Abstract
Lipid transfer inhibitor protein (LTIP) exists in both active and inactive forms. Incubation (37°C) of plasma causes LTIP to transfer from a 470 kDa inactive complex to LDL where it is active. Here, we investigate the mechanisms underlying this movement. Inhibiting LCAT or cholesteryl ester transfer protein (CETP) reduced incubation-induced LTIP translocation by 40–50%. Blocking both LCAT and CETP completely prevented LTIP movement. Under appropriate conditions, either factor alone could drive maximum LTIP transfer to LDL. These data suggest that chemical modification of LDL, the 470 kDa complex, or both facilitate LTIP movement. To test this, LDL and the 470 kDa fraction were separately premodified by CETP and/or LCAT activity. Modification of the 470 kDa fraction had no effect on subsequent LTIP movement to native LDL. Premodification of LDL, however, induced spontaneous LTIP movement from the native 470 kDa particle to LDL. This transfer depended on the extent of LDL modification and correlated negatively with changes in the LDL phospholipid + cholesterol-to-cholesteryl ester + triglyceride ratio. We conclude that LTIP translocation is dependent on LDL lipid composition, not on its release from the inactive complex. Compositional changes that reduce the surface-to-core lipid ratio of LDL promote LTIP binding and activation.
Keywords: cholesteryl ester transfer protein, lipoprotein composition, lecithin:cholesterol acyltransferase, lipid packing
Cholesteryl ester transfer protein (CETP) mediates the exchange of cholesteryl ester (CE) and triglyceride (TG) between lipoproteins (1, 2). Because the selection of CE or TG as substrate is defined by the relative abundance of these lipids in the lipoprotein, when CETP promotes lipid transfer between TG-rich and CE-rich lipoproteins, there is a net flux of TG toward the CE-rich particle with the converse holding true for the flux of CE (3, 4). Lipid transfer inhibitor protein (LTIP), also known as apolipoprotein F (5), modulates CETP function by preferentially blocking CETP activity on LDL (6). LTIP does not bind to CETP, but rather inhibits its activity by preventing CETP from binding to the lipoprotein surface (7). By selectively blocking lipid transfer to/from LDL, LTIP enhances the net flux of CE from HDL to VLDL. Because VLDL has a relatively short plasma lifetime, this flux is proposed to facilitate the clearance of HDL-derived CE, thus promoting reverse cholesterol transport. Recent studies of LTIP overexpression in mice with a human-like lipoprotein profile and transgenic for human CETP are consistent with this role for LTIP in cholesterol metabolism (8).
We previously reported that plasma LTIP exists in two forms, one active and the other inactive (9). Active LTIP is bound to LDL, whereas LTIP bound in a 470 kDa lipoprotein complex is inactive. In normolipidemic plasma, up to 25% of LTIP is associated with LDL, but this percentage is markedly higher in hypercholesterolemic subjects (9). This altered distribution is not due simply to the presence of elevated LDL in these subjects because the addition of normolipidemic LDL to plasma has little effect on LTIP status. However, the fraction of LTIP associated with LDL can be increased by incubating plasma at 37°C (9). Collectively, these findings suggest that lipoprotein composition is an important determinant of LTIP distribution.
In the present study, we examined mechanisms that control the association of LTIP with LDL and therefore regulate its activation status. We observed that both CETP and LCAT activities can promote the activation of LTIP. The movement of LTIP from the inactive complex to the LDL-associated active form is driven by changes in LDL composition.
METHODS
Materials
[1α, 2α(n)-3H]cholesteryl oleate was purchased from Perkin-Elmer (Boston, MA). Mouse monoclonal antibody to human CETP (TP2) was purchased from the Ottawa Heart Institute (Ottawa, Ontario, Canada). Paraoxon (diethyl p-nitrophenyl phosphate), DTNB, and all buffers, salts and detergents were purchased from Sigma (St. Louis, MO). Paraoxon stock solutions (100 mM) were prepared in 50% methanol, 25 mM Tris-HCl, pH 6.0, and stored at −80°C. Stock solutions of DTNB (14 mM) were freshly prepared in 0.2 M phosphate buffer, pH 7.1 (10). For LTIP quantification, ELISA plates (Nunc Maxisorp) were purchased from Nalge Nunc International, (Rochester, NY) and goat anti-rabbit IgG (horseradish peroxidase conjugate) was obtained from Calbiochem (San Diego, CA).
Human plasma and lipoproteins
Blood was drawn from volunteers following written informed consent. The Institutional Review Board of the Cleveland Clinic approved the study protocol. Whole blood was collected in EDTA-containing tubes and plasma was isolated by centrifugation. Freshly isolated human plasma was also obtained from the Cleveland Clinic Blood Bank. VLDL, LDL, HDL, HDL3, and the d > 1.21 g/ml (lipoprotein-free) fraction were prepared by sequential ultracentrifugation (11). Samples were extensively dialyzed against 0.9% NaCl, 0.02% EDTA, 0.02% NaN3, pH 7.4. LDL was radiolabeled with [3H]CE by a dispersion transfer method (12).
CETP and LTIP were partially purified from frozen plasma as previously described (13). CETP and LTIP activities were measured by their ability to stimulate CE transfer from [3H]CE-LDL to HDL, or to inhibit CETP-mediated CE transfer from [3H]CE-LDL to HDL, respectively, as previously published (12, 14, 15).
Gel filtration
Plasma and other plasma-derived samples were fractionated by fast protein liquid chromatography (FPLC) on tandem Superose 6 HR 10/30 columns (GE Healthcare, Piscataway, NJ) at room temperature (9). Samples (≤ 0.5 ml) were applied to columns equilibrated in 0.9% NaCl, 0.02% EDTA, 0.02% NaN3, pH 7.4, and eluted at a rate of 0.3 ml/min. Beginning 50 min after sample injection, 1 ml fractions were collected. Fractions were analyzed for cholesterol content by enzymatic assay (ThermoFisher Scientific, Middletown, VA). LTIP content was determined by immunoassay utilizing rabbit anti-human LTIP as previously described (16).
The inactive fraction of LTIP, referred to as the 470 kDa LTIP complex, was isolated from HDL3 (9). HDL3 was purified by sequential ultracentrifugation as the 1.125–1.21 g/ml density fraction. HDL3 (0.5 ml, ∼2 mg cholesterol) was then applied to the FPLC columns described above and the distribution of LTIP and cholesterol in the eluate determined. The LTIP 470 kDa complex is partially separated from the bulk HDL3 peak by this method (9). The three fractions with highest LTIP content, which contained approximately 30% of the HDL3 cholesterol, were pooled.
LTIP movement assays
Plasma or specified plasma components were incubated at 4°C or 37°C for the indicated time and then stored on ice for no more than 24 h before being separated by gel filtration FPLC as described above. In some experiments, CETP was inhibited by preincubating samples with TP2 monoclonal antibody, as indicated in the figure legends, prior to initiating the LTIP movement phase of the experiment. Preincubation with TP2 overnight at 4°C or for 1 h at 37°C inhibited CETP to a similar extent. CETP activity was typically inhibited 70–85%. LCAT activity was quantitatively inhibited with 1 mM paraoxon or 2.8 mM DTNB. LTIP mass in eluted FPLC fractions was determined in triplicate by immunoassay (16). All FPLC fractions for a given sample were assayed on a single microtiter plate along with a standard curve. LTIP mass data were plotted versus fraction number. Area under the curve values were determined for LTIP associated with the LDL and 470 kDa complex peaks. LTIP was routinely observed in only these two fractions. Data are presented as the mass or the percentage of LTIP recovered in the LDL peak. The latter was calculated from the mass values using the equation: LDL LTIP / (LDL LTIP + 470 kDa LTIP) × 100. For the experiments reported, LTIP recovery after FPLC was 101.8 ± 10.5% of that for 4°C controls.
