Abstract
Most current assays of HDL functional properties are cell-based. We have developed a fluorometric biochemical assay based on the oxidation of dihydrorhodamine 123 (DHR) by HDL. This cell-free assay assesses the intrinsic ability of HDL to be oxidized by measuring increasing fluorescence due to DHR oxidation over time. The assay distinguishes the oxidative potential of HDL taken from different persons, and the results are reproducible. Direct comparison of this measurement correlated well with results obtained using a validated cell-based assay (r2 = 0.62, P < 0.001). The assay can be scaled from a 96-well format to a 384-well format and, therefore, is suitable for high-throughput implementation. This new fluorometric method offers an inexpensive, accurate, and rapid means for determining the oxidative properties of HDL that is applicable to large-scale clinical studies.
Keywords: dysfunctional high density lipoprotein, oxidation, dihydrorhodamine 123, HDL function
There is a continuing search for new biomarkers of increased risk for atherosclerotic disease. High-density lipoproteins (HDL) can become dysfunctional during acute-phase responses (1, 2). We have previously shown a correlation between the lipid hydroperoxides produced by oxidized lipids such as low-density lipoproteins (LDL) in cell culture supernatant of macrophages and the recruitment of inflammatory mediators that are conducive to atherosclerosis (1, 2). Thus, oxidized lipids produce reactive oxygen species (ROS) and can induce monocyte migration, which is an important event in atherosclerosis (1, 2). Normally functioning HDL removes ROS from low-density lipoproteins (LDL), reducing both the oxidation of LDL and the recruitment of inflammatory mediators that are important in the pathogenesis of atherosclerosis (3, 4). However, dysfunctional HDL is defective in this function (1); this HDL can be pronounced in some persons, including those with chronic inflammatory conditions that predispose to atherosclerosis (1, 5–7). Thus, although plasma HDL cholesterol levels are inversely related to risk across large populations, HDL function rather than absolute level may be a more accurate indicator for risk of developing atherosclerosis (1).
Currently, HDL functional properties are most often determined by cell-based assays (8–12). However, the limitations and labor of these cell-based assays render them inaccessible to many researchers, and they are difficult to scale up for large-scale clinical trials or routine clinical use.
Previously a cell-free assay that measures functional properties of HDL by testing the effect of HDL on the production of reactive oxygen species after oxidation and conversion of dichlorodihydrofluorescein diacetate (DCF-DA) to fluorescent DCF (2’,7’-dichlorofluorescein) was developed to provide an alternative to cell-based assays (8). This was based on our demonstration that levels of reactive oxygen species (such as lipid hydroperoxides produced from oxidation of lipoproteins) are significantly associated with the monocyte chemotactic activity and functional properties of HDL that are measured by a cell-based assay (8). Thus this measurement of reactive oxygen species after HDL exposure as reflected by the increased DCF fluorescence reflects the oxidative properties of different types of HDL that vary in their capacity to engage in redox cycling (1).
This approach promised to allow a direct biochemical assessment of functional properties of HDL without relying on a biologic readout. Although this assay yielded results that correlated to the cell-based assay, it did not achieve widespread usage due to i) the oxidative instability of DCF-DA and the resulting tendency for minor variations in experimental conditions to cause inconsistency of the assay and ii) the sensitivity of the assay to any degree of hemolysis.
Here we describe an approach to quantify the oxidative activity of HDL in a cell-free, biochemical assay. The products of redox cycling are detected as time-dependent oxidation of the fluorogenic probe dihydrorhodamine 123 (DHR) to fluorescent rhodamine 123 (13). The rate of DHR oxidation in the presence of HDL reflects the oxidative/antioxidative activity of HDL. This assay provides a readout that is highly correlated to a validated cell-based assay (5, 11, 12), and it is highly reproducible and amenable to high-throughput implementation.
MATERIALS AND METHODS
Subjects
Blood samples were collected from patients with coronary artery disease (CAD) or equivalent as defined by National Cholesterol Education Program Adult Treatment Panel III criteria (14) and were collected from patients referred to the cardiac catheterization laboratory at the Center for Health Sciences at the University of California, Los Angeles (UCLA). After signing a consent form approved by the Human Research Subject Protection Committee of UCLA, patients donated a fasting blood sample collected in a heparinized tube. Plasma samples were also randomly selected from pretreatment samples remaining from a previously described study in which all patients had coronary artery disease or equivalent (12). All of these patients were on a statin (12). Rheumatoid arthritis (RA) patients were recruited from the rheumatology offices at UCLA via flyers posted in the offices and in the UCLA Medical Center. All RA patients met the American College of Rheumatology criteria for RA, which was verified by chart review. Human immunodeficiency virus (HIV)-infected subjects had HIV-1 RNA ≥ 10,000 copies/ml; were not receiving antiretroviral therapy; had no documented coronary atherosclerosis; had normal total cholesterol (200 mg/dl), LDL cholesterol (130 mg/dl), HDL cholesterol (males, >45 mg/dl; females, >50 mg/dl), and triglycerides (<150 mg/dl); were not receiving hypolipidemic medications; and were not diabetic. Normal volunteers were recruited under a protocol approved by the Human Research Subject Protection Committee of UCLA.
Mice
ApoE- and LDLR-null mice originally purchased from the Jackson Laboratories on a C57BL/6J background were obtained from the breeding colony of the Department of Laboratory and Animal Medicine at the David Geffen School of Medicine at UCLA as previously described (15). The mice were maintained on a Western diet (Teklad, Harlan, catalog #TD88137) for two weeks; a group of mice was also treated with pravastatin at 12 μg/ml drinking water (or approximately 50 μg per day) for two weeks. All experiments were performed using protocols approved by the Animal Research Committee at UCLA.
Reagents
Dihydrorhodamine 123 (DHR) and dihydrodichlorofluorescein diacetate (DCFH-DA) were obtained from Molecular Probes (Eugene, OR). DHR was prepared as concentrated stock of 50 mM in dimethyl sulfoxide (DMSO), and DCFH was prepared as previously described (16). Iron-free HEPES (N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid)-buffered saline (HBS, HEPES 20 mM, NaCl 150 mM, pH 7.4) was prepared as previously described (13). The DHR stock was diluted 1:1000 in HEPES saline solution to prepare a working solution of 50 µM. Purified apolipoprotein (apo)A-I from human plasma was obtained from Sigma-Aldrich (St. Louis, MO). Pravastatin sodium (Lot No. M000301, Catalog #P6801) was purchased from LKT Laboratories, Inc.
HDL and LDL purification
HDL and LDL were isolated from cryopreserved human plasma (with or without added sucrose) by ultracentrifugation (UC), fast-performance liquid chromatography (FPLC), or dextran sulfate and precipitation with polyethylene glycol. These were aliquoted and stored as previously described (4, 17–20). HDL cholesterol was quantified using a standard colorimetric assay (Thermo DMA Co., San Jose, CA) as previously described (8). HDL cholesterol was oxidized by use of copper ions (OxHDL) as previously described (21).
HDL inflammatory index
The HDL inflammatory index was determined for each subject's HDL as described previously (11, 12). In this assay, a value >1.0 is considered proinflammatory, and a value <1.0 is considered anti-inflammatory.
DCF-based cell-free assay of HDL function
The DCF-based cell-free assay was performed as previously described (8, 16).
