Abstract
Both prolonged exposure to hyperoxia and large tidal volume mechanical ventilation can each independently cause lung injury. However, the combined impact of these insults is poorly understood. We recently reported that preexposure to hyperoxia for 12 h, followed by ventilation with large tidal volumes, induced significant lung injury and epithelial cell apoptosis compared with either stimulus alone (Makena et al. Am J Physiol Lung Cell Mol Physiol 299: L711–L719, 2010). The upstream mechanisms of this lung injury and apoptosis have not been clearly elucidated. We hypothesized that lung injury in this model was dependent on oxidative signaling via the c-Jun NH2-terminal kinases (JNK). We, therefore, evaluated lung injury and apoptosis in the presence of N-acetyl-cysteine (NAC) in both mouse and cell culture models, and we provide evidence that NAC significantly inhibited lung injury and apoptosis by reducing the production of ROS, activation of JNK, and apoptosis. To confirm JNK involvement in apoptosis, cells treated with a specific JNK inhibitor, SP600125, and subjected to preexposure to hyperoxia, followed by mechanical stretch, exhibited significantly reduced evidence of apoptosis. In conclusion, lung injury and apoptosis caused by preexposure to hyperoxia, followed by high tidal volume mechanical ventilation, induces ROS-mediated activation of JNK and mitochondrial-mediated apoptosis. NAC protects lung injury and apoptosis by inhibiting ROS-mediated activation of JNK and downstream proapoptotic signaling.
Keywords: apoptosis, mechanical stretch, alveolar epithelium
the critical signaling pathways of either hyperoxia (HO) or high tidal volume (Vt) mechanical ventilation (HV), leading to acute lung injury (ALI) or ventilator-induced lung injury (VILI), have been extensively reported; however, despite their near ubiquitous concomitant use, few studies have focused on the combined effect of both HO and HV (28, 30, 31, 36, 42). Our laboratory recently reported that preexposure to HO (12 h), followed by large Vt mechanical ventilation (25 μl/g for 4 h), caused significant lung injury greater than either stimulus alone or with combined HV and HO, not preceded by HO preexposure (31). This severe lung injury was associated with caspase-mediated apoptosis of alveolar epithelial cells both in vivo and in vitro.
Recent studies have established that mitogen-activated protein kinases play an important regulatory role for various physical and chemical stimuli to mount specific response to maintain homeostasis. The c-Jun NH2-terminal kinases (JNK), also known as stress-activated protein kinases, are encoded by three genes. The Jnk1 and Jnk2 genes are expressed ubiquitously, whereas Jnk3 has a more limited pattern of expression and is largely restricted to brain, heart, and testis (14). Reactive oxygen species (ROS) can induce sustained JNK activation and induce both apoptotic and necrotic cell death (25). JNK activation has been implicated in various animal models of lung injury (11, 13, 27, 28) and in cultured cells in response to HO (29, 38).
The goals of this study are to better characterize our model of VILI caused by 12-h exposure to HO followed by 4 h of HV. We hypothesized that lung injury in this model is mediated by increased production of ROS, leading to increased apoptosis via activation of JNK. To test this hypothesis, we showed that N-acetyl-cysteine (NAC), a glutathione precursor, significantly protected against HO/mechanical ventilation-induced lung injury and apoptosis by reducing ROS-mediated activation of JNK. Additionally, we showed that pharmacological JNK inhibition, independent of antioxidant treatment, also inhibited mitochondrial-dependent apoptosis.
MATERIALS AND METHODS
Animals.
Male C57BL/J6 mice, aged 8–10 wk (26–30 g body wt), were obtained from the Charles River Laboratories (Wilmington, MA). All animals received care in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 86-23, rev. 1985). The animal care and use committee of the University of Tennessee Health Science Center approved the study.
Experimental conditions.
Mice were divided into three groups: a control group [spontaneously breathing room air; inspired O2 fraction (FiO2) = 0.21], a lung injury condition (LIC) group, in which mice were preexposed to HO (FiO2 = 0.9) for 12 h before 4 h of large Vt mechanical ventilation (25 μl/g) with HO, and a group in which mice were treated with an intraperitoneal injection of NAC (150 μg/g body wt) before HO preexposure, then subjected to conditions identical to the LIC group (NAC + LIC). LIC mice were injected with an equal volume of saline as a control. Positive end-expiratory pressure (PEEP) was set at zero for all mechanically ventilated animals. Our laboratory has previously reported significant lung injury produced by LIC (31).
A total of 20 mice were used in this study. Two mice (one each meant for the LIC and NAC + LIC groups) died during surgical preparation (excess blood loss) before initiation of mechanical ventilation. Each was replaced, and neither was included in the final analysis. For the remaining 18 mice, 3 were included in each of the following six groups: 1) control; 2) LIC; 3) NAC + LIC; 4) control-Flexivent; 5) LIC-Flexivent; and 6) NAC + LIC-Flexivent. Lung homogenates, bronchoalveolar lavage fluid (BALF) and histological sections were collected from groups 1–3. Flexivent data and additional lung sections for immunohistochemistry were obtained from groups 4–6.
Ventilation protocol.
Mice were anesthetized with intraperitoneal ketamine/xylazine (1:1) (0.15 ml/10 g). A tracheotomy was performed using an 18-Ga needle, and mice were ventilated via a mouse ventilator (UGO BASILE, Comario, VA, Italy). Vt was 25 μl/g in all mechanically ventilated groups, and respiratory rate was kept at 60 breaths/min. End-tidal CO2 was measured at time = 0 and every 30 min thereafter (Micro-CapnoGraph CI240 Columbus Instruments, Columbus, OH). Inspiratory gas mixture was maintained using a precision gas mixer (PEGAS 4000 MF Columbus Instruments). Heart rate and arterial oxygen saturation were monitored using a small-animal oximeter (Mouse OX STARR Life Science). A catheter was placed in the peritoneal cavity for crystalloid administration (100 μl of 0.9% NaCl at time = 0 and every 1 h thereafter). Adequate anesthesia was maintained with inhalation of 1% isofluorane. Rectal temperature was maintained within the normal range with a heat lamp.
