Abstract
We and others have shown that moderate passive whole body heating (i.e., increased internal temperature ∼0.7°C) increases muscle (MSNA) and skin sympathetic nerve activity (SSNA). It is unknown, however, if MSNA and/or SSNA continue to increase with more severe passive whole body heating or whether these responses plateau following moderate heating. The aim of this investigation was to test the hypothesis that MSNA and SSNA continue to increase from a moderate to a more severe heat stress. Thirteen subjects, dressed in a water-perfused suit, underwent at least one passive heat stress that increased internal temperature ∼1.3°C, while either MSNA (n = 8) or SSNA (n = 8) was continuously recorded. Heat stress significantly increased mean skin temperature (Δ∼5°C, P < 0.001), internal temperature (Δ∼1.3°C, P < 0.001), mean body temperature (Δ∼2.0°C, P < 0.001), heart rate (Δ∼40 beats/min, P < 0.001), and cutaneous vascular conductance [Δ∼1.1 arbitrary units (AU)/mmHg, P < 0.001]. Mean arterial blood pressure was well maintained (P = 0.52). Relative to baseline, MSNA increased midway through heat stress (Δ core temperature 0.63 ± 0.01°C) when expressed as burst frequency (26 ± 14 to 45 ± 16 bursts/min, P = 0.001), burst incidence (39 ± 13 to 48 ± 14 bursts/100 cardiac cyles, P = 0.03), or total activity (317 ± 170 to 489 ± 150 units/min, P = 0.02) and continued to increase until the end of heat stress (burst frequency: 61 ± 15 bursts/min, P = 0.01; burst incidence: 56 ± 11 bursts/100 cardiac cyles, P = 0.04; total activity: 648 ± 158 units/min, P = 0.01) relative to the mid-heating stage. Similarly, SSNA (total activity) increased midway through the heat stress (normothermia; 1,486 ± 472 to mid heat stress 6,467 ± 5,256 units/min, P = 0.03) and continued to increase until the end of heat stress (11,217 ± 6,684 units/min, P = 0.002 vs. mid-heat stress). These results indicate that both MSNA and SSNA continue to increase as internal temperature is elevated above previously reported values.
Keywords: muscle sympathetic nerve activity, neural control, skin sympathetic nerve activity
heat stress elicits a range of highly coordinated autonomic nervous system responses to maintain both internal temperature and blood pressure within safe limits (32). Heat stress-induced elevations in internal and skin temperatures profoundly increase skin blood flow and sweat rate, which are necessary for heat dissipation (18, 40). At the same time, elevations in cardiac output and vascular resistance of noncutaneous beds ensure the maintenance of arterial blood pressure (34, 36). Appropriate adjustments of the autonomic nervous system are critical in mediating these cardiovascular and thermoregulatory responses to heat stress. Given that these neural responses are primarily governed by increases in sympathetic nervous system activity, heat stress has been referred to as a “hyperadrenergic” state (33).
Whole body heating causes pronounced increases in skin sympathetic nerve activity (SSNA), which is the neural signal responsible for increasing sudomotor and perhaps cutaneous active vasodilator activity during this exposure (16, 30). Similarly, heat stress elevates sympathetic nerve activity to muscle, i.e., muscle sympathetic nerve activity (MSNA). We and others have shown that moderate passive whole body heating in humans (i.e., increased internal temperature ∼0.7°C) increases MSNA and SSNA (1, 6–8, 29, 46). We are unaware of any studies in which MSNA or SSNA responses to more severe heat stress were evaluated. It is unknown, therefore, if MSNA and/or SSNA continue to increase with further increases in internal temperature, when even greater demands are placed on the thermoregulatory and cardiovascular systems, or whether these responses plateau following moderate increases in internal temperature.
Progressive increases in renal, lumbar, and splanchnic sympathetic nerve activity during passive heat stress in rats have been recorded over the course of 3°C elevations in core temperature (20, 21, 23). Based upon these observations, it may be that in humans SSNA and MSNA would continue to rise beyond previously reported elevations in core temperature of ∼0.7°C. Similarly, sympathoexcitation to non-heat-stress interventions, such as hypoxia and exercise, increase in proportion to graded levels of that intervention (37, 39), further supporting the hypothesis of a continued increase in sympathetic nerve activity with increasing severity of a sympathoexcitatory stimulus, as may be the case with passive heating.