DHR-based cell-free assay of HDL function
Quadruplicates of HDL (5 µg of cholesterol, unless otherwise specified) were added to 96-well plates (polypropylene, flat bottom, black, Fisher Scientific). HBS was added to each well to a final volume of 150 µl, followed by addition of 25 µl of the working solution of 50 µM DHR, for a total volume of 175 µl (final DHR concentration of 7 µM). Immediately following DHR addition, the plate was protected from light and placed in a fluorescence plate reader. The fluorescence of each well was assessed at two minute intervals for an hour with either a DTX 800/880 Multimode Detector (Beckman Coulter, CA) or Synergy 2 Multi-Mode Microplate Reader (Biotek, VT), using a 485/538 nm excitation/emission filter pair with the photomultiplier sensitivity set at medium. Both readers gave equivalent readings (r2 = 0.99). Using Microsoft Excel software, the oxidation rate was calculated for each well as the slope for the linear regression of fluorescence intensity between 10 and 50 min, expressed as FU minute−1 (fluorescence units per minute). HDL oxidative function was calculated as the mean of quadruplicates for the wells containing the HDL sample.
The assay was also run in a 384-well format (polypropylene, flat bottom, black, Fisher Scientific), using 1.5-3 µg of HDL cholesterol with 15 µl of the DHR working stock in a total volume of 100 µl of HBS per well.
LC/MS/MS analysis
LC/MS/MS was performed using a 4000 QTRAP quadruple mass spectrometer (Applied Biosystems) equipped with an electrospray ionization source. The HPLC system utilized an Agilent 1200 series LC pump equipped with a thermostatted autosampler (Agilent Technologies). Chromatography was performed using a Luna C-18(2) column (3 µm particle, 150 × 3.0 mm, Phenomenex) with a security guard cartridge (C-18; Phenomenex) at 40°C. Mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in acetonitrile. The autosampler was set at 4°C. The injection volume was 20 µl, and the flow rate was controlled at 0.4 ml/min. The gradient program was as follows: 0-8 min, linear gradient 0-95% B; 8-9 min, 95% B; 9-9.15, 95-0% B; 9-12 min, 0% B. Data acquisition and instrument control were accomplished using Analyst 1.4.2 software (Applied Biosystems). Detection was accomplished by using the multiple reaction monitoring (MRM) mode with positive ion detection. The parameter settings used were the following: ion spray voltage = 5500 V; curtain gas = 22 (nitrogen); ion source gas 1 = 34; ion source gas 2 = 25; ion source gas 2 temperature = 250°C. Collision energy, declustering potential, and collision cell exit potential were optimized to obtain optimum sensitivity. The transition monitored was m/z 347.05 to m/z 315.1 for DHR.
LC/MS/MS experiments
HDL from control and CAD-patient donors was combined with DHR, and the amount of DHR remaining in the samples after 2 h incubation was determined by LC/MS/MS. First, UC- and FPLC-isolated HDLs were combined in triplicate with DHR. Each sample contained 2.5 μg HDL and 50 μM DHR in a final volume of 175 μl HEPES-buffered saline. The samples were incubated in the dark for 2 h. Urate was then added (final concentration 25 μM) to slow further oxidation of DHR (22). The samples were transferred in minimal light to autosampler vials (Fisher Scientific) for LC/MS/MS analysis. The samples were ordered so that HDL-control samples were measured last, to ensure that any additional air oxidation of DHR did not result in a false positive as regards the difference between HDL-CAD and HDL-Ct with respect to DHR. In a separate experiment, UC-isolated HDL from a healthy donor was serially diluted, in triplicate, from 10 to 0.625 μg cholesterol. The dilutions were combined with DHR and incubated in the dark for 2 h as above. After incubation, urate was added, the samples were transferred to autosampler vials, and the amount of DHR remaining was determined by LC/MS/MS.
Statistical analysis
Statistical analysis was performed using Excel (Microsoft Corp., Seattle, WA). Group means were compared using Student t-test for unpaired variates with P < 0.05 considered statistically significant. Correlation coefficients between variables were calculated using least-squares linear regression.
RESULTS
HDL samples reduce the oxidation rate of DHR consistently by differing amounts
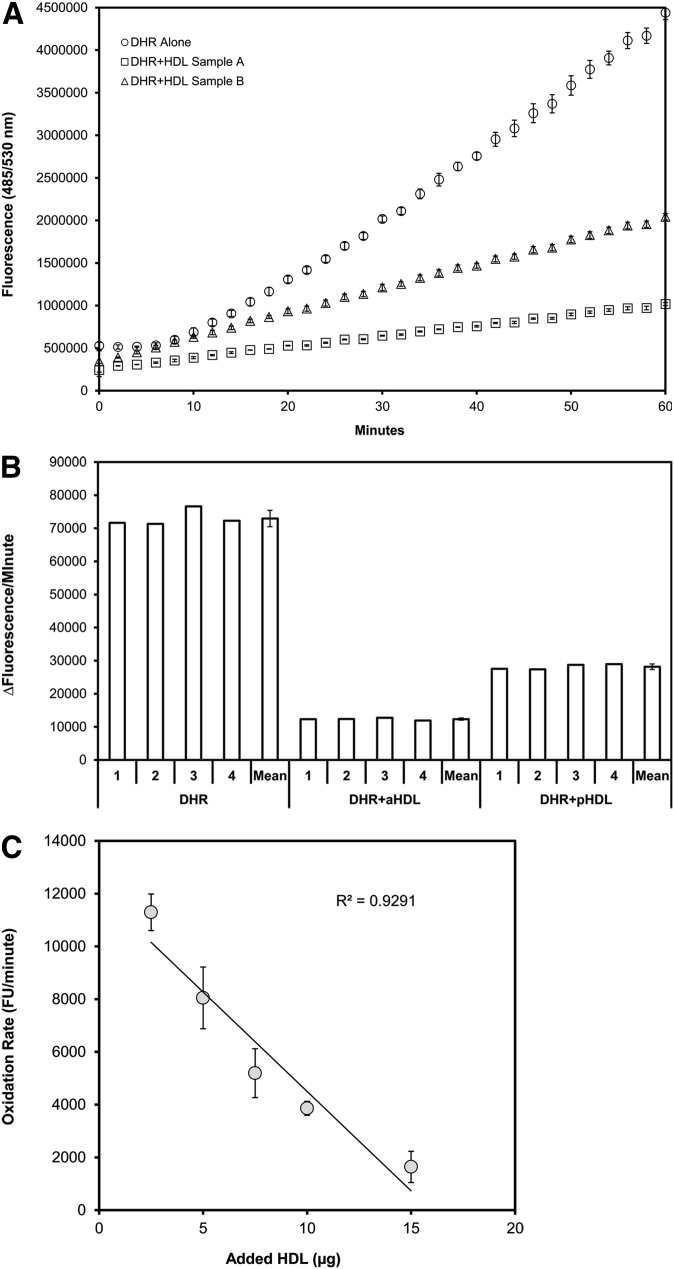
To assess whether the oxidation rate of DHR is affected by the properties of HDL, HDL samples were tested for their effect on DHR oxidation rate. Alone, DHR exposed to air became oxidized (and therefore fluorescent) at a linear rate between 10 and 50 min (Fig. 1). The rate of oxidation of DHR was significantly less with added HDL, and the two HDL samples (an example of anti-inflammatory HDL (aHDL) compared with proinflammatory HDL (pHDL)] showed clearly different effects in this regard (Fig. 1A). Furthermore, when the amount of added HDL was varied, there was a clear dose dependence in the oxidative effects on DHR that was linear in the range 2.5-15 µg (cholesterol) of added HDL per well in the assay (Fig. 1C).
Fig.1.
