideshima T.; Ishitsuka K.; Ocio E. M.; Raje N.; Catley L.; Li C.-Q.; Trudel L. J.; Yasui H.; Vallet S.; Kutok J. L.; Chauhan D.; Mitsiades C. S.; Saavedra J. E.; Wogan G. N.; Keefer L. K.; Shami P. J.; Anderson K. C. (2007) JS-K, a GST-activated nitric oxide generator, induces DNA double strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood 110, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shami P. J.; Saavedra J. E.; Bonifant C. L.; Chu J.; Udupi V.; Malaviya S.; Carr B. I.; Kar S.; Wang M.; Jia L.; Ji X.; Keefer L. K. (2006) Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J. Med. Chem. 49, 4356–4366. [DOI] [PubMed] [Google Scholar]
- Showalter B. M.; Reynolds M. M.; Valdez C. A.; Saavedra J. E.; Davies K. M.; Klose J. R.; Chmurny G. N.; Citro M. L.; Barchi J. J. Jr.; Merz S. I.; Meyerhoff M. E.; Keefer L. K. (2005) Diazeniumdiolate ions as leaving groups in anomeric displacement reactions: a protection-deprotection strategy for ionic diazeniumdiolates. J. Am. Chem. Soc. 127, 14188–14189. [DOI] [PubMed] [Google Scholar]
- Valdez C. A.; Saavedra J. E.; Showalter B. M.; Davies K. M.; Wilde T. C.; Citro M. L.; Barchi J. J. Jr.; Deschamps J. R.; Parrish D.; El-Gayar S.; Schleicher U.; Bogdan C.; Keefer L. K. (2008) Hydrolytic reactivity trends among potential prodrugs of the O2-glycosylated diazeniumdiolate family. Targeting nitric oxide to macrophages for antileishmanial activity. J. Med. Chem. 51, 3961–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. E.; Billiar T. R.; Williams D. L.; Kim Y.-M.; Watkins S. C.; Keefer L. K. (1997) Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-α-induced apoptosis and toxicity in the liver. J. Med. Chem. 40, 1947–1954. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li C.; Waalkes M. P.; Clark J.; Myers P.; Saavedra J. E.; Keefer L. K. (2003) The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology 37, 324–333. [DOI] [PubMed] [Google Scholar]
- Ricciardi R.; Foley D. P.; Quarfordt S. H.; Saavedra J. E.; Keefer L. K.; Wheeler S. M.; Donohue S. E.; Callery M. P.; Meyers W. C. (2001) V-PYRRO/NO: an hepato-selective nitric oxide donor improves porcine liver hemodynamics and function after ischemia reperfusion. Transplantation 71, 193–198. [DOI] [PubMed] [Google Scholar]
- Wang Y.-N.; Bohle D. S.; Bonifant C. L.; Chmurny G. N.; Collins J. R.; Davies K. M.; Deschamps J.; Flippen-Anderson J. L.; Keefer L. K.; Klose J. R.; Saavedra J. E.; Waterhouse D. J.; Ivanic J. (2005) Chemistry of the diazeniumdiolates: Z/E isomerism. J. Am. Chem. Soc. 127, 5388–5395. [DOI] [PubMed] [Google Scholar]
- Nandurdikar R. S.; Keefer L. K.; Saavedra J. E. (2011) Novel protection-deprotection strategies in diazeniumdiolate chemistry: synthesis of V-IPA/NO. Chem. Commun. (Cambridge, U. K.) 47, 6710–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabie J. A.; Saavedra J. E.; Roller P. P.; Southan G. J.; Keefer L. K. (1999) Conversion of proteins to diazeniumdiolate-based nitric oxide donors. Bioconjugate Chem. 10, 838–842. [DOI] [PubMed] [Google Scholar]
- Baek S. H.; Hrabie J. A.; Keefer L. K.; Hou D.; Fineberg N.; Rhoades R.; March K. L. (2002) Augmentation of intrapericardial nitric oxide level by a prolonged-release nitric oxide donor reduces luminal narrowing after porcine coronary angioplasty. Circulation 105, 2779–2784. [DOI] [PubMed] [Google Scholar]