Assessment of lung injury: respiratory system compliance.
A pressure-volume (P-V) curve was constructed for each mechanically ventilated animal, at time = 0 and 4 h, using eight equal volume steps up to total lung capacity (based on body weight), followed by an identical stepwise volume decrease back to functional residual capacity. A maximum pressure limit of 40 cmH2O was also in place and, if reached, would preclude further volume increases. “Plateau” pressure and volume were recorded at each step and used to construct the P-V curve. P-V curves were performed twice at each time point. Each set of two P-V curve measurements was preceded by two inflations to total lung capacity to insure a standard volume history for each measurement. Quasi-static compliance was estimated at time = 0 and at the end of mechanical ventilation by fitting data derived from the P-V loops to the Salazar-Knowles equation (39).
For spontaneously breathing control groups, mice were prepared for mechanical ventilation as described above and subjected to mechanics measurements after a brief period of baseline mechanical ventilation (Vt = 10 μl/g; respiratory rate = 150; PEEP = 0; FiO2 = 0.21 for ∼5 min before performing P-V curve). All measurements and calculations were performed using a Flexivent system and their proprietary software (Scireq USA, Chandler, AZ).
BALF protein concentration and cell count.
At the end of each experiment, the lungs were lavaged three times with 1.0 ml aliquots of PBS with 0.6 mM EDTA at 37°C, and the recovered fluid volume was recorded. The BALF was centrifuged at 14,000 g for 5 min at 4°C, and cell-free supernatant was removed and stored in aliquots at −80°C. Total protein was measured with the Bradford assay. Total cell counts were performed on BALF using a hemacytometer after 1:1 mix with Turk's solution.
Lung histology.
At the experiment's end, the lungs were removed and prepared for histological examination, as previously described (31). Stained lung sections (n = 3 per condition) were scored by a pathologist blinded to the experimental condition. In each section, 10 fields were randomly scored for 1) interstitial edema, 2) alveolar edema, 3) hemorrhage, and 4) neutrophil infiltrates at ×40 magnification under microscope and digitized slides. The scores from each field were averaged and presented on a scale of 0–4 (0 being none present; 4 being severe and diffuse throughout the chosen field). The histological composite scores (Fig. 1D) represent the sum of the mean injury subtype scores for each condition on a scale of 0–16 (42).
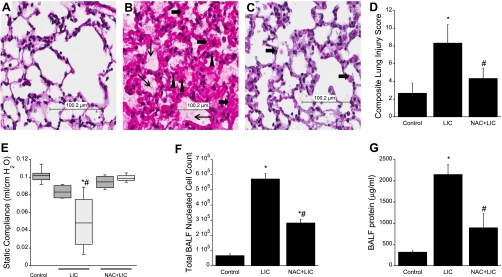
Fig. 1.
N-acetyl-cysteine (NAC)-mediated protection of lung injury. n = 3 Mice per group. Hematoxylin-eosin (H&E)-stained, formalin-fixed, paraffin-embedded lung tissues are from mice subjected to spontaneous breathing with normoxia (control; A); preexposure to hyperoxia followed by high tidal volume (Vt) mechanical ventilation (HV) with hyperoxia (HO) [lung injury conditions (LIC); B]; or NAC pretreatment followed by LIC (NAC + LIC; C) (scale 100 μM). LIC shows evidence of extensive lung injury with interstitial and alveolar edema (thin arrows), hemorrhage (▲), and acute inflammatory cell infiltration (thick arrows), whereas NAC protected mice subjected to LIC. D: composite lung injury scores (LIS) represent the sum of the mean injury subtype scores for each condition on a scale of 0–16. The LIS were significantly higher in animals exposed to LIC, whereas NAC treatment significantly reduced LIS. E: box plot of quasi-static compliance (QSC) of the three experimental conditions at time 0 and at the end of the experiment. Dark gray bars indicate time = 0 (immediately before starting mechanical ventilation); light gray bars indicate time = 4 h (immediately after end of mechanical ventilation). Horizontal lines indicate median, shaded boxes indicate inner quartile, and whiskers indicate data range. The QSC was decreased in LIC, compatible with observed increased lung injury, whereas NAC treatment prevented this decrease in QSC. Total bronchoalveolar lavage fluid (BALF) cells (F) and BALF protein concentration (G) were significantly high in LIC. NAC + LIC decreased both BALF total cell counts and also protein concentration. Values are means ± SD. *P < 0.05 compared with control. #P < 0.05 compared with LIC.
Cell culture.
MLE-12 cells (American Type Culture Collection, ATCC, Manassas, VA), an SV40-transformed mouse lung epithelial cell line of alveolar type II cell lineage, were cultured to 85–100% confluence on BioFlex plates coated with collagen type I (Flexcell International, Hillsborough, NC). Cells were cultured in DMEM containing 100 U/ml penicillin, 0.1 μg/ml streptomycin, and 10% fetal bovine serum at 37°C with 5% CO2.
Cyclic mechanical strain.
Cyclic strain was performed on MLE-12 cells using a Flexcell strain unit 4000 (Flexcell International), as previously described (31). Cell monolayers were exposed to either room air or HO (90% O2 and 5% CO2, balance N2) in a contained chamber for 12 h, followed by 4 h of cyclic stretch in HO. MLE-12 cells were stretched to 20% elongation at 30 cycles/min. These conditions were an attempt to approximate our in vivo experimental conditions. Control cells were exposed to room air and kept static throughout. Cells were preexposed to HO for 12 h, followed by 4 h of cyclic mechanical stretch (CS), both with (NAC + HO-CS) and without (HO-CS) treatment with NAC (1 mM), before cyclic stretch.