Lipoprotein characterization
Protein was quantified by a modification of the Lowry et al. method (17) with BSA as standard. Total cholesterol and triglyceride were determined by enzymatic kits from ThermoFisher Scientific (Middletown, VA), and free cholesterol (FC) was measured by an enzymatic kit from Wako Chemical (Richmond, VA). CE was calculated from the difference between total and FC measurements. Phospholipid (PL) was quantified by the method of Bartlett (18).
RESULTS
Dependence of LTIP movement on LCAT and CETP activities
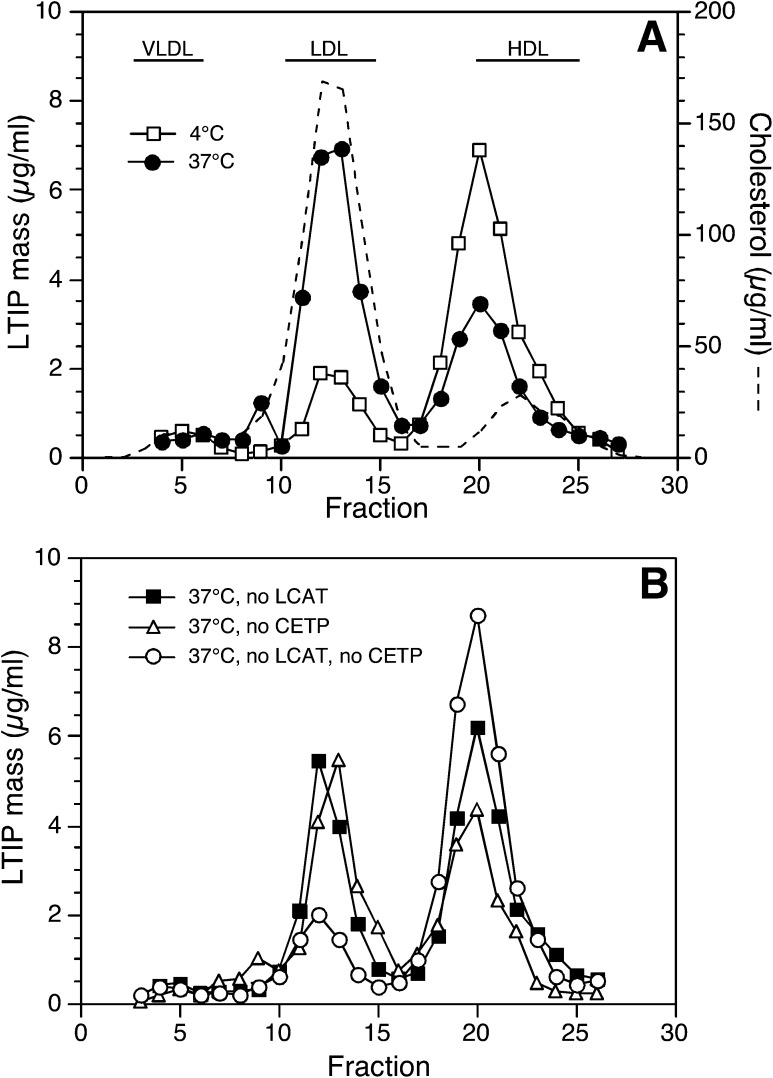
We previously reported that LTIP moves from the inactive 470 kDa complex to LDL during incubation of plasma in vitro (9). Further, this movement was partially prevented when LCAT activity was inhibited with paraoxon, a serine esterase inhibitor. These results suggested that lipid modification of lipoproteins promotes LTIP movement. To investigate this further, we incubated plasma with inhibitors of LCAT, CETP, or a combination of the two. Representative FPLC data from such an experiment are shown in Fig. 1. These data were analyzed to determine the mass of LTIP in the LDL and 470 kDa fractions and to calculate the percentage of plasma LTIP coeluting with LDL after incubation (Table 1). As previously seen, 1 mM paraoxon inhibited 40–50% of the LTIP movement that occurred when plasma was incubated at 37°C for 24 h. To confirm that the effect of paraoxon was specifically due to its inhibition of LCAT, we took advantage of the unique susceptibility of this enzyme to both serine and thiol reactive agents (19). Quantitative suppression of LCAT activity by DTNB (2.8 mM) resulted in the same inhibition of incubation-induced LTIP movement as seen with paraoxon (data not shown). Inhibiting plasma CETP activity with a blocking antibody also prevented a portion of the incubation-induced LTIP movement (Fig. 1 and Table 1). The extent of this inhibition varied among samples, but typically blocking CETP reduced incubation-induced LTIP movement by about half. Most importantly, simultaneous inhibition of both LCAT and CETP activities prevented all LTIP movement to LDL (Fig. 1 and Table 1), showing that these two plasma proteins quantitatively account for all LTIP translocation.
Fig. 1.
Effect of LCAT and CETP inhibition on LTIP movement to LDL in plasma. Plasma (750 µl) was preincubated overnight at 4°C with or without anti-CETP monoclonal antibody (27.5 µg). This blocked CETP activity by ∼70%. These treated samples, ± 1 mM paraoxon to block LCAT activity, were subsequently incubated at 37°C for 24 h to induce LTIP movement. Control samples were held at 4°C. A 500 µl aliquot of each sample was then fractionated by FPLC gel filtration (see Methods) and eluted fractions were assayed in triplicate for LTIP by immunoassay. A and B show the LTIP elution profiles. The recovery of LTIP in 37°C incubated samples was 92–105% of 4°C controls. A also shows the distribution of cholesterol in control plasma. The elution position of lipoproteins was not altered by these incubation conditions. Data are representative of three similar experiments.
TABLE 1.
LTIP association with LDL in incubated plasma
| Activity Present |
LTIP mass (µg) |
Plasma LTIP associated with LDL (%) | |||
| CETP | LCAT | Temp. | LDL | 470 kDa | |
| + | + | 4°C | 5.7 ± 1.1 | 29.3 ± 2.9 | 16.3 ± 1.3 |
| + | + | 37°C | 22.5 ± 1.7 | 10.2 ± 0.8 | 68.7 ± 5.2a |
| + | – | 37°C | 12.9 ± 1.0 | 19.4 ± 1.5 | 40.0 ± 3.0a |
| – | + | 37°C | 17.1 ± 1.3 | 16.7 ± 1.3 | 50.6 ± 3.8a |
| – | – | 37°C | 4.9 ± 0.4 | 31.8 ± 2.4 | 13.3 ± 1.0 |
After incubation of plasma for 24 h at 4°C or 37°C as described in Fig. 1, the distribution of LTIP among LDL and 470 kDa fractions isolated by FPLC was measured by immunoassay. Negative symbols indicate that CETP or LCAT activity was inhibited by blocking antibody or paraoxon, respectively. Plus symbols designate that the indicated activity was present at endogenous levels. Standard deviations reflect the accuracy of LTIP determinations, which were measured in triplicate. Data are representative of 3 similar experiments.
P < 0.01, compared with native plasma at 37°C.