- Tang X.; Xian M.; Trikha M.; Honn K. V.; Wang P. G. (2001) Synthesis of peptide-diazeniumdiolate conjugates: towards enzyme activated antitumor agents. Tetrahedron Lett. 42, 2625–2629. [Google Scholar]
- Velázquez C.; Praveen Rao P. N.; Knaus E. E. (2005) Novel nonsteroidal antiinflammatory drugs possessing a nitric oxide donor diazen-1-ium-1,2-diolate moiety: design, synthesis, biological evaluation, and nitric oxide release studies. J. Med. Chem. 48, 4061–4067. [DOI] [PubMed] [Google Scholar]
- Saavedra J. E.; Shami P. J.; Wang L. Y.; Davies K. M.; Booth M. N.; Citro M. L.; Keefer L. K. (2000) Esterase-sensitive nitric oxide donors of the diazeniumdiolate family. In vitro antileukemic activity. J. Med. Chem. 43, 261–269. [DOI] [PubMed] [Google Scholar]
- Miranda K. M.; Paolocci N.; Katori T.; Thomas D. D.; Ford E.; Bartberger M. D.; Espey M. G.; Kass D. A.; Feelisch M.; Fukuto J. M.; Wink D. A. (2003) A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. U.S.A. 100, 9196–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolocci N.; Katori T.; Champion H. C. St.; John M. E.; Miranda K. M.; Fukuto J. M.; Wink D. A.; Kass D. A. (2003) Positive inotropic and lusitropic effects of HNO/NO– in failing hearts: independence from β-adrenergic signaling. Proc. Natl. Acad. Sci. U.S.A. 100, 5537–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K. M. (2005) The chemistry of nitroxyl (HNO) and implications in biology. Coord. Chem. Rev. 249, 433–455. [Google Scholar]
- Miranda K. M.; Katori T.; Torres de Holding C. L.; Thomas L.; Ridnour L. A.; McLendon W. J.; Cologna S. M.; Dutton A. S.; Champion H. C.; Mancardi D.; Tocchetti C. G.; Saavedra J. E.; Keefer L. K.; Houk K. N.; Fukuto J. M.; Kass D. A.; Paolocci N.; Wink D. A. (2005) Comparison of the NO and HNO donating properties of diazeniumdiolates: primary amine adducts release HNO in vivo. J. Med. Chem. 48, 8220–8228. [DOI] [PubMed] [Google Scholar]
- Salmon D. J.; Torres de Holding C. L.; Thomas L.; Peterson K. V.; Goodman G. P.; Saavedra J. E.; Srinivasan A.; Davies K. M.; Keefer L. K.; Miranda K. M. (2011) HNO and NO release from a primary amine-based diazeniumdiolate as a function of pH. Inorg. Chem. 50, 3262–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. E.; Bohle D. S.; Smith K. N.; George C.; Deschamps J. R.; Parrish D.; Ivanic J.; Wang Y.-N.; Citro M. L.; Keefer L. K. (2004) Chemistry of the diazeniumdiolates. O- versus N-alkylation of the RNH[N(O)NO]− ion. J. Am. Chem. Soc. 126, 12880–12887. [DOI] [PubMed] [Google Scholar]
- Bohle D. S.; Keefer L. K.; Saavedra J. E. (2009) Primary amine diazeniumdiolate ions of structure {RNN(O)NOR′}− as ambident nucleophiles. Tetrahedron Lett. 50, 5917–5919. [Google Scholar]
- Andrei D.; Salmon D. J.; Donzelli S.; Wahab A.; Klose J. R.; Citro M. L.; Saavedra J. E.; Wink D. A.; Miranda K. M.; Keefer L. K. (2010) Dual mechanisms of HNO generation by a nitroxyl prodrug of the diazeniumdiolate (NONOate) class. J. Am. Chem. Soc. 132, 16526–16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A. S.; Suhrada C. P.; Miranda K. M.; Wink D. A.; Fukuto J. M.; Houk K. N. (2006) Mechanism of pH-dependent decomposition of monoalkylamine diazeniumdiolates to form HNO and NO, deduced from the model compound methylamine diazeniumdiolate, density functional theory, and CBS-QB3 calculations. Inorg. Chem. 45, 2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. E.; Southan G. J.; Davies K. M.; Lundell A.; Markou C.; Hanson S. R.; Adrie C.; Hurford W. E.; Zapol W. M.; Keefer L. K. (1996) Localizing antithrombotic and vasodilatory activity with a novel, ultrafast nitric oxide donor. J. Med. Chem. 39, 4361–4365. [DOI] [PubMed] [Google Scholar]