Spontaneous oxidation of DHR and effect of added HDL. In a 96-well flat-bottom plate 50 µM DHR was added to each well alone or with 5 µg (cholesterol) of FPLC-purified HDL from a donor with anti-inflammatory HDL (aHDL) and from a donor with proinflammatory HDL (pHDL) in a total volume of 175 µl (saline with 150 mM NaCl and HEPES 20 mM, pH 7.4), each in quadruplicate. Spontaneous air-oxidation of DHR at 37°C was then followed in 2 min intervals using a fluorescence microplate reader set to detect 485/538 nm excitation/emission. A: The means and standard deviations of the quadruplicate fluorescence measurements are plotted over time. B: The rates of change in fluorescence between 20 and 50 min (calculated by linear regression) are plotted for the quadruplicates, as well as means/standard deviations. C: FPLC-purified HDL was added in varying concentrations (cholesterol) to 50 µM DHR in 175 µl in a 96-well flat-bottom plate, and the rate of change in fluorescence was measured. The rates of change in fluorescence (means and standard deviations) are plotted against the amounts of added HDL. DHR with no added HDL demonstrated a rate of 25,342 ± 2,619 fluorescence units/minute (not plotted).
Lipid-probe interactions explain the reduction in the oxidation rate of DHR after addition of HDL
We observed a significant reduction in fluorescence signal and oxidation rate of DHR after addition of HDL (Fig. 1). To determine whether the reduction in fluorescence signal of DHR after addition of HDL is caused by antioxidant effect of HDL or nonspecific lipid-probe interactions, we tested the effect of addition of different lipids with known oxidative properties on the oxidation rate of DHR. We found that addition of LDL, HDL (Fig. 1, supplementary Fig. I), and other lipids that are known not to be antioxidant, such as oxidized L-α-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) (data not shown), leads to significant decrease in oxidation signal of DHR. In addition, coincubation of a stable amount of DHR with different concentrations of two lipids simultaneously (HDL with LDL, supplementary Fig. I; HDL with oxPAPC, data not shown) caused significant decrease in the oxidation signal of DHR. Similar lipid-probe interactions were confirmed with DCFH (data not shown). The lipid-probe interaction between DHR and HDL was confirmed with an independent experiment (supplementary Fig. II).
To determine whether the lipid-probe interactions were dose dependent, increasing doses of DHR (1-50 μM) were added to specific amount of HDL or LDL (2.5 μg) (supplementary Fig. III). We found that decreasing concentrations of DHR resulted in decreasing the oxidation rate of both LDL and HDL and that the oxidation rate of LDL was higher than HDL (supplementary Fig. III). To increase the sensitivity of the assay and to detect smaller differences in oxidative properties of lipoproteins, we used a concentration of 50 μM of DHR for all our experiments; this concentration has also been previously used to quantify redox activity (13).
To further investigate the dose-dependent interaction between DHR and lipoproteins, increasing concentrations of lipoproteins (HDL and LDL) were incubated with 50 μM of DHR. Fig. 1C and supplementary Fig. I show that the fluorescent signal generated by HDL was dose dependent but that increasing the concentrations of lipoproteins resulted in a statistically significant decrease in fluorescence for each concentration of lipoprotein used. The correlation observed between the quantity of lipoprotein added and DHR fluorescence and oxidation rate was inverse and highly significant (Fig. 1C, supplementary Fig. I-B). These results are consistent with lipid-probe interactions and possible fluorescence quenching as explanation of reduction in fluorescence signal of DHR after addition of lipids.
Differences in fluorescence after addition of the same amount of different HDL samples correspond to real differences in the degree of oxidation of DHR
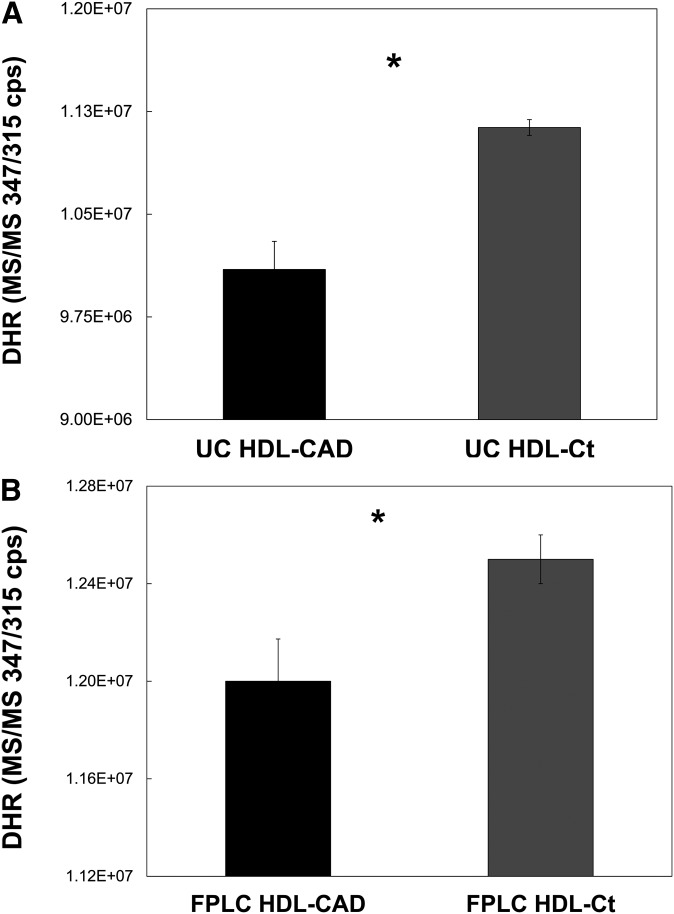
To ensure that the differences we observed with respect to fluorescence corresponded to real differences in the degree of oxidation of DHR, we subjected samples of HDL-CAD and HDL-non-CAD that had been combined with DHR in the manner of the fluorometric assay to LC/MS/MS analysis to determine the amount of DHR remaining in each (Fig. 2). Regardless of whether the HDLs were isolated by sequential UC (Fig. 2A) or by FPLC (Fig. 2B), HDL from healthy non-CAD donors contained significantly more DHR than HDL from CAD-patient donors after an initial 2 h incubation with 50 μM DHR (P = 0.001, n = 3 for each pair). As the amount of DHR remaining in the samples corresponds in this setting to the degree of oxidation of DHR, it follows that HDL from non-CAD donors significantly inhibited the oxidation of DHR compared with HDL from CAD patients. In separate experiments, these same pairs of HDL were shown to exhibit significant differences in both the rate of increase of fluorescent signal and in fluorescent signal after 2 h incubation during the standard fluorometric assay. Both UC- and FPLC-isolated HDL-CAD exhibited a significantly greater rate of increase in fluorescent signal and significantly more final fluorescence than the respective HDL-non-CAD samples (data not shown). Finally, the dose-dependent interaction between DHR and HDL was confirmed by LC/MS/MS (supplementary Fig. IV).
Fig.2.
HDL from healthy donors significantly inhibits the oxidation of DHR compared with HDL from CAD patients, as determined by LC/MS/MS. HDL was isolated from the serum of healthy and CAD-patient donors by either sequential UC or FPLC. Control (non-CAD) and CAD HDL pairs (HDL-CAD and HDL-Ct), one for each method of isolation, were combined in triplicate with DHR and incubated in the dark for 2 h. Each sample contained 2.5 µg HDL and 50 µM DHR in 175 total µl HEPES-buffered saline. After the incubation, urate was added (0.025 mM final concentration) to slow the further oxidation of DHR. The amount of DHR remaining in each sample was then determined by LC/MS/MS. For both the HDL-CAD and HDL-Ct pair isolated by UC (A) and the pair isolated by FPLC (B), the samples of the HDL-Ct contained significantly more DHR than the samples of the HDL-CAD (*P = 0.001, n = 3).