SP600125 (Sigma-Aldrich) is a specific inhibitor of JNK1,2 phosphorylation to its active form (2). To determine the effect of direct inhibition of JNK activation on apoptosis in cells exposed to HO-CS, we first performed dose-response experiments (see Fig. 7) to determine the effective dose to be used in subsequent experiments (50 μM).
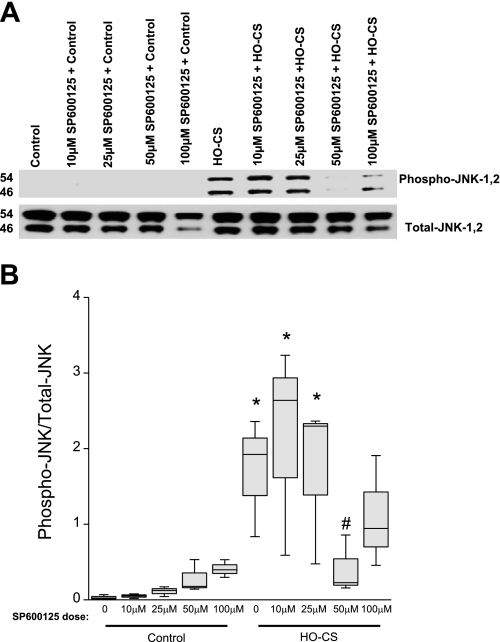
Fig. 7.
Pharmacological inhibition of JNK phosphorylation by SP600125 (A) in MLE 12 cells. n = 3 Independent experiments in which 4 wells were pooled for each condition. A: SP600125 treatment in control cells (lane 1), control cells in the presence of several doses of SP600125–10 μM (lane 2), SP600125–25 μM (lane 3), SP600125–50 μM (lane 4), and SP600125–100 μM (lane 5); HO-CS without SP600125 (lane 6), and HO-CS with several doses of SP600125 pretreatment SP600125–10 μM (lane 7), SP600125–25 μM (lane 8), SP600125–50 μM (lane 9), and SP600125–100 μM (lane 10). B: box plot of densitometry of the Western blot in A shows that the 50 μM dose of SP600125 significantly inhibited JNK phosphorylation. Horizontal lines indicate median, shaded boxes indicate inner quartile, and whiskers indicate data range. *P < 0.05 compared with control. #P < 0.05 compared with HO-CS.
Immunoblot (Western) analysis.
The left lung of each mouse was removed, homogenized (OMNI TH tissue homogenizer OMNI International) with 1 ml of lung lysis buffer [50 mM Tris (pH 7.4), 4 mM KCl, 1% Nonidet P-40 (vol/vol), 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 0.2 U/ml aprotinin, 1 μg/ml pepstatin, 1 mM Na3VO4, 1 mM NaF], sonicated for 10 s (20 pulses) (OMNI Ruptor 250 ultrasonic homogenizer OMNI International), and prepared for Western analysis, as previously described (31). For the in vitro experiments, MLE-12 cells were lysed on ice using RIPA buffer (50 mM Tris·HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS) with protease inhibitor cocktail (Roche). Lysates were centrifuged at 4°C and 16,000 g for 15 min and processed for Western blot analysis, as previously reported (31). Primary antibodies anti-procaspase and cleaved caspases-3, poly(ADP-ribose) polymerase-1 (PARP-1), phospho-JNK and total JNK, cytochrome c, cytochrome-c oxidase subunit IV (COX IV), and GAPDH were obtained from Cell Signaling Technology and incubated overnight at 4°C. Proteins were visualized with horseradish peroxidase-coupled anti-rabbit IgG secondary antibody (Cell Signal Technology) and Luminol chemiluminescent substrate (Thermo Fisher Scientific, Rockford, IL). Densitometry analysis was performed with Image J 1.42 image processing software (National Institutes of Health; Web link; http://rsbweb.nih.gov/ij/) using gel analysis method.
Tissue immunofluorescence and confocal microscopy.
Lung tissue immunofluorescence was performed as previously described (33). Lung sections were incubated overnight in a moist chamber with 200 μl of 1:100 anti-cleaved caspase-3, PARP-1 (Asp214) antibody (murine specific; Cell Signaling Technology, Danvers, MA), and 8-hyroxy-2-guanosine (SantaCruz Biotechnology, Santa Cruz, CA). The primary antibody was removed and washed three times with PBS-Tween 20 (0.01% Tween 20 in PBS) and incubated with 200 μl of 2 μg/ml secondary antibody conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA) for 1 h. Slides were mounted with Vectashield and stored at −20°C in the dark. Confocal images were obtained 48 h later using a BioRad MRC 1024 (Hercules, CA) imaging system, attached to BX50 (Olympus, Downington, PA) microscope. Fluorophores were excited using the 488-nm line of a Krypton/Argon laser. Emitted fluorescence was collected using a ×60 objective lens (NA-1.4 oil), and the images were recorded using LaserSharp2000 software (BioRad, Hercules, CA). All images were captured using the same gain/intensity settings.
Statistical analysis.
All values are expressed as mean ± SD, unless otherwise specified. If data passed both a normality test and an equal variance test, then one-way ANOVA was used to compare mean values for the different groups, followed by Scheffé's test for multiple comparisons between groups, with significance set at P < 0.05. Data that failed either the normality or equal variance test were considered nonparametric data and analyzed using the Kruskal-Wallis one-way analysis of variance on ranks with post hoc pairwise comparisons made using the Holm-Sidak method. Nonparametric data are presented using box plots showing median, range, and interquartile. All statistics were performed using SigmaStat 3.5 software (Systat Software, San Jose, CA).
RESULTS
NAC pretreatment reduced lung injury caused by LIC.