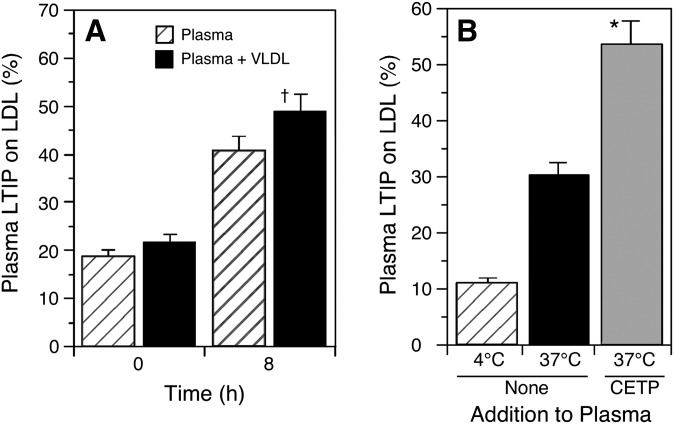
Lipoprotein modification by CETP is dependent on both the levels of VLDL and CETP (20, 21). Supplementing plasma with additional VLDL enhanced the extent of LTIP movement that occurred during an 8 h incubation at 37°C (Fig. 2A). After 24 h incubation, the addition of VLDL has stimulated LTIP movement by 30% above control (not shown). Increasing the CETP content of plasma several-fold also promoted greater LTIP association with LDL (Fig. 2B). These data show that the activation of LTIP, i.e., its movement from an inactive pool to the LDL-associated pool, is enhanced under conditions where CETP activity is elevated.
Fig. 2.
Effect of VLDL and CETP on LTIP movement in plasma. A: Plasma ± VLDL (600 µg cholesterol/ml plasma) was incubated at 37°C for 8 h. † P < 0.05 compared with native plasma incubated for the same time. Panel B: Plasma was incubated with or without partially purified CETP for 8 h at the indicated temperature. The CETP addition increased plasma CETP activity 3-fold. Because partially purified CETP contains low levels of LTIP, the increase in LDL-associated LTIP in 4°C samples due to CETP addition has been subtracted from the data shown. * P < 0.01 compared with native plasma at 37°C. Data are representative of at least two similar experiments.
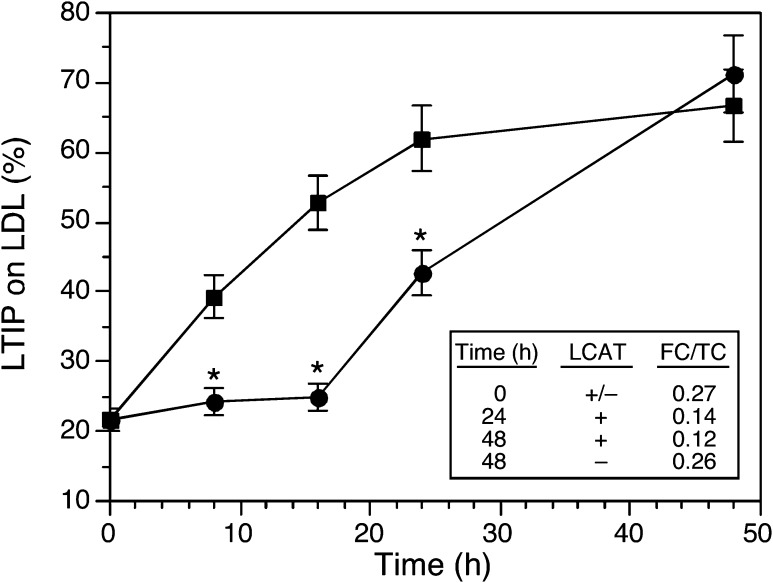
If LCAT and CETP activities promote LTIP association with LDL by similar mechanisms, then extended activity of one should be able to compensate for loss of the other. In native plasma, LTIP movement to LDL increased almost linearly for the first 16–24 h and then plateaued (Fig. 3). In plasma where only CETP is active, LTIP translocation lagged, resulting in ∼50% lower LTIP association with LDL at 24 h. However, with continued incubation of LCAT-inhibited plasma, LTIP associated with LDL became equivalent to that seen in native plasma (Fig. 3). Thus, in the absence of LCAT activity, CETP activity alone can drive maximum LTIP movement when incubation time is extended.
Fig. 3.
Effect of LCAT inhibition on the time course of LTIP movement to LDL in plasma. Plasma without (squares) or with (circles) 1 mM paraoxon to inhibit LCAT was incubated for the indicated times. Inset: ratio of free cholesterol (FC) to total cholesterol (TC) in samples incubated at 37°C for the indicted time. * P < 0.01 compared with native plasma. Data are representative of three time course experiments.
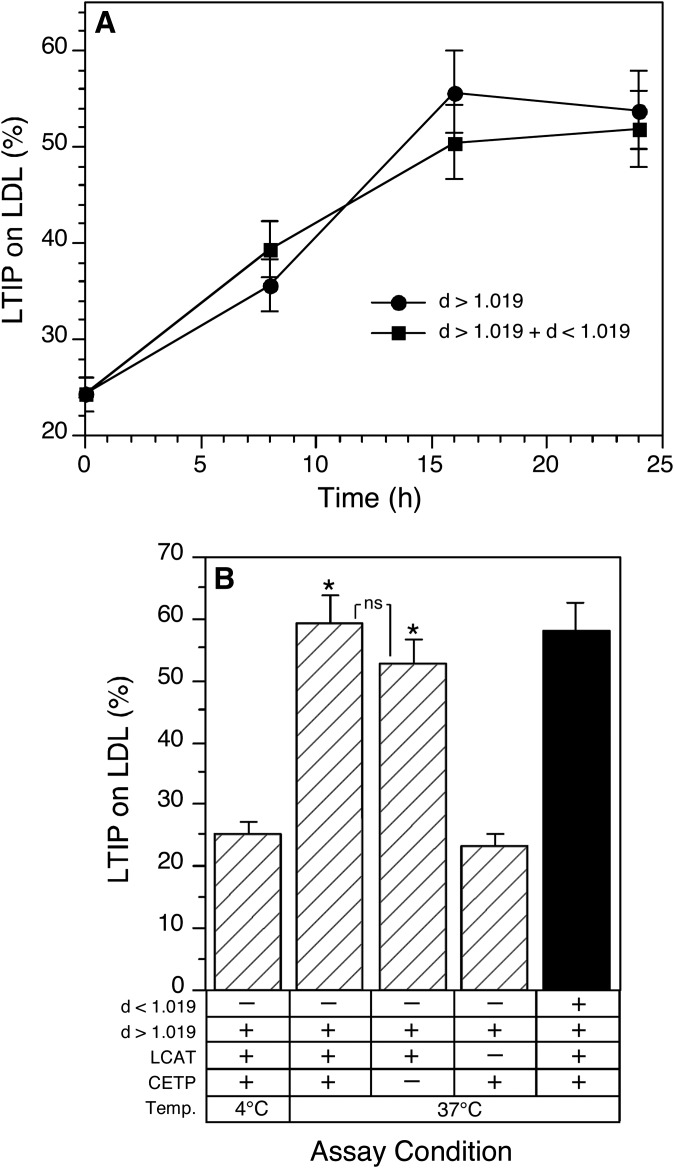
A different approach was taken to determine if LCAT activity alone could compensate for inactive CETP. This approach was needed because we have been unable to establish conditions where plasma CETP activity is fully inhibited by the mouse TP2 blocking antibody. In our experience, CETP activity is inhibited by 70–85% by this antibody depending on the plasma source. A blocking antibody we previously prepared in rabbit (22), although useful in quantitatively suppressing CETP activity, could not be used because it interfered with the subsequent detection of LTIP by immunoassay. As an alternative approach, CETP-mediated lipid remodeling was prevented by removing VLDL from plasma. Without this TG-rich lipoprotein, CE-TG exchange is negligible even though CETP itself remains active. Plasma was fractionated at density 1.019 g/ml and the d > 1.019 g/ml and d < 1.019 g/ml fractions were combined to reconstitute plasma, or the d > 1.019 g/ml fraction was combined with an equivalent amount of buffer to yield VLDL-deficient plasma. Surprisingly, when incubated at 37°C, LTIP movement to LDL in reconstituted plasma and in the d > 1.019 g/ml fraction were the same (Fig. 4A). Thus, although supplementing plasma with VLDL augments CETP activity and promotes LTIP movement (Fig. 2), removing VLDL was without effect. This was not because the removal of VLDL altered LCAT activity because CE formation was not different in the presence or absence of VLDL (81 vs. 82 µg cholesterol esterified/ml/6 h).