Changes in skin blood flow and sweat rate are closely coupled to alterations in internal temperature (18, 40). The skin blood flow/sweat rate to internal temperature relationship is generally not linear throughout heat stress, as the slope of these relationships is often reduced or tends to plateau with further elevations in internal temperature greater than ∼0.7°C (17, 40). It is thus possible that SSNA plateaus, or continues to increase, albeit at a reduced rate, beyond relatively minor elevations in internal temperature. In addition, total peripheral resistance and blood flows to renal and splanchnic vascular beds tend to decrease at a lower rate as internal temperature further increases beyond moderate levels of passive heating (28, 35). If MSNA is reflective of sympathetic activity to these regions, these findings suggest that relatively minor increases in MSNA would occur beyond moderate levels of passive heating. To address these questions, the aim of this investigation was to test the hypothesis that during passive heating MSNA and SSNA continue to increase while individuals progress from a moderate to a more severe level of heat stress.
METHODOLOGY
Subjects.
A total of 13 healthy subjects (8 men, 5 women; mean ± SD; age 35 ± 9 yr, height 175 ± 10 cm and weight 78 ± 10 kg) underwent one or two passive heat stresses during which successful MSNA (n = 8; 5 men) or SSNA (n = 8; 4 men) recordings were obtained throughout the heat stress. Three subjects participated in both MSNA and SSNA trials, but on different days. MSNA and SSNA recordings from male and female subjects were not separately analyzed given the low numbers in each group and sex differences not being the primary objective of the evaluation. Subjects were free from cardiovascular, neurological, or metabolic diseases. Subjects were asked to refrain from alcohol and exercise 24 h and caffeine 12 h before the study. Written informed consent was obtained from all participants before they enrolled in the study. All procedures and the consent form were approved by the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas.
Instrumentation and measurements.
Each subject was placed in a tube-lined suit (Med-Eng, Ottawa, Canada) that permitted the control of skin temperature by changing the temperature of the water perfusing the suit. The suit covered the entire body surface with the exception of the head, one forearm, the hands, the lower leg from which sympathetic recordings were obtained, and the feet. Mean skin temperature was measured via the electrical integration of six thermocouples attached to the skin on the lateral gastrocnemius, anterior quadriceps, lower and upper back, abdomen, and upper chest regions (42). Core temperature was measured from an ingestible telemetry pill (HTI Technologies, Palmetto, FL) that was swallowed at least 2 h before data collection began. Heart rate was obtained from an electrocardiogram (SpaceLabs, Redmond, WA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Intermittent arterial blood pressure was measured from the brachial artery by electrosphygmomanometry (SunTech, Raleigh, NC). Cutaneous blood flow was indexed using a multifiber laser-Doppler probe (Perimed, North Royalton, OH) placed on a dorsal forearm not covered by the suit.
Multifiber recordings of MSNA and SSNA were obtained using a tungsten microelectrode positioned in the common peroneal nerve. A reference electrode was placed subcutaneously ∼2–3 cm from the recording electrode. The position of the recording electrode was adjusted until a site was attained in which bursts of MSNA or SSNA were identified using previously established criteria (13, 43). The main criteria for the identification and differentiation of MSNA relative to SSNA recordings were 1) synchronicity of discharges with pulse rate, 2) increases in (or lack of) activity during inspiratory apnea, and 3) responsiveness to mental or somatosensory stimulation (e.g., loud sound and light stroking of the innervated region). Needle adjustments were made for recordings that contained evidence of both MSNA and SSNA, until just MSNA or just SSNA recordings were evident. Nerve signals were amplified, passed through a bandpass filter with a band width of 700–2,000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA). Mean voltage neurograms were displayed on a chart recorder. Nerve signals were also routed to an oscilloscope and a loudspeaker for monitoring throughout the study. If evidence of a position change in the recording electrode was identified, which occurred in a few subjects, the protocol was immediately ended and these data were not analyzed. Subjects in whom this occurred are not included in the aforementioned subject numbers.
Experimental protocols.
Experiments were performed in a temperature-controlled laboratory (26 ± 1°C) in the morning or early afternoon at least 2 h postprandial. After instrumentation, subjects rested in the supine position while 34°C water was perfused through the tube-lined suit for ∼15 min. Thereafter, subjects were heat stressed by perfusing 48°C water through the suit. Subjects were also covered with blankets during this heating protocol. The heat stress ended once subjects had reached the target internal temperature increase (∼1.3–1.5°C). This temperature was chosen given our experience that at temperatures much past this level subjects are often agitated making it difficult to maintain sympathetic nerve recordings.
Data analysis.