We compared severity of lung injury in the presence and absence of NAC by evaluating lung histology (hematoxylin-eosin, Fig. 1, A–C), composite lung injury scores (Fig. 1D), lung physiological changes (static compliance, Fig. 1E), total BALF cell counts (Fig. 1F), and protein concentrations (Fig. 1G). Figure 1 shows photomicrographs of hematoxylin-eosin-stained lung sections from control (Fig. 1A), LIC (Fig. 1B), and NAC + LIC (Fig. 1C) mice. The lung sections of control mice show normal lung parenchyma (Fig. 1A), whereas LIC lungs (Fig. 1B) show severe and diffuse alveolar and interstitial edema (thin arrows), neutrophil infiltration (solid triangles), and hemorrhage (thick arrows). In contrast, in NAC + LIC lungs, identical injury conditions (Fig. 1C) resulted in markedly decreased alveolar and interstitial edema, neutrophil infiltration, and hemorrhage (n =3 mice/group). Although some injury was observed in the NAC + LIC group, composite lung injury scores (Fig. 1D) were significantly lower compared with LIC. Lung mechanics were similar in all experimental groups at 0 h, but the static compliance markedly decreased in LIC mice after 4 h of mechanical ventilation compared with control. NAC pretreatment ameliorated this deterioration in lung mechanics (Fig. 1E) (n =3 mice/group). A significant increase in total BALF cells and protein concentration was observed in mice exposed to LIC compared with control mice (Fig. 1, F and G). A significant decrease in BALF total cell count (Fig. 1F) and protein concentration (Fig. 1G) was observed in NAC + LIC compared with LIC.
NAC pretreatment protected the lung from oxidative stress.
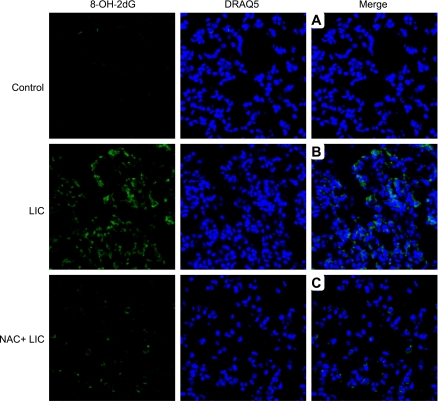
Next, we tested the hypothesis that lung injury caused by LIC was mediated through ROS. We evaluated oxidative modification of DNA using anti-8-hydroxy-2-deoxyguanosine (8-OH-dG) antibody. 8-OH-dG is a product of oxidative damage of DNA by reactive oxygen and nitrogen species and serves as a well-known marker of oxidative stress (47). As shown in Fig. 2, immunofluorescence of lung sections demonstrates that 8-OH-dG staining was more abundant in mice exposed to LIC (Fig. 2B) compared with controls (Fig. 2A). NAC + LIC showed a marked reduction in 8-OH-dG staining (Fig. 2C).
Fig. 2.
Confocal images of 8-hydroxy 2-deoxy guanosine (8-OH-2dG) immunofluorescence-stained lung sections of control mice (top row), LIC (middle row), and NAC + LIC (bottom row). n = 3 Individual sections per group. 8-OH-2dG was stained with Alexa (green), and nuclei were stained with DRAQ5 (blue). Merged images for control (A), LIC (B), and NAC + LIC (C) are in the right-hand column. Control produced no positive staining for 8-OH-2dG, while sections of LIC mouse lungs showed positive staining for 8-OH-2dG. NAC + LIC markedly reduced the positive cells for 8-OH-2dG.
NAC pretreatment inhibited cleavage of caspase-3 and PARP-1 in vivo.
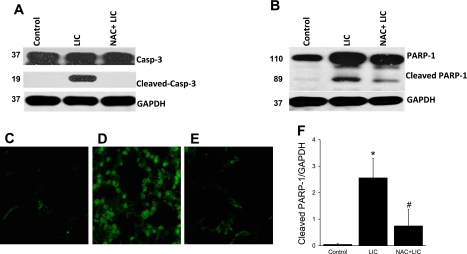
Our laboratory recently reported an association of lung injury and apoptosis in mice exposed to 12 h of HO, followed by large Vt mechanical ventilation (31). In the present study, we performed Western blotting of cleaved caspase-3 and PARP-1 to determine whether NAC pretreatment could prevent apoptosis by reducing oxidative stress. Caspase-3 is an executioner caspase that activates PARP-1, a nuclear enzyme and marker of apoptosis (26, 41). Mice subjected to LIC exhibited increased cleavage of both caspase-3 (Fig. 3A) and PARP-1 (Fig. 3B). NAC + LIC completely inhibited the activation of caspase-3 (Fig. 3A) and significantly reduced PARP-1 cleavage (Fig. 3, B and F) compared with LIC alone. Western blots were performed on whole lung homogenates and, therefore, gave no information as to which cell types were undergoing apoptosis. To determine the affected cell type(s), we performed immunofluorescence staining of cleaved PARP-1. Consistent with our laboratory's previous study (31), mice in the LIC group exhibited significant staining with anti-cleaved PARP-1, and this was predominantly localized in epithelial cells (Fig. 3D). NAC pretreatment markedly reduced staining for cleaved PARP-1 under the same injury conditions as LIC (Fig. 3E).
Fig. 3.
Cleaved caspase-3 (A) and its downstream substrate poly(ADP-ribose) polymerase-1 (PARP-1; B and D) in whole lung homogenates of mice subjected to control (lane 1), LIC (lane 2), and NAC + LIC (lane 3). n = 3 Mice per group. Significantly increased cleavage of caspase-3 and PARP-1 was observed in LIC. NAC + LIC completely inhibited caspase-3 cleavage. Immunofluorescent confocal image of cleaved PARP-1 in control (C), LIC (D), NAC + LIC (E) F: cleaved PARP-1 is increased in LIC compared with control. NAC pretreatment markedly reduced staining for cleaved PARP-1 under the same conditions as LIC. *P < 0.05 compared with control. #P < 0.05 compared with LIC.