Fig. 4.
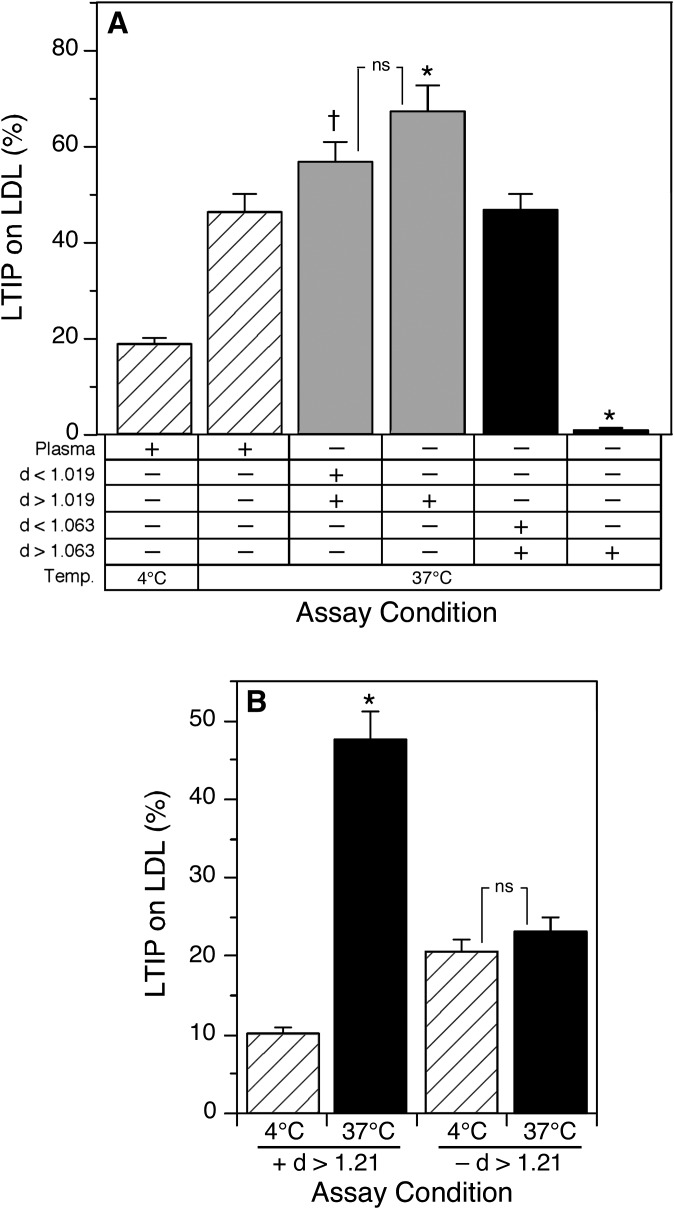
Effect of VLDL removal on incubation-induced association of LTIP with LDL. Plasma was centrifuged at density 1.019 g/ml to generate VLDL (d < 1.019 g/ml) and VLDL-free (d > 1.019 g/ml) plasma fractions. A: The d > 1.019 g/ml plasma fraction was combined with the d < 1.019 g/ml fraction, to reconstitute plasma, or with buffer alone. Samples were incubated at 37°C for the indicated times. B: One milliliter aliquots of the d > 1.019 g/ml fraction were incubated ± 45.9 µg anti-CETP for 1 h at 25°C. Subsequently, samples received 1 mM paraoxon as indicated and were incubated at 4°C or 37°C for 16 h. Negative symbols indicate that CETP or LCAT activities were inhibited by blocking antibody or paraoxon, respectively. Plus symbols designate that the indicated activities are present at endogenous levels. * P < 0.01 compared with native plasma at 4°C. ns, not significant.
To examine the possibility that CETP promotes LTIP movement by a mechanism independent of VLDL, the effects of LCAT and CETP inhibition on LTIP movement in VLDL-deficient plasma (d > 1.019 g/ml fraction) were examined. Unlike that seen in intact plasma, in the absence of VLDL incubation-induced LTIP association with LDL was not affected by the addition of a CETP blocking antibody, whereas LCAT inhibition completely blocked all LTIP movement (Fig. 4B). Thus, in the absence of functional CETP (i.e., absence of VLDL), LTIP movement is normal and fully dependent on LCAT activity. These data show that LCAT is sufficient to promote normal LTIP movement in the absence of functional CETP.
LTIP movement in a defined system
Collectively, the data above show that the lipoprotein modifying activities of CETP and LCAT promote LTIP redistribution. To investigate the mechanism underlying this effect, we sought to determine a minimal system in which LTIP movement occurred. The incubation-induced movement of LTIP from the inactive complex to fractions coeluting with LDL required that LDL was present during the incubation. Whereas removing VLDL did not impair LTIP movement (Fig. 4), the removal of LDL completely prevented the release of LTIP from the inactive complex (Fig. 5A). Also, LTIP movement did not require the presence of other components in the bulk HDL fraction. LTIP movement could be reconstituted in a mixture of LDL, the 470 kDa inactive LTIP fraction, and the d > 1.21 g/ml fraction of plasma (Fig. 5B). No LTIP movement occurred when the LCAT-containing d > 1.21 g/ml fraction was omitted or when LCAT activity in this fraction was inhibited (data not shown). Likewise, purified CETP could not substitute for the d > 1.21 g/ml fraction (data not shown), consistent with the data above showing that CETP activity had no effect on LTIP movement in the absence of VLDL.
Fig. 5.
Reconstitution of LTIP movement activity. A: Plasma was sequentially fractioned by ultracentrifugation to generate VLDL (d < 1.019 g/ml) and VLDL-free plasma (d > 1.019 g/ml), or VLDL+LDL (d < 1.063 g/ml) and HDL + protein (d > 1.063 g/ml) fractions. Dialyzed samples were recombined as indicated at equivalent original plasma volumes. Samples were incubated at 4°C or 37°C for 24 h. † P < 0.05, * P < 0.01 compared with native plasma at 37°C. ns, not significant. B: LDL (220 µg cholesterol), the d > 1.21 g/ml lipoprotein-free fraction of plasma (0.25 ml) and the 470 kDa fraction of LTIP (200 µg protein) were combined and incubated at 4°C or 37°C for 24 h. * P < 0.01 compared with the corresponding 4°C sample. ns, not significant. Data are representative of two-three similar experiments.