The concentration-dependent effect of addition of HDL on oxidation of DHR is not secondary to lipid-free apoAI
To determine whether lipid-free apoAI has a concentration-dependent effect on oxidation of DHR at concentrations that correspond to the physiologic range of concentration of apoAI in normal subjects (90-130 mg/dl) (1, 23), commercially available apoAI from human plasma was obtained, and the effect of different concentrations of apoAI on oxidation of DHR was determined. We found that although addition of apoAI caused reduction in the oxidation rate of DHR, the reduction was not concentration dependent and not specific to the addition of increasing concentrations of apoAI, consistent with nonspecific probe-apoAI interactions (supplementary Fig. V).
The DHR-based assay of HDL oxidation function yields highly reproducible measurements despite fluorescence quenching
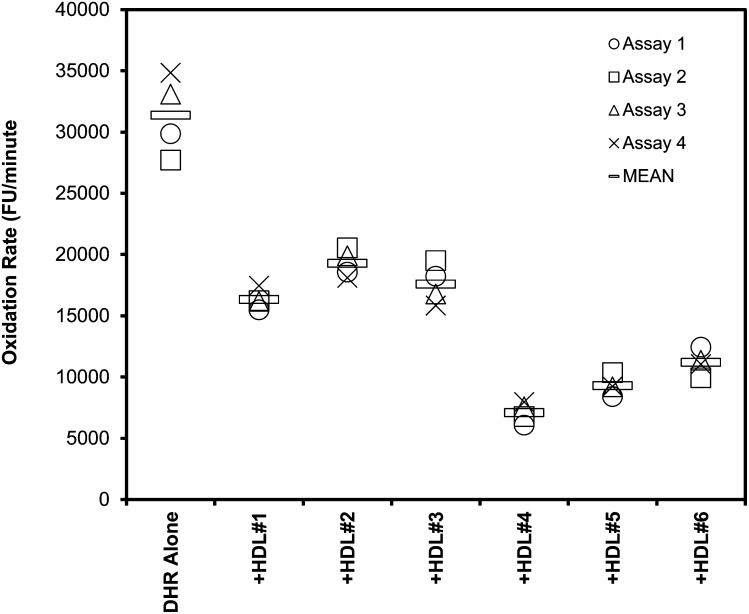
To counteract the effect of fluorescence quenching between HDL and DHR, relative differences in the oxidation rate of DHR for a specific amount of HDL (2.5 μg or 5 μg) were studied between different samples. To assess whether the assay is reproducible so that results of different experiments are comparable, six HDL samples were assessed in four independent experiments (Fig. 3). Again, all HDL samples reduced the oxidation rate of DHR with low intra-assay variability between the quadruplicates, which ranged 5.8-7.6% (mean 6.6%). Between independent replicates of the experiment, inter-assay variability for each of the samples ranged 5.0-11.9% (mean 8.6%).
Fig.3.
Low inter-assay variability between measurements of HDL effects. Oxidation of DHR in the presence of six different samples of HDL was assessed as described in Fig. 1, using 2.5 µg (cholesterol) of added HDL. The data (means of quadruplicates) from four independent experiments are plotted. The mean inter-assay variability for these six samples was 8.6% (range 5.0-11.9%), and the mean intra-assay variability was 6.6% (range 5.8-7.6%).
To determine the effect of the variance from HDL isolation method, six samples were isolated with dextran/polyethylene glycol (PEG) method four different times, as described in “Materials and Methods.” Using dextran and PEG, the mean inter-assay variability for these six samples was 9.1% (range 6.0-12.1%) and 8.6% (range 5.4-11.8%), respectively. The mean intra-assay variability for these six samples was 8.3% (range 4.9-11.1%) and 7.1% (range 4.7-10.3%), respectively. These results indicated that the assay could reliably measure HDL oxidative function.
Different diluents affect the readout of the assay
Different methods of HDL purification and storage utilize varying compositions of diluents, so this DHR-based assay was tested for effects of common types of diluents utilized for HDL suspension and storage in preparation for HDL functional assays. Compared with the buffered saline diluent (150 mM NaCl and HEPES 20 mM, pH 7.4) used for the results in Fig. 1, added sodium azide reduced the oxidation rate of DHR (supplementary Fig. VI-A). Standard phosphate-buffered saline (PBS) also reduced the oxidation rate, which was further reduced by the addition of sodium azide (supplementary Fig. VI-A). Oxidation of DHR was also pH dependent (supplementary Fig. VI-B). Testing a range of pH values from 3 to 9, the oxidation rate was highest at pH 3, lowest at pH 9, and similar at pH 5 and 7. These results suggested that sensitivity of the assay is best using saline without sodium azide and that it is less prone to pH effects near the physiologic range.
Different methods of HDL isolation affect the readout of the assay
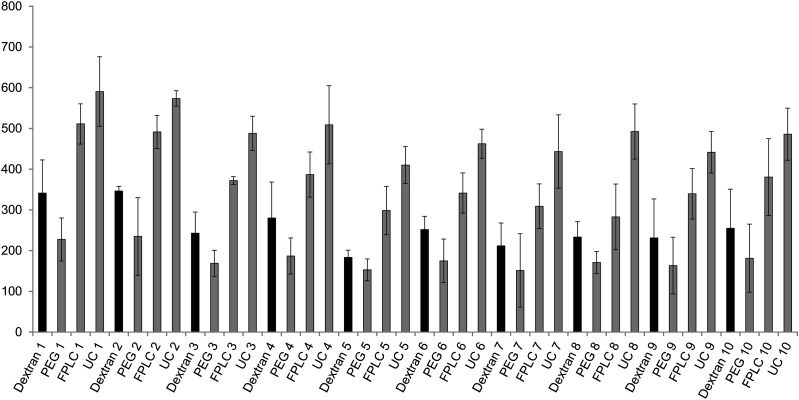
Different methods of HDL purification may affect the functional properties of HDL, so this DHR-based assay was tested for effects of common methods of HDL isolation such as UC, FPLC, and use of dextran sulfate and PEG (Fig. 4). The rate of oxidation of DHR with added HDL was significantly higher in patients compared with controls regardless of whether the HDL was isolated by FPLC or UC (supplementary Fig. VII). In addition, the DHR assay gave consistent results using all different methods of HDL isolation (supplementary Fig. VIII). However, the oxidation rate of DHR was significantly higher when HDL isolated by UC was added compared with when HDL isolated by other methods was added (P < 0.001). The results obtained by all the methods correlated strongly (r2 > 0.8, P < 0.0001; supplementary Fig. VIII).
Fig.4.
Influence of method of HDL isolation on measurements of HDL oxidative activity. Influence of method of HDL isolation on DHR oxidation was assessed using 100 µl in a 384-well flat-bottom plate, and the rate of change in fluorescence was measured as in Fig. 1. The HDL was isolated from 10 healthy volunteers (non-CAD). An amount of 15 µM DHR was exposed to 2.5 µg (cholesterol) of FPLC-purified (FPLC), ultracentrifugation-purified HDL (UC), or HDL that was isolated using dextran sulfate or polyethylene glycol as described in “Materials and Methods.” Rates of oxidation of DHR are plotted as means of quadruplicates for each sample.