NAC pretreatment inhibited apoptosis in MLE-12 cells.
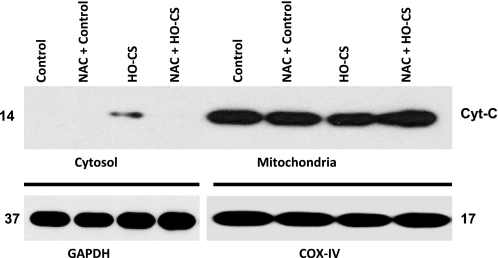
Cytochrome c release from the mitochondria into the cytoplasm is an indicator of mitochondrial-mediated apoptosis (43). To determine whether NAC pretreatment inhibited apoptosis in cultured alveolar epithelial cells, we exposed MLE-12 cells to experimental conditions, as described in materials and methods, and lysates were separated into cytosolic and mitochondrial fractions. Western blotting was performed using anti-cytochrome c antibody (Cell Signaling Technologies), and GAPDH was used as a loading control for cytosolic fraction, whereas COX IV was used as mitochondrial loading control. Cytochrome c release from mitochondria into cytoplasm was observed only in HO-CS. Under the same conditions, cytochrome c release was completely blocked by NAC (Fig. 4) (n = 3 experiments/group).
Fig. 4.
Cytochrome c (Cyt-C) release from mitochondria in MLE-12 cells subjected to control, HO-cyclic mechanical stretch (CS), and NAC + HO-CS (1 mM). Cytoplasmic and mitochondrial fractions were separated for Western blotting. Top: cytoplasmic fractions are shown of control (lane 1), NAC + control (lane 2), HO-CS (lane 3), and NAC + HO-CS (lane 4); mitochondrial fractions are shown of control (lane 5), NAC + control (lane 6), HO-CS (lane 7), and NAC + HO-CS (lane 8). Bottom: GAPDH was the loading control for cytoplasmic fractions (lanes 1–4), and cytochrome-c oxidase subunit IV (COX IV) was the loading control for mitochondrial fractions (lanes 5–8). n = 3 Independent experiments in which 4 wells were pooled for each condition. Cytochrome c release was observed in HO-CS, whereas NAC + HO-CS completely blocked cytochrome c release from mitochondria.
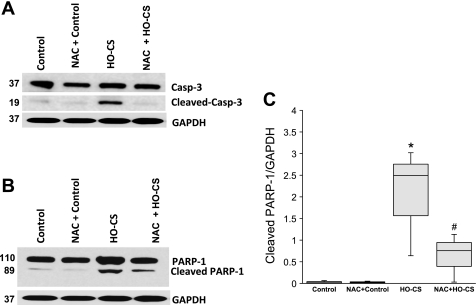
We further examined cleavage of caspase-3 and PARP-1, the downstream activators of apoptosis, by Western blotting in this in vitro model. Consistent with the in vivo data, significant caspase-3 (Fig. 5A) and PARP-1 cleavage (Fig. 5B) were seen in MLE-12 cells exposed to HO-CS. Pretreatment with NAC completely inhibited cleavage of caspase-3 and significantly reduced PARP-1 cleavage (Fig. 5C).
Fig. 5.
Cleaved caspase-3 (A) and its downstream substrate PARP-1 (B) in mouse lung epithelial cells (MLE-12) subjected to control (lane 1), NAC + control (lane 2), HO-CS (lane 3), and NAC + HO-CS (lane 4). Significant cleavage of caspase-3 and PARP-1 was observed in HO-CS. In NAC + HO-CS, activation of caspase-3 was completely inhibited. C: box plots of cleaved PARP-1 densitometry show NAC + HO-CS significantly reduced cleaved PARP-1 compared with HO-CS. Horizontal lines indicate median, shaded boxes indicate inner quartile, and whiskers indicate data range. Representative blots and densitometry were from 3 independent experiments in which 4 wells were pooled for each condition. *P < 0.05 compared with control. #P < 0.05 compared with HO-CS.
NAC pretreatment inhibited JNK activation in both in vivo and in vitro models.
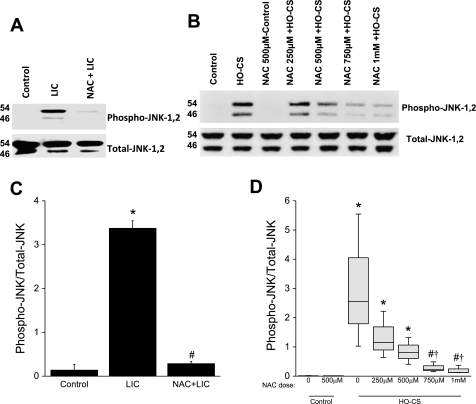
To test our hypothesis that JNK activation mediates apoptosis due to LIC, we performed Western blotting to determine JNK activation (phosphorylation) in each experimental group. JNK is a mitogen-activated protein kinase, which responds to various stress stimuli and induces inflammation and apoptosis (18, 49). As shown in Fig. 6A, increased JNK phosphorylation was observed in LIC mice compared with controls. NAC pretreatment significantly inhibited JNK phosphorylation in NAC + LIC mice (Fig. 6C). We further investigated JNK activation in our in vitro injury model. MLE-12 cells exposed to HO-CS produced increased JNK activation. In NAC + HO-CS cells, activation of JNK was inhibited in a dose-dependent fashion [Fig. 6B, (densitometry Fig. 6D)].
Fig. 6.