Influence of 470 kDa chemical composition on LTIP movement
Given that LTIP movement could be reconstituted in an assay containing only LDL, the 470 kDa fraction of LTIP, and LCAT activity, we investigated whether modification of the chemical composition of 470 kDa complex might be the mechanism by which LCAT activity promotes LTIP movement. Isolated 470 kDa LTIP was incubated for 24 h with the d > 1.21 g/ml fraction to induce LCAT modification of 470 kDa composition. This caused a 50% decrease in FC, a 20% decrease in PL content, and a modest increase in CE content compared with control 470 kDa incubated at 4°C. When combined with native LDL and the d > 1.21g/ml fraction, the incubation-induced movement of LTIP was not different between control versus premodified 470 kDa. Incubation at 37°C for 12 h increased the LTIP associated with LDL from 7.8% to 27.9% for native 470 kDa and from 3.1% to 24.3% for modified 470 kDa.
To further rule out a contribution of 470 kDa lipid composition to LTIP movement, premodification conditions were altered to increase the extent of 470 kDa modification. The d > 1.063 g/ml fraction of plasma, which contains CETP and LCAT, was incubated for 24 h at 4°C or 37°C to induce lipoprotein modification. This incubation resulted in marked changes in the lipid composition of HDL and the 470 kDa subfraction. Compared with 4°C control, the premodification incubation increased the CE+TG / protein ratio (0.17 ± 0.01 vs. 0.23 ± 0.01, P <0.02), and decreased the FC+PL/protein (0.40 ± 0.02 vs. 0.30 ± 0.01, P < 0.02), and FC+PL/CE+TG ratios (2.34 ± 0.07 vs. 1.32 ± 0.03, P <0.02) of the 470 kDa fraction containing inactive LTIP. Native and premodified d > 1.063 g/ml fractions were then recombined with native d < 1.063 g/ml plasma fraction and incubated to induce LTIP movement to LDL. No significant difference was observed in the movement of LTIP to LDL from native versus chemically premodified 470 kDa (27.7% vs. 30.1% at 8 h, respectively, and 45.3% vs. 41.1%, respectively, at 16 h). Collectively, these studies indicate that changes in 470 kDa lipid composition do not appear to regulate the release of LTIP from the inactive complex.
Influence of LDL chemical composition on LTIP movement
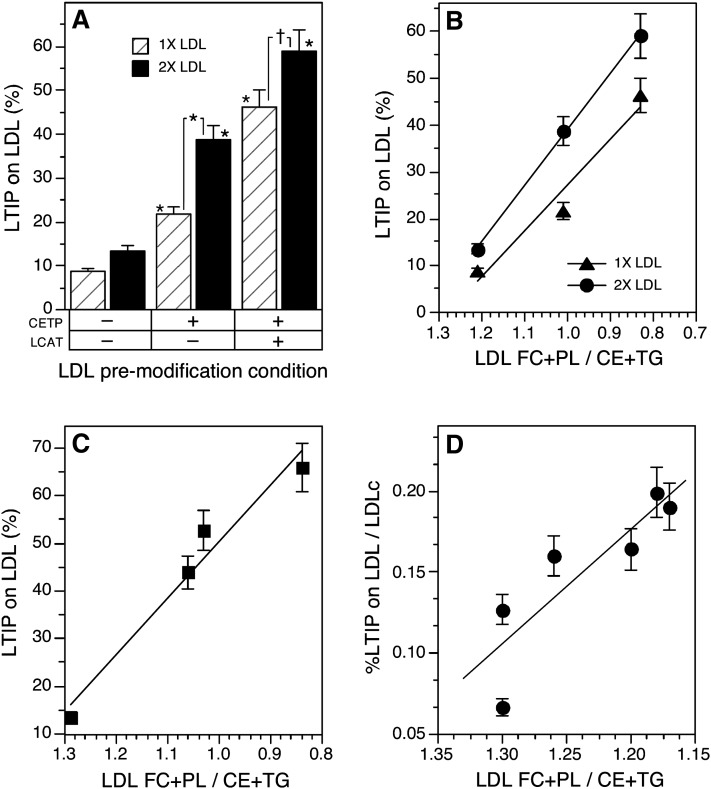
The alternative possibility, that modification of LDL renders it a better binding site for LTIP, was investigated. LDL was modified by incubating the d < 1.063 g/ml plasma fraction with the d >1.21 g/ml fraction under conditions where the activities of LCAT and CETP were individually manipulated. In this approach, the 470 kDa fraction was omitted, thus preventing LTIP movement during the modification process. LDL from such reactions was modified in surface and core lipids depending on which modifying factors were active (Table 2). To measure LTIP movement, LDL isolated from these modification reactions was incubated for a short time with native 470 kDa LTIP complex plus d > 1.21 g/ml plasma fraction in the presence of both paraoxon and TP2 antibody. Thus, during the subsequent LTIP movement phase of this experiment, both LCAT and CETP were inactive. Under these conditions, very little LTIP was associated with native LDL after incubation with the 470 kDa complex (Fig. 6A). However, modified LDLs bound much higher levels of LTIP. The extent of LTIP association with LDL was dependent on both the amount of LDL incubated with the 470 kDa fraction and the extent of LDL modification. Like that seen in incubations of whole plasma, LTIP association with LDL was highest when both LCAT and CETP were active during the premodification process and reduced when LCAT was inhibited (Fig. 6A). In similar studies, we observed that the association of LTIP with premodified LDL was not dependent on assay temperature. The percentage of sample LTIP coeluting with premodified LDL was the same when it and the native 470 kDa complex were coincubated at 4°C or 37°C [30.8% ± 2.3 vs. 32.4% ± 2.5, respectively, compared with native LDL (13.4% ± 1.0)]. This indicates that the temperature dependence of LTIP movement seen in previous experiments is because 37°C promotes lipoprotein remodeling by CETP and LCAT, not because it is required for the actual movement of LTIP.
TABLE 2.
Modification of LDL composition
| Condition | Active factor | FC | CE | TG | PL |
| µg/µg protein | |||||
| 4°C | None | 0.43 ± 0.01 | 1.08 ± 0.07 | 0.21 ± 0.01 | 1.12 ± 0.02 |
| 37°C + paraoxon | CETP | 0.43 ± 0.01 | 0.99 ± 0.03 | 0.62 ± 0.02 a | 1.19 ± 0.01 b |
| 37°C | CETP, LCAT | 0.32 ± 0.01 a | 0.96 ± 0.02 | 0.56 ± 0.01 a | 0.93 ± 0.02 ac |
The d < 1.063 g/ml and d > 1.21 g/ml fractions of plasma were coincubated for 22 h at 4°C or 37°C in the presence or absence of 1 mM paraoxon to inhibit LCAT activity. LDL was subsequently isolated by sequential ultracentrifugation, dialyzed, and chemically characterized. Values are the mean ± SD of duplicate determinations. Based on protein, LDL recovery was 92–109%. Results are representative of three similar experiments.
P < 0.01 or
P < 0.05 versus 4°C control
P < 0.05 versus 37°C + paraoxon value. FC, free cholesterol; PL, phospholipids; CE, cholesteryl ester; TG, triglyceride.
Fig. 6.