Data were sampled at 200 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA) and analyzed using a statistical software package (SPSS v17, Chicago, IL). Mean arterial blood pressure (MAP) was calculated as one-third pulse pressure + diastolic blood pressure. Cutaneous vascular conductance (CVC) was calculated from the ratio of skin blood flux to MAP. Mean body temperature (Tb) was calculated as (0.9 × Tc) + (0.1 x Tsk), where Tsk is mean skin temperature. MSNA data were evaluated using LabView software (National Instruments, Austin, TX) that identified bursts on a beat-by-beat basis from the integrated neurogram established by fixed criteria, including an appropriate latency after the R-wave of the ECG and signal-to-noise ratio (7, 10). MSNA was expressed as burst frequency (bursts/min), burst incidence (number of bursts per 100 cardiac cycles), and total activity (identified from burst area of the integrated neurogram). To assess SSNA (expressed as total activity), a period of normothermic baseline in the absence of SSNA bursts was identified. The area of the integrated bursts above this baseline was measured at each stage of the heat stress. This approach was required given that for some subjects during more severe heating sequential SSNA bursts did not consistently return to baseline, and thus the area of individual bursts could not be identified. Segments of the digitized MSNA and SSNA records from normothermia (4–6 min), from the middle level (2–3 min) of heating, and the end of the heat stress (2–3 min) were selected for analysis. A shorter duration was used for heat stress periods to avoid substantial increases in Tc over the evaluation period. MSNA data were expressed relative to the increase in Tc whereas the SSNA data were expressed relative to the increases in mean Tb and Tc, given the recognized influence of skin temperature on cutaneous vasodilator and sudomotor responses (44, 46). For the MSNA recordings, the middle of heat stress was defined as half of the magnitude of the increase in Tc. For SSNA recordings, the middle of the heat stress was defined as half of the magnitude of the increase in both Tc and Tb. Thermoregulatory, MSNA, and SSNA responses during normothermia, at the mid-point of the heat stress, and the end of heat stress were statistically compared using a 1-way repeated-measures ANOVA followed by post hoc comparisons, where necessary. All values are reported as means ± SD unless otherwise indicated. P values of <0.05 were considered statistically significant.
RESULTS
Thermoregulatory and cardiovascular responses to heat stress.
Thermoregulatory and cardiovascular responses to passive heating, during the periods when SSNA and MSNA were analyzed, are summarized in Tables 1 and 2, respectively. Heat stress significantly increased Tc, Tsk, and Tb at the middle level and at the end of heating. Similarly, heart rate and cutaneous vascular conductance increased at both stages of heating (both P < 0.05). Mean arterial blood pressure was maintained throughout heating (P > 0.05).
Table 1.
Mean thermoregulatory and cardiovascular responses to normothermia and heat stress when SSNA was recorded
| Normothermia | Mid Heat Stress (Tb) | Mid Heat Stress (Tc) | End Heat Stress | |
|---|---|---|---|---|
| Core temperature, °C | 37.10 ± 0.18 | 37.37 ± 0.29* | 37.70 ± 0.22*‡ | 38.35 ± 0.30*† |
| Mean skin temperature, °C | 34.6 ± 0.7 | 38.5 ± 0.5* | 38.7 ± 0.6* | 39.3 ± 0.5*‡ |
| Mean body temperature, °C | 36.6 ± 0.2 | 37.6 ± 0.2* | 37.9 ± 0.2* | 38.6 ± 0.3*† |
| Heart rate, beats/min | 55 ± 10 | 73 ± 14* | 80 ± 13* | 94 ± 16*† |
| Systolic blood pressure, mmHg | 112 ± 14 | 114 ± 11 | 115 ± 10 | 120 ± 16 |
| Diastolic blood pressure, mmHg | 68 ± 6 | 65 ± 4 | 65 ± 6 | 66 ± 10 |
| Mean arterial blood pressure, mmHg | 82 ± 8 | 81 ± 5 | 81 ± 6 | 84 ± 11 |
| Forearm CVC, AU/mmHg | 0.41 ± 0.35 | 1.05 ± 0.67* | 1.19 ± 0.61* | 1.51 ± 0.59*‡ |
Values are means ± SD; n = 8. SSNA, skin sympathetic nerve activity; Tb, mean body temperature; Tc, mean core temperature; CVC, cutaneous vascular conductance; AU, arbitrary units.
P < 0.05 vs. normothermia;
P < 0.05 vs. mid heat stress (Tb and Tc);
P < 0.05 vs. mid heat stress (Tb only).
Table 2.