A: phosphorylation of JNK in mice subjected to control (lane 1), LIC (lane 2), and NAC + LIC (lane 3). n = 3 Mice per group. Phosphorylation of JNK was significantly increased in LIC. In NAC + LIC, phosphorylation of JNK was significantly inhibited. B: phosphorylation of JNK in MLE-12 cells subjected to control (lane 1), NAC + control (lane 3), LIC (lane 2), and NAC + HO-CS at several different doses; HO-CS + 200 μM NAC (lane 4), 500 μM NAC (lane 5), 750 μM NAC (lane 6), and 1 mM NAC (lane 7). JNK phosphorylation was observed in HO-CS, and a dose-dependent inhibition of JNK phosphorylation was observed in HO-CS + NAC. C: densitometry for phospho-JNK Western blots from whole lung homogenates. NAC treatment significantly inhibited phospho-JNK in vivo. D: box plots for densitometry of phospho-JNK Western blots from MLE-12 cell lysates. Horizontal lines indicate median, shaded boxes indicate inner quartile, and whiskers indicate data range. In these in vitro experiments, a dose-dependent inhibition of JNK phosphorylation was observed with NAC treatment. n = 3 Mice per group (in vivo) and 3 independent experiments in which 4 wells were pooled for each condition (in vitro). *P < 0.05 compared with control. #P < 0.05 compared with LIC (in vivo) or HO-CS (in vitro). †P < 0.05 compared with HO-CS 250 μM NAC.
JNK inhibitor (SP600125) blocked cytochrome c release, caspase-3, and PARP-1 cleavage in vitro.
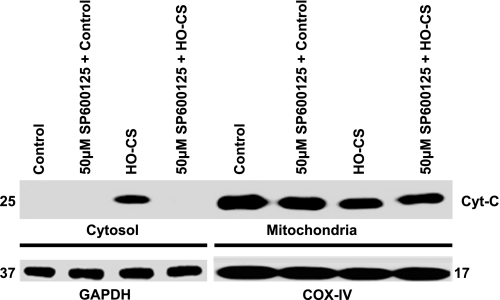
To confirm whether JNK activation was associated with the observed caspase-3 and PARP-1-mediated apoptosis when MLE-12 cells were subjected to HO-CS, we pretreated MLE-12 cells with SP600125, a potent and specific inhibitor of JNK1, 2, and 3 (2) before exposure to HO-CS. To estimate the inhibition of JNK1 and 2, Western blotting was performed using anti-phospho-JNK1, 2, and anti-total JNK antibodies. SP600125 inhibited the activation of JNK1 and 2 in a dose-dependent fashion (Fig. 7). We selected the 50 μM concentration of SP600125 to use in subsequent experiments. Consistent with NAC + HO-CS data, pretreatment with SP600125 completely blocked cytochrome c release from mitochondria compared with HO-CS alone (Fig. 8).
Fig. 8.
Cytochrome c release from mitochondria in MLE-12 cells subjected to control, SP600125 + control, HO-CS, and SP600125 + LIC. Cytoplasmic and mitochondrial fractions were separated, and Western blotting performed. Top: cytoplasmic fractions of control (lane 1), SP600125 + control (lane 2), HO-CS (lane 3), and SP600125 + HO-CS (lane 4); mitochondrial fractions of control (lane 5), SP600125 + control (lane 6), HO-CS (lane 7), and SP600125 + HO-CS (lane 8). Bottom: GAPDH was the loading control for cytoplasmic fractions (lanes 1–4), and COX IV was the loading control for mitochondrial fractions (lanes 5–8). n = 3 Independent experiments, and 4 wells were pooled for each condition. Cytochrome c release was observed in HO-CS, whereas SP600125 + HO-CS completely blocked cytochrome c release from mitochondria. SP600125 dose was 50 μM final concentration based on dose-response data.
DISCUSSION
Although several studies have reported more severe lung injury with combined HO and HV than with either insult alone (28, 30, 31, 36, 42), the underlying mechanisms of the observed lung injury have not been clearly elucidated. Our laboratory recently reported augmented lung injury caused by preexposure to HO followed by HV and its association with epithelial cell apoptosis (31). Although our laboratory's previous study did not provide a causal link between apoptosis and lung injury, it determined that HO preexposure “primed” the lungs for injury caused by subsequent exposure to HV. In the present study, we show a strong association between injury and apoptosis. Using both in vivo and in vitro injury models, we demonstrate that treatment with NAC, a potent antioxidant and glutathione precursor, inhibits ROS production, JNK phosphorylation, epithelial cell apoptosis, and lung injury. Furthermore, we show that both the observed lung injury and apoptosis are dependent on activation of JNK.
This experimental model has been previously published (31) and is both stable and reproducible. Additionally, given that patients admitted to the hospital with respiratory failure are usually treated with supplemental oxygen for hours to days before initiation of mechanical ventilation, we feel that this model is more clinically relevant than many reported VILI models that do not account for HO before or during mechanical ventilation. We chose this large Vt model of mechanical ventilation-induced lung injury for several reasons. First, it is compatible with multiple previously published models in this field (1, 6, 9, 16, 23). Second, it allows isolation of a single additional variable (HO) and its impact on the development of lung injury without clouding the results with other potential variables (i.e., saline lavage, oleic acid, LPS, bacterial infection, PEEP level, etc.), and, third, unlike patients with acute respiratory distress syndrome (ARDS)/ALI, we are ventilating animals with normal lungs before mechanical ventilation (no significant injury seen with 12 h of HO preexposure). However, the experimental model and study design have several potential shortcomings.
We did not include all of the possible control groups in this study. In our first report using this experimental model (31), we found that only mice exposed to 12 h of HO, followed by 4 h of HV, produced significant lung injury and apoptosis. No significant increase in lung injury or apoptosis was observed with HO alone, 4 h of HV alone, 4 h of combined HO and HV, or with 12 h of HO followed by 4 h of low Vt (6 ml/kg) mechanical ventilation compared with controls. In the present study, our objective was to characterize the effect of antioxidant treatment (NAC) on the observed injury phenotype. Therefore, to reduce animals used, we limited the study to conditions in which this injury phenotype was observed, both with and without NAC treatment. Additionally, we also limited our animal numbers per experimental group to the minimum number required to achieve a statistically significant difference in our primary outcome measure (lung injury score) between LIC and controls.