Influence of LDL composition on LTIP association with LDL. A: The d < 1.063 g/ml (VLDL+LDL) fraction of plasma was recombined with a plasma equivalent volume of d > 1.21 g/ml (lipoprotein-free fraction) of plasma ± 1 mM paraoxon and incubated at 4°C or 37°C for 22 h. Under these conditions, LDL was either unmodified (4°C), modified by CETP (37°C + paraoxon) or modified by CETP and LCAT activities (37°C, no paraoxon). LDL was isolated from these mixtures by ultracentrifugation. Native or modified LDL (160 (1×) or 320 (2×) µg protein) was then combined with native 470 kDa LTIP fraction (200 µg protein) and 500 µl of lipoprotein-free plasma (d > 1.21 fraction). All samples received anti-CETP (21 µg) and 1 mM paraoxon so that CETP and LCAT were inactive during this phase of the experiment. Samples were incubated at 37°C for 6 h. † P < 0.05, * P < 0.01 compared with unmodified LDL or as indicated. B: The percent LTIP on LDL measured in A is plotted versus the ratio of free cholesterol (FC) + phospholipid (PL) divided by cholesteryl ester (CE) + triglyceride (TG) in LDL. The reverse x axis places native LDL on the left side of the graph. Correlation coefficients for both regression lines are ≥ 0.98. C: The percent LTIP on LDL after incubation of whole plasma as described in Table 3 is plotted versus the FC + PL-to-CE + TG ratio of the LDL isolated from those incubations. Correlation coefficient for the regression line is 0.99, P = 0.02. Data are representative of three similar experiments. D: The percent LTIP on LDL in normolipidemic subjects and those with borderline high cholesterol or TG is plotted versus LDL composition. Values on the y axis have been normalized by dividing the %LTIP on LDL by the LDL cholesterol (LDLc) content of each sample. Correlation coefficient for the regression line is 0.87, P = 0.025. Of the seven plasma samples analyzed, six are plotted here. The remaining sample, perhaps due to its very low HDL and LTIP content, did not fit this correlation.
LCAT modification of LDL primarily affects surface lipids (PC and FC) levels whereas CETP induces CE-TG heteroexchange causing LDL to become TG enriched (Table 2). Because the exchange of CE for TG is equimolar (23, 24) and TG is a larger molecule, this process expands the lipoprotein core. However, both modifications promote LTIP association with LDL. To identify common compositional changes induced by CETP and LCAT, we assessed the relationship between changes in LDL lipid composition induced by the premodification process and the extent of subsequent LTIP association with LDL. LTIP association with modified LDL did not correlate with the relative amount of any individual lipid component (lipid/protein ratio), the ratio of TG/CE, FC/PL, PL+FC/protein or the CE+TG/protein ratio of LDL. LTIP association with LTIP did correlate well with the ratio of LDL surface lipids to core lipids (i.e., PL/CE+TG, FC/CE+TG, and FC+PL/CE+TG). Among multiple experiments, the FC+PL/CE+TG ratio was consistently the best predictor of LTIP association with LDL (Fig. 6B).
To determine if this association held true in a more physiologic environment, we investigated whether the incubation-induced translocation of LTIP in whole plasma was also correlated with this change in LDL lipid composition. CETP and LCAT activities caused similar changes in the LDL surface to core lipid ratio, and this change was additive when both factors were active (Table 3). As shown in Fig. 6C, in these incubated plasmas, the same strong correlation was observed between the LTIP content of LDL and LDL FC+PL/CE+TG composition.
TABLE 3.
Composition of LDL isolated from incubated whole plasma
| Active factor | FC | CE | TG | PL | FC+PL/protein | CE+TG/protein | FC+PL/CE+TG |
| µg/µg protein | |||||||
| None | 0.29 ± 0.01 | 0.72 ± 0.02 | 0.15 ± 0.01 | 0.84 ± 0.01 | 1.13 ± 0.03 | 0.87 ± 0.02 | 1.29 ± 0.04 |
| CETP | 0.30 ± 0.01 | 0.65 ± 0.01 | 0.41 ± 0.01 a | 0.82 ± 0.03 | 1.12 ± 0.03 | 1.06 ± 0.02 a | 1.06 ± 0.03 b |
| LCAT | 0.19 ± 0.01 a | 0.74 ± 0.02 | 0.16 ± 0.01 | 0.74 ± 0.02 b | 0.93 ± 0.02 b | 0.91 ± 0.02 | 1.03 ± 0.02 b |
| CETP, LCAT | 0.20 ± 0.01 a | 0.71 ± 0.01 | 0.37 ± 0.01 a | 0.70 ± 0.01 a | 0.90 ± 0.02 a | 1.07 ± 0.02 a | 0.84 ± 0.02 a |
Plasma was incubated (37°C) for 16 h in the presence or absence of 1 mM paraoxon to inhibit LCAT and/or TP2 (46 µg/ml plasma) to inhibit CETP activity. LDL was isolated by sequential ultracentrifugation and chemically characterized as described in the Methods. Based on protein, LDL recovery was 98–111%. Values are the mean ± SD of duplicate determinations. Results are representative of 3 similar experiments.
P < 0.01 or
P < 0.05 versus control (CETP and LCAT inactive). FC, free cholesterol; CE, cholesteryl ester; TG, triglyceride; PL, phospholipid.
To evaluate the in vivo relevance of these in vitro findings, we sought to determine whether the same correlation might be observed in native plasmas isolated from different individuals. To ensure a range of LDL compositions, we obtained plasma from both normolipidemic individuals and those with borderline high levels of cholesterol and/or triglyceride. Lipid parameters for this group were: cholesterol, 126–233 mg/dl (LDL cholesterol, 68–150 mg/dl; HDL cholesterol, 45–69 mg/dl); and triglyceride, 33–179 mg/dl. The percentage of plasma LTIP associated with LDL ranged from 5 to 22%. In these native plasma samples, LDL levels varied more than 2-fold. Because the association of LTIP with LDL depends on both LDL composition and LDL concentration (Fig. 6 A, B), LDL LTIP values were normalized by dividing the percent of LTIP on LDL by the LDL pool size. Following this adjustment, we again observed a statistically significant correlation between the ratio of FC+PL/CE+TG in LDL and the LTIP content of that LDL (Fig. 6D).
DISCUSSION
Most apolipoproteins are exchangeable and their association with specific lipoproteins is determined by the chemical and physical properties of these particles. For example, lipoprotein size is a strong determinate of apolipoproteins A-I, A-II, C-II, and C-III association. Apolipoprotein A-I binds preferentially to small particles (i.e., high surface curvature), whereas apolipoproteins A-II, C-II, and C-III prefer larger particles (25). Also, apolipoprotein A-I binds better to monolayers composed of surface lipids isolated from HDL than from LDL. The more condensed packing of these lipids from LDL prevents apolipoprotein A-I binding, providing a structural basis for the absence of this protein on LDL (26). Apolipoprotein A-IV binding is also highly dependent on the molecular packing of lipoprotein phospholipids, decreasing as lateral packing increases with no binding occurring above a surface pressure of 28–29 mN/m (27). HDL surface pressure may oscillate about a narrow range that spans the exclusion pressure of apolipoprotein A-IV as a result of LCAT and CETP activities (27). It is proposed that LCAT activity promotes the unbound to bound transition of apolipoprotein A-IV by creating “gaps” in the packing of phospholipid head groups (28).
The binding of CETP to lipoproteins is the initial and indispensable step in the lipid transfer process (29, 30). LTIP (apolipoprotein F) suppresses CETP by binding to lipoproteins and preventing or disrupting the interaction of CETP with its lipoprotein substrate (6, 31). This LTIP mechanism, combined with LTIP's preferential suppression of lipid transfer events involving LDL (6, 7, 24), is consistent with our finding that in normolipidemic plasma active LTIP resides exclusively on LDL (9). However, a second, several-fold larger pool of LTIP resides on a unique 470 kDa particle that is isolated in the HDL3 fraction. This LTIP is inactive (9). Given the importance of lipoprotein structural properties in determining the binding of many other apolipoproteins, it seems likely that similar mechanisms control the distribution of LTIP between these two metabolically distinct pools.