Results from the DHR assay correlate with a validated cell-based assay
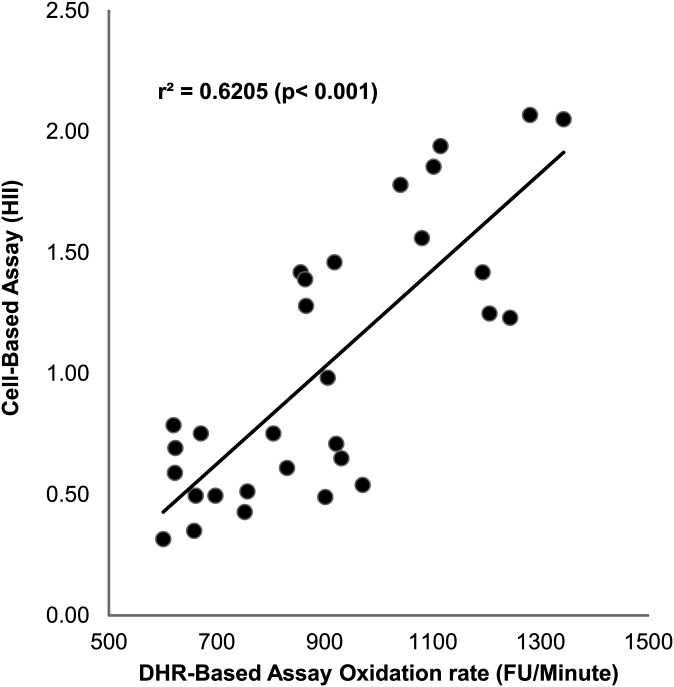
To compare the results of this DHR assay to those obtained using a validated cell-based assay (11, 12), 30 HDL samples were assessed using both assays (Fig. 5). Comparing DHR oxidation rate with the HDL inflammatory index, there was a strong positive correlation (r2 = 0.62, P < 0.001).
Fig.5.
Correlation of DHR method with cell-based method. Thirty samples of FPLC-purified HDL were assessed for their ability to inhibit DHR oxidation as shown in Fig. 1, and their HDL inflammatory index was determined in a cell-based assay as described in “Materials and Methods.” The values from each assay are plotted against each other.
Oxidized HDL is proinflammatory HDL
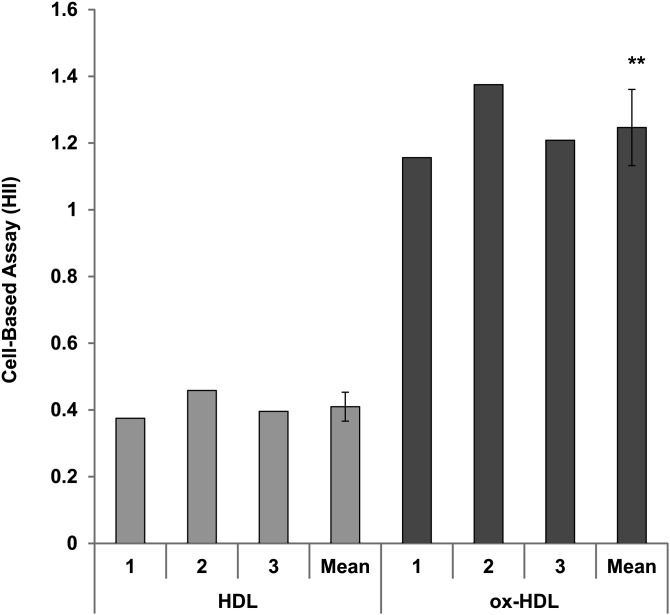
To determine whether there is an association between oxidative and inflammatory properties of HDL, HDL was oxidized as described in “Materials and Methods,” and inflammatory properties were determined by a validated cell-based assay (11, 12) (Fig. 6). Anti-inflammatory HDL from healthy volunteers became proinflammatory after oxidation (Fig. 6). Thus, the DHR assay determines functional, oxidative properties of HDL that are associated with inflammatory properties of HDL.
Fig.6.
Oxidized HDL has inflammatory properties. The HDL inflammatory index was determined for three samples of FPLC-purified HDL from healthy volunteers in a cell-based assay as described in “Materials and Methods.” The same HDL samples were oxidized as described in “Materials and Methods” (oxHDL), and the HDL inflammatory index was determined. The oxidized HDL had significantly higher HDL inflammatory index (**P = 0.002, paired t-test).
The DHR assay can detect dysfunctional HDL in different conditions
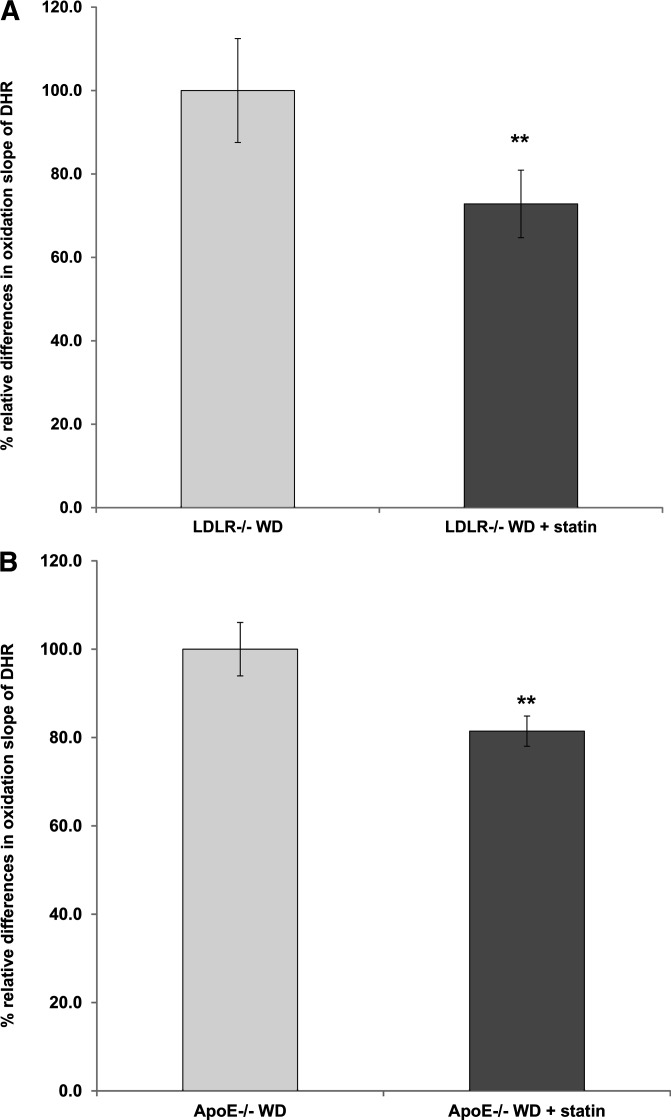
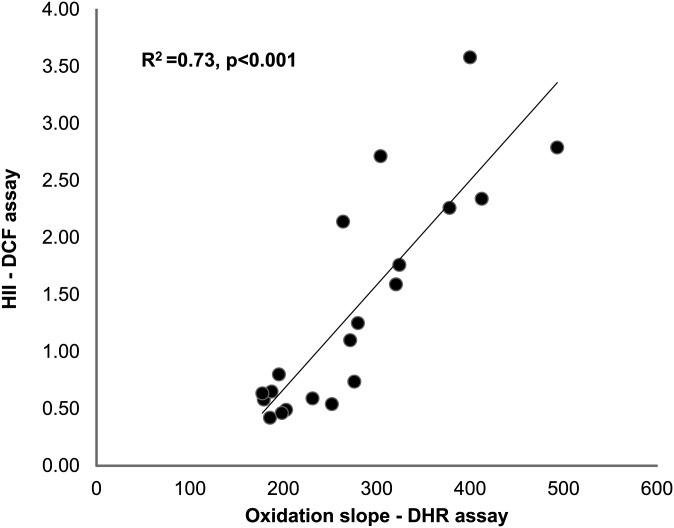
To further validate the new method, we used the DHR assay to detect dysfunctional HDL in conditions in which dysfunctional HDL is known to be present, such as established animal models of atherosclerosis (1, 23), RA (16), and HIV infection (24). The DHR assay detected the established effect of statins on functional properties of HDL (1, 23) in animal models of atherosclerosis such as LDLR−/− (Fig. 7A) and ApoE−/− mice (Fig. 7B). In addition, the functional properties of HDL in patients with RA as determined by a previously validated cell-free assay using DCF correlated significantly with the functional properties of HDL as determined by the oxidation slope of DHR (Fig. 8). Finally, the DHR assay detected dysfunctional HDL in HIV infection, even when the HDL from these patients was mixed with HDL from non-HIV patients at various ratios (supplementary Fig. IX).