We also recognize that, unlike others, we reproducibly see no significant lung injury or apoptosis with 4 h of HV alone. This may be due to variations in experimental technique, particularly exposure to HO before mechanical ventilation, species/strain differences, or how lung injury is assessed. Other studies that specifically looked at oxygen as a variable in VILI also showed significantly less lung injury with room air ventilation compared with ventilation with HO (3, 28). The large Vt values used in this study are clearly not clinically applicable, especially in light of the proven mortality benefit with low Vt ventilation in ARDS (1). In the ARDS lung, spatial heterogeneity of injury results in very diseased lung regions, primarily in dependent regions, and relatively normal lung regions (19). During mechanical ventilation, the relatively unaffected lung with its higher regional compliance will be more distended for a given Vt than its diseased counterparts. Higher Vt values are, therefore, required to mimic these conditions experimentally with lungs that are uninjured before initiation of mechanical ventilation.
It is also unlikely that a patient with ALI would be ventilated without the benefit of PEEP. In addition, most patients with ARDS or ALI have another risk factor (i.e., sepsis, trauma, aspiration, etc.) for lung injury besides mechanical ventilation. The experimental protocol was also limited to 4 h of mechanical ventilation, which may be insufficient time to document prevention vs. delay in the development of VILI.
Our in vitro stretched cell model obviously does not directly reproduce what occurs in the in vivo setting. First, we use a transformed murine alveolar type II cell line, which does not behave identically to primary alveolar epithelial cells. Second, these cells are grown in submersion culture rather than at an air-liquid interface, so they are not polarized, as they would be in vivo. Unfortunately, an adequate substrate that would allow air-liquid interface culture and significant cyclic stretch is not currently available. Third, the culture system only includes alveolar type II-like cells and does not account for the potential effects of other cells, such as alveolar macrophages, alveolar type I cells, etc. Finally, these cells were grown on collagen type 1 coated silastic-bottomed plates. The actual alveolar-capillary basement membrane contains multiple components, including collagen, laminin-enactin/nidogen complexes, and proteoglycans (34). NAC has been reported to be protective in many drug-induced toxicities and other diseases, especially liver injury caused by acetaminophen (48), carbon tetra chloride (37), and antituberculosis drugs (4). In the lung, Sciuto et al. (40) reported that NAC treatment protects rabbit lungs after phosgene exposure. Hagiwara et al. (21) reported NAC was effective in preventing lung fibrosis in animal models. NAC also exerts protective effects and prevents lung redox imbalance and peroxynitrite generation in endotoxemic rats (10).
To test our hypothesis that NAC could exert a protective effect on lung injury and apoptosis by reducing ROS, we administered intraperitoneal NAC just before HO exposure. As shown in the Fig. 1, C and D, NAC significantly inhibited edema, hemorrhage, and neutrophil influx. A likely reason for the inhibition of lung injury by NAC is the suppression of ROS. Many studies have reported that both HO and lung stretch independently or combined could induce ROS production (7, 8, 12, 35). To confirm whether the injury conditions in this model increased ROS production, we measured 8-hydroxy 2-guanosine, an oxidative DNA marker. Our results (Fig. 2) indicate that LIC increased ROS production, and this was effectively decreased by NAC treatment. In this model of lung injury, the NAC-induced reduction in ROS was associated with marked reduction in lung injury. Syrkina et al. (45) reported that 20 ml/kg ventilation in rats produced ALI associated with increased serum isoprostane and decreased lung glutathione, the major antioxidant in the lung. NAC treatment prevented the decrease in lung glutathione, significantly lowered serum isoprostane levels, and reduced lung injury, including lung neutrophil infiltration, bronchoalveolar lavage cytokine concentration, and airway epithelial apoptosis. Davreux et al. (15) studied the effect of NAC on LPS-induced lung injury in a rat model and reported significant attenuation of LPS-induced lung permeability and lipid peroxidation. Although neutrophil influx was unaffected, neutrophil activation was significantly downregulated by NAC. In our study, LIC induced significant lung injury, as indicated by decreased lung compliance, increased BALF cell counts and protein concentrations, and lung histology, all of which were markedly attenuated by NAC pretreatment (Fig. 1, B and D–G).
Our next objective was to evaluate whether inhibition of lung injury by NAC could also inhibit apoptosis. We evaluated the activation of the executioner caspase, caspase-3, and its downstream substrate PARP-1 as evidence of apoptosis. NAC pretreatment inhibited cleavage of caspase-3 and PARP-1 in vivo, indicating ROS as an important upstream activator of apoptosis and lung injury. In our laboratory's previous report (31), preexposure to HO followed by HV induced apoptosis in alveolar epithelial cells. In the present study, immunofluorescence (Fig. 3D) confirmed that cleaved PARP-1 was seen primarily in epithelial cells and was suppressed by NAC pretreatment (Fig. 3E). These findings were confirmed in our in vitro epithelial cell injury model (Fig. 5). The presence of some PARP-1 activation after NAC treatment is likely due to noncaspase-dependent mechanisms (24).
We have shown cytochrome c release from mitochondria in response to HO-CS, which is characteristic of mitochondrial-mediated apoptosis. NAC pretreatment inhibited cytochrome c release in HO-CS, suggesting involvement of ROS in alveolar epithelial apoptosis (Fig. 4). Many studies have reported cytochrome c release from mitochondria as a hallmark of early apoptosis via the intrinsic pathway that requires JNK activation (17, 22, 25, 46). Mice, as well as MLE-12 cells exposed to LIC or HO-CS, respectively, produced increased JNK phosphorylation (Fig. 6). NAC dose-dependently reduced JNK phosphorylation, indicating ROS-dependent JNK activation plays an important role in the observed mitochondrial-mediated apoptosis. However, Fas R (receptor)-mediated caspase-8 cleavage can also induce apoptosis via the mitochondrial pathway (20, 50). To confirm the essential role of JNK in this model, we studied the effect of pretreatment with the specific JNK inhibitor SP600125 on mitochondrial cytochrome c release in MLE-12 cells exposed to HO-CS. Pretreatment with SP600125 completely inhibited cytochrome c release, confirming the importance of JNK in this model (Fig. 8).