We report here that both LCAT and CETP activities drive the movement of LTIP from the inactive 470 kDa complex to LDL. On average, each activity contributes equally to the LTIP movement that occurs when plasma is incubated in vitro. Although these two activities have an additive effect on LTIP movement, under appropriate conditions, either activity alone is capable of facilitating maximum LTIP redistribution. LTIP movement could be reconstituted in a simple system containing only LDL, partially purified 470 kDa complex, and LCAT. Collectively, these findings strongly suggest that changes in the chemical composition of LDL, the 470 kDa complex, or both, are responsible for LTIP movement. When these two lipoprotein fractions were independently modified prior to the LTIP movement assay, only the extent of LDL compositional change correlated with LTIP movement. Altered 470 kDa composition had no effect. These data indicate that the generation of a suitable binding surface on LDL, not LTIP release from the inactive complex, is the major driving force controlling the activation status of LTIP.
The observation that both LCAT and CETP activity could modify LDL to induce LTIP association is interesting given that these two activities affect different regions in LDL. The consequence of LCAT activity, which occurs in large part on HDL (32), is to deplete LDL of the surface lipids, PL and FC. These lipids transfer to HDL to replenish those consumed by the LCAT reaction. Conversely, CETP promotes CE for TG exchange resulting in LDL that contain higher levels of TG and lower CE. Because this transfer is largely an equimolar exchange reaction, and a molecule of TG occupies 47% more volume than a molecule of CE (33), this results in an increase in the volume of the LDL core. Thus, LCAT and CETP activities both promote a decrease in the ratio of LDL surface lipid to LDL core lipid, one by depleting the surface and the other by expanding the core. We observed that the extent of LTIP movement to LDL is highly correlated with this lipid ratio in LDL.
A decrease in the surface to core lipid ratio (FC+PL/CE+TG) would be expected to result in a less densely packed surface lipid domain with altered capacity to accommodate apolipoproteins. Indeed, under very similar conditions to those used here, Deckelbaum et al. (34) showed that modified LDL gain apolipoproteins C, A-I, and E, which were derived from VLDL. These findings suggest that as reduced lipid packing occurs on the LDL surface during lipid remodeling, these “gaps” are filled by apolipoproteins, thus maintaining lipid packing in a narrow range. Our data suggest that once 470 kDa LTIP is added to these modified LDL, LTIP can displace some of these acquired apoproteins, resulting in LTIP movement to LDL. This mechanism is consistent with our seemingly conflicting observations that LTIP movement to LDL is reduced when CETP activity is inhibited by antibody (Figs. 1 and 4B), but the removal of VLDL has no effect on LTIP movement (Fig. 4A) even though VLDL is required for CETP activity. These data could be explained if VLDL serves as both a positive regulator of LTIP movement by supporting CETP-mediated LDL remodeling and also a negative regulator of LTIP movement by providing a reservoir of exchangeable apolipoproteins that compete with LTIP binding to the modified LDL surface. We conclude that CETP and LCAT promote LTIP binding to LDL by creating a lipid surface that can accept this apolipoprotein. The failure of LTIP to bind to VLDL even though it has lower surface lipid packing than native LDL (35, 36) suggests that additional factors such as surface curvature (i.e., lipoprotein particle size) contribute to the binding surface preference of LTIP. In this way, LTIP binding is regulated by mechanisms similar to those controlling binding of A-I, A-II, C-II, C-III, and A-IV (25, 27, 28).
LTIP, LCAT (37), and phospholipid transfer protein (38) share a common regulatory mechanism: sequestration in an inactive complex. LCAT and phospholipid transfer protein appear to enter plasma in high activity states and then are transferred to inactive complexes. At least for LCAT, sequestration in the inactive complex may be a mechanism for receptor-mediated removal from plasma. Whether these proteins are ever released from their inactive complexes is not known. In contrast, LTIP can be readily induced to translocate from the inactive to the active pool by LCAT or CETP. Enhanced LCAT modification of LDL causes LDL to acquire LTIP and therefore, participate less in CETP-mediated transfers. This exclusion of LDL from CETP reactions promotes the removal of HDL cholesteryl ester (the LCAT product) because of enhanced CETP-mediated CE flux from HDL to VLDL (7, 24) combined with the rapid clearance of VLDL and its remnants from plasma. Thus, depletion of LDL surface lipids by LCAT signals the need to process HDL-derived CE for removal.
In a similar way, acquisition of LTIP by LDL may regulate LDL synthesis. LDL is a product of VLDL catabolism. In humans, the conversion of VLDL to LDL primarily involves the hydrolysis of TG. The resulting CE-rich remnants continue to be reduced in size by the combined action of CETP-mediated CE-TG exchange followed by lipolysis of the acquired TG (7). We show that LTIP does not bind to VLDL, binds modestly to LDL, but has enhanced binding affinity toward modified LDL that are chemically analogous to LDL precursors (34). We suggest that the acquisition of LTIP by VLDL remnants as they near LDL in size may be an important mechanism for regulating LDL size. This hypothesis is consistent with the observations of Berneis and Krauss (39) that the formation of small dense LDL is enhanced when the levels of TG-rich remnants are high and HDL levels are low. It follows, if HDL concentrations are low, then the removal of excess surface lipid generated during TG-rich lipoprotein catabolism would be impaired. This excess surface would keep the ratio of surface lipid to core lipid of remnants high and prevent LTIP from binding as they near LDL in size. The failure to suppress CETP permits the continued exchange of CE out of, and TG into, these remnants. When this TG is hydrolyzed by lipase the remnants continue to decrease in size, resulting in abnormally small LDL. We conclude that modulation of LTIP (apolipoprotein F) binding to LDL and its precursors by lipoprotein surface properties may play an important role in regulating lipoprotein metabolism.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- FC
- free cholesterol
- FPLC
- fast protein liquid chromatography
- LTIP
- lipid transfer inhibitor protein
- PL
- phospholipid
- TG
- triglyceride
This research was supported in part by grant #HL60934 from the National Heart, Lung and Blood Institute, the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Morton R. E. 1990. Interaction of lipid transfer protein with plasma lipoproteins and cell membranes. Experientia. 46: 552–560. [DOI] [PubMed] [Google Scholar]
- 2.Tall A. 1995. Plasma lipid transfer proteins. Annu. Rev. Biochem. 64: 235–257. [DOI] [PubMed] [Google Scholar]
- 3.Morton R. E., Steinbrunner J. V. 1990. Concentration of neutral lipids in the phospholipid surface of substrate particles determines lipid transfer protein activity. J. Lipid Res. 31: 1559–1567. [PubMed] [Google Scholar]
- 4.Morton R. E., Serdyuk A. P. 1997. Cholesteryl ester transfer protein (CETP) has no preference for cholesteryl esters in high density- versus low density- lipoproteins. Circulation. 96: I-108. [Google Scholar]