Mean thermoregulatory and cardiovascular responses to normothermia and heat stress when MSNA was recorded
| Normothermia | Mid Heat Stress | End Heat Stress | |
|---|---|---|---|
| Core temperature, °C | 37.01 ± 0.22 | 37.64 ± 0.22* | 38.37 ± 0.22*† |
| Mean skin temperature, °C | 34.6 ± 0.6 | 38.7 ± 0.7* | 39.2 ± 0.6*† |
| Mean body temperature, °C | 36.6 ± 0.2 | 37.9 ± 0.2* | 38.6 ± 0.2*† |
| Heart rate, beats/min | 63 ± 11 | 92 ± 11* | 105 ± 10*† |
| Systolic blood pressure, mmHg | 122 ± 16 | 125 ± 12 | 130 ± 14 |
| Diastolic blood pressure, mmHg | 77 ± 6 | 68 ± 5* | 68 ± 9 |
| Mean arterial blood pressure, mmHg | 92 ± 10 | 87 ± 6 | 89 ± 7 |
| Forearm CVC, AU/mmHg | 0.34 ± 0.40 | 1.45 ± 0.66* | 1.52 ± 0.69* |
Values are means ± SD; n = 8. MSNA, muscle sympathetic nerve activity.
P < 0.05 vs. normothermia;
P < 0.05 vs. mid heat stress.
SSNA responses to heat stress (N = 8).
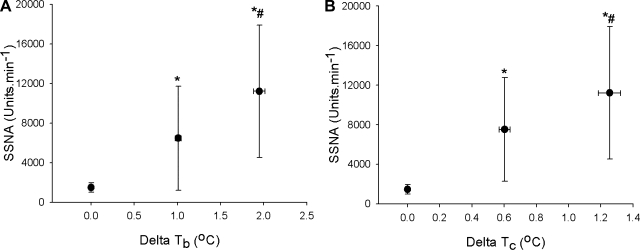
Compared with normothermia, when expressed against mean body temperature, SSNA increased midway through the heat stress (ΔTb 1.01 ± 0.10°C; baseline 1,486 ± 472 to mid heat stress 6,467 ± 5,256 units/min, P = 0.03, Fig. 1A) and continued to increase until the end of heating (ΔTb 1.95 ± 0.19°C, 11,217 ± 6,684 units/min, P = 0.01 vs. baseline and P = 0.002 vs. mid-heat stress). Similar responses were identified when changes in SSNA were expressed against the elevation in Tc (Fig. 1B). The change in SSNA for the latter half of heating (e.g., moderate to severe heating) was not different from the first half of heating (e.g., normothermia to moderate heating; 4,795 ± 4,435 vs. 5,075 ± 2476 units·min−1·°C−1, P > 0.05).
Fig. 1.
Group (± SD) averaged skin sympathetic nerve activity (SSNA) data during normothermia, and mid and end of heat stress, expressed as total activity (arbitrary units) against increases in mean body temperature (Tb; A) or increases in core temperature (Tc; B). Regardless of how it is expressed, SSNA continues to increase throughout heat stress with no evidence of a plateau following the midlevel of heating. *P < 0.05 vs. normothermia, #P < 0.05 vs. mid-heat stress (n = 8).
MSNA responses to heat stress (n = 8).
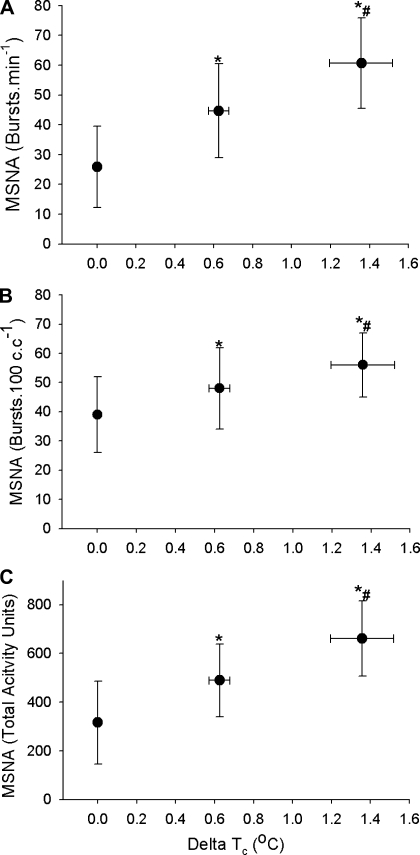
Relative to normothermic conditions, MSNA increased midway through the heat stress (ΔTc 0.63 ± 0.05°C, see Fig. 2) when expressed as burst frequency (26 ± 14 to 45 ± 16 bursts/min, P = 0.001), burst incidence (39 ± 13 to 48 ± 14 bursts/100 cardiac cyles, P = 0.03), or total activity (317 ± 170 to 489 ± 150 units/min, P = 0.02). Relative to mid-heat stress, MSNA was further elevated at the end of heating when expressed as burst frequency (61 ± 15 bursts/min, P = 0.01), burst incidence (56 ± 11 bursts/100 cardiac cyles, P = 0.04), and total activity (648 ± 158 units/min, P = 0.01). The change in MSNA for the latter half of heating (e.g., moderate to severe heating) was not different from the first half of heating (e.g., normothermia to moderate heating) regardless of the expression of MSNA, e.g., frequency (30 ± 12 vs. 19 ± 21 bursts·min−1·°C−1, P > 0.05), incidence (14 ± 15 vs. 10 ± 19 bursts·100 cardiac cycles−1·°C−1, P > 0.05), or total activity (276 ± 210 vs. 202 ± 217 units·min−1·°C−1, P > 0.05).