We are certainly not the first to suggest that antioxidants, particularly NAC, might help prevent or treat ALI or VILI. In fact, antioxidants studied in small clinical trials of ALI have been encouraging (5, 32, 44). However, until a large multicenter trial is performed, their clinical efficacy in ALI remains uncertain. Our primary rationale for NAC treatment was to demonstrate the importance of oxidative signaling in the development of alveolar epithelial apoptosis and lung injury in this model.
In conclusion, this study reveals a strong association between ROS production, lung injury, and apoptosis in this model of lung injury and provides evidence that JNK activation plays a significant role. Further studies are required to better understand the causal link between lung injury and apoptosis.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grants HL-081297, HL-094366, and HL-75503.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Linda White for technical assistance with immunohistochemistry and Crystal Stanton and Dr. Anand Kulkarni for assistance with the slide digitizer.
REFERENCES
- 1. An L, Liu CT, Qin XB, Liu QH, Liu Y, Yu SY. Protective effects of hemin in an experimental model of ventilator-induced lung injury. Eur J Pharmacol 661: 102–108, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Assi K, Pillai R, Gomez-Munoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology 118: 112–121, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey TC, Martin EL, Zhao L, Veldhuizen RA. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J Appl Physiol 94: 975–982, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, Velayati AA. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol 22: 1235–1238, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 112: 164–172, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 155: 44–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 34: 453–463, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem 279: 6753–6760, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cannizzaro V, Hantos Z, Sly PD, Zosky GR. Linking lung function and inflammatory responses in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 300: L112–L120, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Carbonell LF, Diaz J, Hernandez I, Cuevas S, Valero F, Quesada T, Fenoy F, Salom MG. N-acetylcysteine exerts protective effects and prevents lung redox imbalance and peroxynitrite generation in endotoxemic rats. Med Chem 3: 29–34, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L834–L841, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Chen LW, Chang WJ, Wang JS, Hsu CM. Interleukin-1 mediates thermal injury-induced lung damage through C-Jun NH2-terminal kinase signaling. Crit Care Med 35: 1113–1122, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Davreux CJ, Soric I, Nathens AB, Watson RW, McGilvray ID, Suntres ZE, Shek PN, Rotstein OD. N-acetyl cysteine attenuates acute lung injury in the rat. Shock 8: 432–438, 1997 [PubMed] [Google Scholar]
- 16. dos Santos CC, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med 183: 471–482, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res 63: 5940–5949, 2003 [PubMed] [Google Scholar]
- 18. Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science 265: 806–808, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Gattinoni L, Mascheroni D, Torresin A, Marcolin R, Fumagalli R, Vesconi S, Rossi GP, Rossi F, Baglioni S, Bassi F, et al. .Morphological response to positive end expiratory pressure in acute respiratory failure. Computerized tomography study. Intensive Care Med 12: 137–142, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Gong XM, Choi J, Franzin CM, Zhai D, Reed JC, Marassi FM. Conformation of membrane-associated proapoptotic tBid. J Biol Chem 279: 28954–28960, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162: 225–231, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem 275: 26576–26581, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Huang CS, Kawamura T, Lee S, Tochigi N, Shigemura N, Buchholz BM, Kloke JD, Billiar TR, Toyoda Y, Nakao A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit Care 14: R234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson DE. Noncaspase proteases in apoptosis. Leukemia 14: 1695–1703, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371: 346–347, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Lee HS, Kim HJ, Moon CS, Chong YH, Kang JL. Inhibition of c-Jun NH2-terminal kinase or extracellular signal-regulated kinase improves lung injury. Respir Res 5: 23, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care 11: R25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA. Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 29: 779–783, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-kappaB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res 154: 228–240, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Makena PS, Luellen CL, Balazs L, Ghosh MC, Parthasarathi K, Waters CM, Sinclair SE. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol 299: L711–L719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 236: 814–822, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 116: 2193–2200, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol 27: 93–127, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 93: 517–525, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Ritter C, Reinke A, Andrades M, Martins MR, Rocha J, Menna-Barreto S, Quevedo J, Moreira JC, Dal-Pizzol F. Protective effect of N-acetylcysteine and deferoxamine on carbon tetrachloride-induced acute hepatic failure in rats. Crit Care Med 32: 2079–2083, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Romashko J, 3rd, Horowitz S, Franek WR, Palaia T, Miller EJ, Lin A, Birrer MJ, Scott W, Mantell LL. MAPK pathways mediate hyperoxia-induced oncotic cell death in lung epithelial cells. Free Radic Biol Med 35: 978–993, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964 [DOI] [PubMed] [Google Scholar]
- 40. Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med 151: 768–772, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Shah GM, Shah RG, Poirier GG. Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells. Biochem Biophys Res Commun 229: 838–844, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med 32: 2496–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Skulachev VP. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett 423: 275–280, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest 105: 190–194, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Syrkina O, Jafari B, Hales CA, Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology 13: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288: 870–874, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27: 120–139, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Vale JA, Meredith TJ, Crome P, Helliwell M, Volans GN, Widdop B, Goulding R. Intravenous N-acetylcysteine: the treatment of choice in paracetamol poisoning? Br Med J 2: 1435–1436, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi H, Bhalla K, Wang HG. Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res 63: 1483–1489, 2003 [PubMed] [Google Scholar]