Fig.7.
The DHR assay can detect established effect of statins on functional properties of HDL in animal models of atherosclerosis. A: By using FPLC, HDL was isolated from three pooled plasma samples from LDLR−/− mice on Western diet (LDLR−/− WD) for two weeks and from three pooled plasma samples from LDLR−/− mice on Western diet for two weeks that were also treated with pravastatin 12.5 μg/ml for two weeks. Each plasma sample was pooled from 4 mice (12 mice in total). Oxidation of DHR was assessed as in Fig. 1, using 2.5 µg (cholesterol) of added HDL. The oxidation slope of DHR in the presence of HDL from LDLR−/− WD + statin was normalized to the oxidation slope of DHR in the presence of HDL from LDLR−/− WD, and the percent relative differences are shown. The data represent the average of measurements from three independent experiments. There was a statistically significant reduction in the oxidation slope of DHR in the presence of HDL isolated from LDLR−/− WD + statin mice compared with the oxidation slope of DHR in the presence of HDL isolated from LDLR−/− WD mice (**P = 0.01) B: By using FPLC, HDL was isolated from three pooled plasma samples from ApoE−/− female mice on Western diet (ApoE−/− WD) for two weeks and from three pooled plasma samples from ApoE−/− female mice on Western diet for two weeks that were also treated with pravastatin 12.5 μg/ml for two weeks. Each plasma sample was pooled from 4 mice (12 mice in total). Oxidation of DHR was assessed as in Fig. 1, using 2.5 µg (cholesterol) of added HDL. The oxidation slope of DHR in the presence of HDL from ApoE−/− WD + statin was normalized to the oxidation slope of DHR in the presence of HDL from ApoE−/− WD and the percent relative differences are shown. The data represent the average of measurements from three independent experiments. There was a statistically significant reduction in the oxidation slope of DHR in the presence of HDL isolated from ApoE−/− WD + statin mice compared with the oxidation slope of DHR in the presence of HDL isolated from ApoE−/− WD mice (**P = 0.01).
Fig.8.
Correlation of DHR method with previous cell-free method. Twenty samples (10 from healthy volunteers and 10 from patients with rheumatoid arthritis) of HDL isolated using precipitation with dextran sulfate were assessed for their ability to inhibit DHR oxidation as shown in Fig. 1, and their HDL inflammatory index was determined in a cell-free assay using DCFH as described in “Materials and Methods.” The values from each assay are plotted against each other.
The DHR assay can be scaled to a high-throughput, 384-well format
Although inter-assay variability is low (as shown above for the 96-well format), the capability to compare numerous samples in the same experiment could be advantageous for clinical studies of HDL function. Scaling down the assay to the 384-well format yielded results that were comparable to those for the 96-well format when HDL samples were tested in both formats (supplementary Fig. X, r2 = 0.96, P < 0.0001). The intra-assay variability averaged 7.8% and the inter-assay variability was about 10.2% in the 384-well format (data not shown), and thus it was slightly higher than for the 96-well format. The increased variability was not due to the longer time to load the plate manually, because the oxidation rate was similar when the plate was allowed to incubate for 2 h at room temperature (data not shown). Overall, these data suggested that a high-throughput, 384-well format is feasible for assessing large numbers of HDL samples simultaneously.
DISCUSSION
Growing evidence suggests that HDL varies significantly in its phenotype and influence on cardiovascular disease risk (6, 25). Atherosclerosis begins when LDL is trapped in the arterial wall and seeded with ROS (26), resulting in oxidized LDL that attracts monocytes and induces localized inflammation (3, 4). Properly functioning HDL removes ROS from LDL, antagonizing this process. HDL, however, can be dysfunctional under certain clinical conditions, such as acute phase responses (2), and various pathogenic states, such as diabetes. Measuring the functional status of HDL, rather than HDL cholesterol concentration, may be more informative in predicting cardiovascular disease. Unfortunately, the best assays for HDL function have been cell-based assays (9–12), which are technically prohibitive for many researchers and logistically difficult for large-scale studies. The function of HDL has been determined in previous assays based on the capacity of HDL to prevent the formation of or to inactivate oxidized phospholipids produced by LDL (8).
Recent interest has focused on the functional consequences of HDL oxidation. Oxidation could conceivably contribute to the formation of dysfunctional HDL, proposed to be present in humans with cardiovascular disease (27). One potentially important pathway for generating dysfunctional HDL via oxidation involves myeloperoxidase that mediates conversion of protein tyrosine residues to 3-chlorotyrosine and methionine residues to methionine sulfoxide (MetO) (28). MetO can also be formed from exposure of HDL's major protein, apoA-I, to H2O2 (29) or lipid hydroperoxide (30, 31), the latter generated during the oxidation of HDL lipids. ROS, such as 1e-oxidants (i.e., hydroxyl radical and metal ions) and 2e-oxidants (i.e., HOCl, H2O2, and peroxynitrite), have previously been shown to oxidize tyrosine and methionine residues (32), which can have dramatic consequences on the functions of apoA-I/HDL, including reverse cholesterol transport (33).
Dihydrorhodamine 123 (DHR) may be oxidized by a variety of oxidants, including hypochlorous acid generated by myeloperoxidases, peroxynitrite anion formed by oxidation of nitric oxide and hydrogen peroxide in the presence of peroxidases (13, 34). Although DHR has mostly been used to determine oxidation of various molecules in intracellular matrix (34) previous studies have demonstrated that oxidation rate of DHR can be used to quantify redox activity in extracellular matrix such as plasma (13). The oxidation of HDL is the result of oxidation of both the lipid and protein component. During air oxidation of the HEPES-saline-lipoprotein-DHR solution both 1e-oxidants and 2e-oxidants are generated (13). The 1e-oxidants preferentially react with the lipoprotein's lipids, and this causes lipid peroxidation with the resulting accumulation of hydroperoxides of phospholipids and cholesteryl-esters (35) that then oxidize apoA-I's methionine to MetO (30, 31, 36). We demonstrated that oxidation of lipid-free apoAI did not cause a concentration-dependent effect on oxidation of DHR. The measurement of the slope of changes in fluorescence of DHR over time in response to addition of different types of HDL corresponds to the rate of formation of lipid hydroperoxide products and ROS. Thus, the oxidation rate of DHR can be used to quantify the intrinsic ability of HDL to be oxidized, which has been shown to affect the function of apoA-I/HDL (33). Thus, in the current study, we demonstrated a novel cell-free assay that biochemically examines the oxidative ability of HDL, providing a functional measurement that correlates well with a validated cell-based assay. We utilized DHR as a substrate similar to a previously reported assay to measure the redox potential of iron in plasma (13). Using the new method, we demonstrated that addition of normal HDL led to a significantly smaller increase in the fluorescent signal of DHR compared with pHDL.