- Garcia H.; Lijinsky W. (1973) Studies of the tumorigenic effect in feeding of nitrosamino acids and of low doses of amines and nitrite to rats. Z. Krebsforsch. Klin. Onkol. 79, 141–144. [DOI] [PubMed] [Google Scholar]
- Greenblatt M.; Lijinsky W. (1972) Failure to induce tumors in Swiss mice after concurrent administration of amino acids and sodium nitrite. J. Natl. Cancer Inst. 48, 1389–1392. [PubMed] [Google Scholar]
- Mirvish S. S.; Bulay O.; Runge R. G.; Patil K. (1980) Study of the carcinogenicity of large doses of dimethylnitramine, N-nitroso-L-proline, and sodium nitrite administered in drinking water to rats. J. Natl. Cancer Inst. 64, 1435–1442. [DOI] [PubMed] [Google Scholar]
- Nixon J. E.; Wales J. H.; Scanlan R. A.; Bills D. D.; Sinnhuber R. O. (1976) Null carcinogenic effect of large doses of nitrosoproline and nitrosohydroxyproline in Wistar rats. Food Cosmet. Toxicol. 14, 133–135. [DOI] [PubMed] [Google Scholar]
- Nagasawa H. T.; Fraser P. S.; Yuzon D. L. (1973) A new method for nitrosation of proline and related sec-α-amino acids to N-nitrosamino acids with possible oncogenic activity. J. Med. Chem. 16, 583–585. [DOI] [PubMed] [Google Scholar]
- Hecht S. S.; Abbaspour A.; Hoffman D. (1988) A study of tobacco carcinogenesis XLII. Bioassay in A/J mice of some structural analogues of tobacco-specific nitrosamines. Cancer Lett. 42, 141–145. [DOI] [PubMed] [Google Scholar]
- Lijinsky W.; Reuber M. D. (1982) Transnitrosation by nitrosamines in vivo. IARC Sci. Publ. 41, 625–631. [PubMed] [Google Scholar]
- DeRosa F.; Kibbe M. R.; Najjar S. F.; Citro M. L.; Keefer L. K.; Hrabie J. A. (2007) Nitric oxide-releasing fabrics and other acrylonitrile-based diazeniumdiolates. J. Am. Chem. Soc. 129, 3786–3787. [DOI] [PubMed] [Google Scholar]
- Smith D. J.; Chakravarthy D.; Pulfer S.; Simmons M. L.; Hrabie J. A.; Citro M. L.; Saavedra J. E.; Davies K. M.; Hutsell T. C.; Mooradian D. L.; Hanson S. R.; Keefer L. K. (1996) Nitric oxide-releasing polymers containing the [N(O)NO]− group. J. Med. Chem. 39, 1148–1156. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Annich G. M.; Miskulin J.; Osterholzer K.; Merz S. I.; Bartlett R. H.; Meyerhoff M. E. (2002) Nitric oxide releasing silicone rubbers with improved blood compatibility: preparation, characterization, and in vivo evaluation. Biomaterials 23, 1485–1494. [DOI] [PubMed] [Google Scholar]
- Robbins M. E.; Schoenfisch M. H. (2003) Surface-localized release of nitric oxide via sol-gel chemistry. J. Am. Chem. Soc. 125, 6068–6069. [DOI] [PubMed] [Google Scholar]
- Srinivasan A.; Kebede N.; Saavedra J. E.; Nikolaitchik A. V.; Brady D. A.; Yourd E.; Davies K. M.; Keefer L. K.; Toscano J. P. (2001) Chemistry of the diazeniumdiolates. 3. Photoreactivity. J. Am. Chem. Soc. 123, 5465–5472. [DOI] [PubMed] [Google Scholar]
- Pavlos C. M.; Xu H.; Toscano J. P. (2004) Controlled photochemical release of nitric oxide from O2-substituted diazeniumdiolates. Free Radic. Biol. Med. 37, 745–752. [DOI] [PubMed] [Google Scholar]