- 5.Wang X., Driscoll D. M., Morton R. E. 1999. Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J. Biol. Chem. 274: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 6.Morton R. E., Greene D. J. 1994. Regulation of lipid transfer between lipoproteins by an endogenous plasma protein: selective inhibition among lipoprotein classes. J. Lipid Res. 35: 836–847. [PubMed] [Google Scholar]
- 7.Morton R. E. 1999. Cholesteryl ester transfer protein and its plasma regulator: lipid transfer inhibitor protein. Curr. Opin. Lipidol. 10: 321–327. [DOI] [PubMed] [Google Scholar]
- 8.Lagor W. R., Brown R. J., Toh S-A., Millar J. S., Fuki I. V., De la Llera-Moya M., Yuen T., Rothblat G., Billheimer J. T., Rader D. J. 2009. Overexpression of apolipoprotein F reduces HDL cholesterol levels in vivo. Arterioscler. Thromb. Vasc. Biol. 29: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y., Greene D. J., Kinter M., Morton R. E. 2008. Control of cholesteryl ester transfer protein activity by sequestration of lipid transfer inhibitor protein in an inactive complex. J. Lipid Res. 49: 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokke K. T., Norum K. R. 1971. Determination of lecithin:cholesterol acyltransfer in human blood plasma. Scand. J. Clin. Lab. Invest. 27: 21–27. [DOI] [PubMed] [Google Scholar]
- 11.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton R. E., Zilversmit D. B. 1981. A plasma inhibitor of triglyceride and cholesteryl ester transfer activities. J. Biol. Chem. 256: 11992–11995. [PubMed] [Google Scholar]
- 13.Morton R. E., Zilversmit D. B. 1982. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J. Lipid Res. 23: 1058–1067. [PubMed] [Google Scholar]
- 14.Pattnaik N. M., Montes A., Hughes L. B., Zilversmit D. B. 1978. Cholesteryl ester exchange protein in human plasma: isolation and characterization. Biochim. Biophys. Acta. 530: 428–438. [DOI] [PubMed] [Google Scholar]
- 15.Morton R. E., Zilversmit D. B. 1981. The separation of apolipoprotein D from cholesteryl ester transfer protein. Biochim. Biophys. Acta. 663: 350–355. [DOI] [PubMed] [Google Scholar]
- 16.Morton R. E., Gnizak H. M., Greene D. J., Cho K-H., Paromov V. M. 2008. Lipid transfer inhibitor protein (apolipoprotein F) concentration in normolipidemic and hyperlipidemic subjects. J. Lipid Res. 49: 127–135. [DOI] [PubMed] [Google Scholar]
- 17.Peterson G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83: 346–356. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234: 466–468. [PubMed] [Google Scholar]
- 19.Jauhiainen M., Dolphin P. J. 1986. Human plasma lecithin-cholesterol acyltransferase: an elucidation of the catalytic mechanism. J. Biol. Chem. 261: 7032–7043. [PubMed] [Google Scholar]
- 20.Mann C. J., Yen F. T., Grant A. M., Bihain B. E. 1991. Mechanism of plasma cholesteryl ester transfer in hypertriglyceridemia. J. Clin. Invest. 88: 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami T., Michelagnoli S., Longhi R., Gianfranceschi G., Pazzucconi F., Calabresi L., Sirtori C. R., Franceschini G. 1995. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler. Thromb. Vasc. Biol. 15: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 22.Morton R. E. 1988. Interaction of plasma-derived lipid transfer protein with macrophages in culture. J. Lipid Res. 29: 1367–1377. [PubMed] [Google Scholar]
- 23.Morton R. E., Zilversmit D. B. 1983. Inter-relationship of lipids transferred by the lipid-transfer protein isolated from human lipoprotein-deficient plasma. J. Biol. Chem. 258: 11751–11757. [PubMed] [Google Scholar]
- 24.Serdyuk A. P., Morton R. E. 1999. Lipid transfer inhibitor protein defines the participation of lipoproteins in lipid transfer reactions: CETP has no preference for cholesteryl esters in HDL versus LDL. Arterioscler. Thromb. Vasc. Biol. 19: 718–726. [DOI] [PubMed] [Google Scholar]
- 25.Tajima S., Yokoyama S., Yamamoto A. 1983. Effect of lipid particle size on association of apolipoproteins with lipid. J. Biol. Chem. 258: 10073–10082. [PubMed] [Google Scholar]
- 26.Ibdah J. A., Lund-Katz S., Phillips M. C. 1989. Molecular packing of high-density and low-density lipoprotein surface lipids and apolipoprotein A-I binding. Biochemistry. 28: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg R. B., Ibdah J. A., Phillips M. C. 1992. Adsorption of apolipoprotein A-IV to phospholipid monolayers spread at the air/water interface. A model for its labile binding to high density lipoproteins. J. Biol. Chem. 267: 8977–8983. [PubMed] [Google Scholar]
- 28.Bisgaier C. L., Sachdev O. P., Lees E. S., Williams K. J., Blum C. B., Glickman R. M. 1987. Effect of lecithin:cholesterol acyltransferase on distribution of apolipoprotein A-IV among lipoproteins of human plasma. J. Lipid Res. 28: 693–703. [PubMed] [Google Scholar]
- 29.Jiang X. C., Bruce C., Cocke T., Wang S., Boguski M., Tall A. R. 1995. Point mutagenesis of positively charged amino acids of cholesteryl ester transfer protein: conserved residues within the lipid transfer/lipopolysaccharide binding protein gene family essential for function. Biochemistry. 34: 7258–7263. [DOI] [PubMed] [Google Scholar]
- 30.Qiu X., Mistry A., Ammirati M., Chrunyk B., Clark R., Cong Y., Culp J., Danley D., Freeman T., Geoghegan K., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113. [DOI] [PubMed] [Google Scholar]
- 31.Morton R. E. 1985. Binding of plasma-derived lipid transfer protein to lipoprotein substrates: the role of binding in the lipid transfer process. J. Biol. Chem. 260: 12593–12599. [PubMed] [Google Scholar]
- 32.Morton R. E., Greene D. J. 2007. Partial suppression of CETP activity beneficially modifies the lipid transfer profile of plasma. Atherosclerosis. 192: 100–107. [DOI] [PubMed] [Google Scholar]
- 33.Shen B. W., Scanu A. M., Kézdy F. J. 1977. Structure of human serum lipoproteins inferred from compositional analysis. Proc. Natl. Acad. Sci. USA. 74: 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deckelbaum R. J., Eisenberg S., Oschry Y., Butbul E., Sharon I., Olivecrona T. 1982. Reversible modification of human plasma low density lipoproteins toward triglyceride-rich particles: a mechanism for losing excess cholesterol esters. J. Biol. Chem. 257: 6509–6517. [PubMed] [Google Scholar]
- 35.Ben-Yashar V., Barenholz Y. 1991. Characterization of the core and surface of human plasma lipoproteins. A study based on the use of five fluorophores. Chem. Phys. Lipids. 60: 1–14. [DOI] [PubMed] [Google Scholar]
- 36.Massey J. B., Pownall H. J. 1998. Surface properties of native human plasma lipoproteins and lipoprotein models. Biophys. J. 74: 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krimbou L., Marcil M., Davignon J., Genest J., Jr 2001. Interaction of lecithin:cholesterol acyltransferase (LCAT) • alpha 2-macroglobulin complex with low density lipoprotein receptor-related protein (LRP). Evidence for an alpha 2-macroglobulin/LRP receptor-mediated system participating in LCAT clearance. J. Biol. Chem. 276: 33241–33248. [DOI] [PubMed] [Google Scholar]
- 38.Kärkkäinen M., Oka T., Olkkonen V. M., Metso J., Hattori H., Jauhiainen M., Ehnholm C. 2002. Isolation and partial characterization of the inactive and active forms of human plasma phospholipid transfer protein (PLTP). J. Biol. Chem. 277: 15413–15418. [DOI] [PubMed] [Google Scholar]
- 39.Berneis K. K., Krauss R. M. 2002. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 43: 1363–1379. [DOI] [PubMed] [Google Scholar]