In the previous cell-free assay, we demonstrated lipid-probe interactions between the fluorochrome DCFH and various lipids (8). To our knowledge, the interactions of lipids with DHR have not been previously tested. We found that addition of lipids, including pro-oxidant lipids contained in lipoproteins, led to a significant decrease in the oxidation signal of DHR; thus the decrease in the oxidation rate of DHR after addition of HDL was caused by lipid-probe interactions. In addition, changes in fluorescence that were observed with different buffers are likely due to an effect of the buffer on the HDL complex; hence, the interaction between the buffer and the fluorescent indicator were affected by both the temperature of the reaction and the concentration of buffer (data not shown). Moreover, addition of detergent (SDS) in the DHR + HDL reaction caused an increase in fluorescence (data not shown), which could be explained by possible dissociation of the HDL/dye complex and reduction in quenching (personal communication with Molecular Probes). We demonstrated with two different independent methods, including LC/MS/MS (Fig. 2, supplementary Fig. II), that the interaction of DHR-HDL is responsible for the fluorescence quenching in the DHR assay.
However, we show that our DHR-based assay of HDL oxidation yields highly reproducible measurements despite fluorescence quenching. In addition, the inverse correlation between HDL concentration and oxidation rate of DHR allows quantification of functional properties of HDL using very low concentrations of HDL (1.25 μg per sample). Thus, using this assay we were able to quantify relative differences in the oxidative properties of different HDL samples that correlate with their functional properties.
This DHR-based cell-free assay improves upon the prior dichlorofluorescein-diacetate (DCFH-DA)-based cell-free assay (8). Although measuring the ability of HDL to inhibit oxidation, the earlier assay had several technical challenges that limited its utility. It was designed to mimic the cell-based assay and test the effect of HDL in combination with LDL. This introduced additional variability because different preparations of LDL can vary in their oxidative activity and lipid-probe interactions become less consistent with multiple lipids. Mixing LDL and HDL with DHR in our assay, we observed inconsistent results that appeared to be caused by fluorescence quenching (data not shown) compared with the highly consistent results seen with HDL alone. Another technical challenge was the instability of DCFH-DA, which needed to be dissolved in methanol for activation to the active molecule DCFH and protected from room air because DCFH is unstable and prone to auto-oxidation (22, 34, 37–39). In contrast, DHR is relatively stable (22, 34, 37–39) and oxidizes at a predictable rate when exposed to room air. Furthermore, conversion of DCFH-DA to DCFH can be mediated by esterases that are carried over variably during the lipid purification process, adding another variable that is difficult to control, whereas DHR requires no activation and is not prone to this effect (22, 34, 37–39). Another point of improvement is the kinetic approach for measuring oxidation rate during a linear phase, which lends greater precision compared with a single endpoint measurement. Finally, we demonstrated that different diluents for the assay affect the oxidation rate of the indicator compound; this may also help explain the variability observed in HDL measurements using the DCFH-DA assay among research groups.
We also demonstrated that although different methods of HDL isolation affect the oxidation rate of DHR when HDL is added, UC, FPLC, and isolation of HDL using precipitation with dextran sulfate or PEG could be used to determine functional properties of HDL using our DHR-based cell-free assay. The use of dextran sulfate or PDG may allow HDL isolation and use of this method in large-scale studies. The oxidation rate of HDL was higher in samples isolated using UC. Removal of much of the albumin bound to the HDL particle during the process of UC may alter the association of nonpolar and polar substances, including ROS, associated with lipoproteins (40, 41). The process of UC is longer than other methods and may yield additional ROS, which may account for the higher oxidation rate of DHR using UC compared with other methods of HDL isolation when the same amount of HDL is added (40). Finally, we found that addition of sucrose in plasma during cryopreservation did not change the HDL oxidation rate significantly and that sucrose-free samples can be used in our assay (data not shown).
This novel DHR-based assay offers an attractive alternative to current cell-based assays. Because it measures a biochemical rather than biologic phenomenon, it may be more precise. The inter-assay variability of ∼8-10% compares favorably with the cell-based assay, which has a variability of >15% (12).
The correlation of HDL effects on DHR oxidation rate to the biological readout of HDL in a cell-based assay (Fig. 5) is consistent with a proposed mechanism whereby HDL exerts its effects through modulating ROS (8), and it suggests that the assay accurately reflects the balance of aHDL and pHDL. Further support for the biological relevance of this measurement is the finding that oxidation of DHR was higher with LDL than with any of the HDL samples tested (supplementary Fig. I) and that it was significantly reduced after addition of HDL from statin-treated mice with atherosclerosis compared with addition of HDL from nonstatin-treated mice (Fig. 7). Indeed, treatment of these mice with statins has been shown to reduce the inflammatory properties of HDL (15), and we demonstrated that the DHR assay could detect the favorable effect of statins on the functional properties of HDL. Finally, the DHR assay can detect dysfunctional HDL in patients with RA (Fig. 8) and HIV infection (supplementary Fig. IX), confirming our previous results (15, 16).
The fact that our assay quantifies oxidation of HDL by a range of different oxidants increases its applicability to biological samples, at least in the context of cardiovascular diseases. This is because various oxidants contribute to oxidative modifications taking place in the affected arterial wall during atherogenesis (42). Thus, while “oxidized HDL” is not a chemically defined term, the oxidation rate of DHR corresponds to the intrinsic oxidative features of modified lipoproteins. However, we exclusively used in vitro-generated oxidized forms of HDL, whereas the oxidative modifications occurring to HDL in the diseased artery wall are conceivably more complex (42). An additional potential limitation of the present assay is that HDL is subject to continuous remodeling in vivo. This includes dissociation of apoA-I from the lipoprotein particle, a process that could be increased by oxidation (43). Clearly, future studies are required to assess these aspects, as well as the utility of the assay for clinical studies.
In conclusion, this new assay offers a rapid method for measuring the properties of HDL that inhibit oxidation. It yields results that correlate well with a validated cell-based assay. This new technical approach may offer a convenient tool for studies of the role of the HDL functional phenotype in the development of atherosclerosis in vivo.
Supplementary Material
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- DCFH
- dihydrodichlorofluorescein
- DCFH-DA
- dichlorofluorescein-diacetate
- DHR
- dihydrorhodamine 123
- FPLC
- fast-performance liquid chromatography
- FU
- fluorescence unit
- aHDL
- anti-inflammatory HDL
- pHDL
- proinflammatory HDL
- HIV
- human immunodeficiency virus
- PEG
- polyethylene glycol
- RA
- rheumatoid arthritis
- ROS
- reactive oxygen species
- UC
- ultracentrifugation
This work was supported by National Institutes of Health Grant RO1 HL-095132, by Public Health Service Grant HL-30568, and by the University of California at Los Angeles (UCLA) AIDS Institute and UCLA Center for AIDS Research Grant AI-28697. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies. A. M. Fogelman and M. Navab are principals at Bruin Pharma, Inc., and A.M. Fogelman is an officer at Bruin Pharma, Inc.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of ten figures.
REFERENCES
- 1.Navab M., Reddy S. T., Van Lenten B. J., Anantharamaiah G. M., Fogelman A. M. 2009. The role of dysfunctional HDL in atherosclerosis. J. Lipid Res. 50(Suppl): S145–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navab M., Berliner J. A., Watson A. D., Hama S. Y., Territo M. C., Lusis A. J., Shih D. M., Van Lenten B. J., Frank J. S., Demer L. L., et al. 1996. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler. Thromb. Vasc. Biol. 16: 831–842. [DOI] [PubMed] [Google Scholar]
- 3.Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., et al. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41: 1481–1494. [PubMed] [Google Scholar]
- 4.Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., Fogelman A. M. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41: 1495–1508. [PubMed] [Google Scholar]
- 5.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756. [DOI] [PubMed] [Google Scholar]
- 6.Ansell B. J., Fonarow G. C., Fogelman A. M. 2007. The paradox of dysfunctional high-density lipoprotein. Curr. Opin. Lipidol. 18: 427–434. [DOI] [PubMed] [Google Scholar]
- 7.Navab M., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Fogelman A. M. 2009. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes. 58: 2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navab M., Hama S. Y., Hough G. P., Subbanagounder G., Reddy S. T., Fogelman A. M. 2001. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 42: 1308–1317. [PubMed] [Google Scholar]
- 9.Patel S., Drew B. G., Nakhla S., Duffy S. J., Murphy A. J., Barter P. J., Rye K. A., Chin-Dusting J., Hoang A., Sviridov D., et al. 2009. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J. Am. Coll. Cardiol. 53: 962–971. [DOI] [PubMed] [Google Scholar]
- 10.Undurti A., Huang Y., Lupica J. A., Smith J. D., DiDonato J. A., Hazen S. L. 2009. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J. Biol. Chem. 284: 30825–30835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Lenten B. J., Wagner A. C., Navab M., Anantharamaiah G. M., Hama S., Reddy S. T., Fogelman A. M. 2007. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J. Lipid Res. 48: 2344–2353. [DOI] [PubMed] [Google Scholar]
- 12.Watson C. E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S. T., et al. 2011. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 52: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito B. P., Breuer W., Sirankapracha P., Pootrakul P., Hershko C., Cabantchik Z. I. 2003. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 102: 2670–2677. [DOI] [PubMed] [Google Scholar]
- 14.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 15.Navab M., Anantharamaiah G. M., Hama S., Hough G., Reddy S. T., Frank J. S., Garber D. W., Handattu S., Fogelman A. M. 2005. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 16.Charles-Schoeman C., Khanna D., Furst D. E., McMahon M., Reddy S. T., Fogelman A. M., Paulus H. E., Park G. S., Gong T., Ansell B. J. 2007. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J. Rheumatol. 34: 1459–1464. [PubMed] [Google Scholar]
- 17.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedrick C. C., Castellani L. W., Warden C. H., Puppione D. L., Lusis A. J. 1993. Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. J. Biol. Chem. 268: 20676–20682. [PubMed] [Google Scholar]
- 19.Watson A. D., Berliner J. A., Hama S. Y., La Du B. N., Faull K. F., Fogelman A. M., Navab M. 1995. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. 96: 2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widhalm K., Pakosta R. 1991. Precipitation with polyethylene glycol and density-gradient ultracentrifugation compared for determining high-density lipoprotein subclasses HDL2 and HDL3. Clin. Chem. 37: 238–240. [PubMed] [Google Scholar]
- 21.Ren J., Jin W., Chen H. 2010. oxHDL decreases the expression of CD36 on human macrophages through PPARgamma and p38 MAP kinase dependent mechanisms. Mol. Cell. Biochem. 342: 171–181. [DOI] [PubMed] [Google Scholar]
- 22.Kooy N. W., Royall J. A., Ischiropoulos H., Beckman J. S. 1994. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 16: 149–156. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe J., Grijalva V., Hama S., Barbour K., Berger F. G., Navab M., Fogelman A. M., Reddy S. T. 2009. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J. Biol. Chem. 284: 18292–18301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelesidis T., Yang O. O., Currier J. S., Navab K., Fogelman A. M., Navab M. 2011. HIV-1 infected patients with suppressed plasma viremia on treatment have pro-inflammatory HDL. Lipids Health Dis. 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navab M., Ananthramaiah G. M., Reddy S. T., Van Lenten B. J., Ansell B. J., Fonarow G. C., Vahabzadeh K., Hama S., Hough G., Kamranpour N., et al. 2004. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 45: 993–1007. [DOI] [PubMed] [Google Scholar]
- 26.Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H., et al. 1991. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88: 2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. 2004. Antiinflammatory properties of HDL. Circ. Res. 95: 764–772. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. 1994. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 94: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anantharamaiah G. M., Hughes T. A., Iqbal M., Gawish A., Neame P. J., Medley M. F., Segrest J. P. 1988. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J. Lipid Res. 29: 309–318. [PubMed] [Google Scholar]
- 30.Garner B., Witting P. K., Waldeck A. R., Christison J. K., Raftery M., Stocker R. 1998. Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that accompanies lipid peroxidation and can be enhanced by alpha-tocopherol. J. Biol. Chem. 273: 6080–6087. [DOI] [PubMed] [Google Scholar]
- 31.Garner B., Waldeck A. R., Witting P. K., Rye K. A., Stocker R. 1998. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 273: 6088–6095. [DOI] [PubMed] [Google Scholar]
- 32.Wang X. S., Shao B., Oda M. N., Heinecke J. W., Mahler S., Stocker R. 2009. A sensitive and specific ELISA detects methionine sulfoxide-containing apolipoprotein A-I in HDL. J. Lipid Res. 50: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oram J. F., Heinecke J. W. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372. [DOI] [PubMed] [Google Scholar]
- 34.Wardman P. 2008. Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol. 441: 261–282. [DOI] [PubMed] [Google Scholar]
- 35.Bowry V. W., Stanley K. K., Stocker R. 1992. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. USA. 89: 10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sattler W., Christison J., Stocker R. 1995. Cholesterylester hydroperoxide reducing activity associated with isolated high- and low-density lipoproteins. Free Radic. Biol. Med. 18: 421–429. [DOI] [PubMed] [Google Scholar]
- 37.Glebska J., Koppenol W. H. 2003. Peroxynitrite-mediated oxidation of dichlorodihydrofluorescein and dihydrorhodamine. Free Radic. Biol. Med. 35: 676–682. [DOI] [PubMed] [Google Scholar]
- 38.Ischiropoulos H., Gow A., Thom S. R., Kooy N. W., Royall J. A., Crow J. P. 1999. Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol. 301: 367–373. [DOI] [PubMed] [Google Scholar]
- 39.Wardman P. 2007. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic. Biol. Med. 43: 995–1022. [DOI] [PubMed] [Google Scholar]
- 40.Hallberg C., Haden M., Bergstrom M., Hanson G., Pettersson K., Westerlund C., Bondjers G., Ostlund-Lindqvist A. M., Camejo G. 1994. Lipoprotein fractionation in deuterium oxide gradients: a procedure for evaluation of antioxidant binding and susceptibility to oxidation. J. Lipid Res. 35: 1–9. [PubMed] [Google Scholar]
- 41.Sattler W., Mohr D., Stocker R. 1994. Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol. 233: 469–489. [DOI] [PubMed] [Google Scholar]
- 42.Stocker R., Keaney J. F., Jr 2004. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84: 1381–1478. [DOI] [PubMed] [Google Scholar]
- 43.Panzenböck U., Kritharides L., Raftery M., Rye K. A., Stocker R. 2000. Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J. Biol. Chem. 275: 19536–19544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.