Fig. 2.
Group (± SD) averaged muscle sympathetic nerve activity (MSNA) data, expressed as burst incidence (A), bursts per 100 cardiac cycles (B), and total activity (C), during normothermia (first data point) and at mid (second data point) and end (last data point) of heat stress. Regardless of how it is expressed, MSNA continues to increase throughout heat stress with no evidence of a plateau following the mid-level of heating. Delta Tc: Increase in core temperature measured from telemetry pill in the intestines. *P < 0.05 vs. normothermia, #P < 0.05 vs. mid heat stress (n = 8).
DISCUSSION
The aim of this investigation was to test the hypothesis that MSNA and SSNA continue to increase during more severe passive heating relative to that employed in previous studies. MSNA or SSNA were continuously recorded while subjects, dressed in a water-perfused suit, underwent whole body heating that increased internal temperature ∼1.3°C. The main findings of this study are that, relative to the mid-point of heating (e.g., moderate passive heating), MSNA (regardless of whether it was expressed as frequency, incidence, or total activity) and SSNA continued to increase with further heating. These results indicate that MSNA and SSNA do not plateau after an elevation of internal temperature of ∼0.7°C but continue to increase as internal/mean body temperature increase above these previously reported values. These findings are consistent with progressive elevations in sympathetic nerve activity across a wider range of increases in core temperature during passive heating in the rodent model (20, 21, 23).
Appropriate alterations in sympathetic nerve activity are vital for the effective regulation of internal body temperature. Whole body heating causes pronounced increases in SSNA, which presumably cause elevations in skin blood flow and sweating to facilitate heat dissipation (16, 18, 30, 40). The neural signal contained within the SSNA signal includes both vasoconstrictor and vasodilator components (43). Transitioning from normothermic conditions to slight heating initially withdraws vasoconstrictor activity, which is evident by reductions in SSNA (1). More pronounced heating increases the sudomotor, and perhaps the vasodilator component, considerably, resulting in large increases in SSNA that are well above normothermic baseline. Consistent with these changes, in previous studies, which increased internal temperature by no more than ∼0.7°C, we and others have shown that this moderate level of passive whole body heating increases SSNA (1, 6–8, 29, 46). The relationship between skin blood flow/sweat rate and internal temperature is not generally linear during heat stress, as the rate of increase in skin blood flow and sweat rate are often attenuated with further elevations in internal temperature beyond ∼0.7°C (17, 40). It therefore seemed reasonable that SSNA responses may likewise be attenuated during more severe passive heating.
In the present study, more severe passive whole body heating was utilized to almost double the internal temperature increase previously employed, resulting in further increases in SSNA above the midpoint of heating (i.e., increased internal temperature of ∼0.7°C; Fig. 1B). This finding indicates that SSNA continues to increase with more severe whole body heating relative to that previously reported and likely mediates continued elevations in sweat rate and skin blood flow with increasing internal temperature. SSNA, as well as skin blood flow and sweat rate, are very sensitive to changes in mean skin temperature (44, 46). In the present study, there was a significant increase in mean skin temperature between mid- and final heating stages. To account for these changes in mean skin temperature, SSNA responses were also evaluated against mean body temperature (Fig. 1B), which resulted in similar findings relative to that observed when analyzed against intestinal temperature (Fig. 1A).
One could speculate that cardiopulmonary baroreceptor unloading coincident with passive heating, which attenuates heat stress-induced elevations in skin blood flow (5), may alter the elevation in SSNA during both moderate and more severe heat stress. If this were the case, then such unloading would limit the extent to which SSNA increases during heat stress. However, prior findings strongly suggest that baroreceptor unloading with lower body negative pressure or upright tilt does not alter SSNA in heat-stressed humans (9, 46). That said, it remains unknown whether preventing the reduction in cardiopulmonary baroreceptor unloading during passive heating would augment SSNA responses to that heat stress.
Others have shown a reduced rate in the decrease in splanchnic and renal blood flows, as well as total peripheral resistance, as passive heat stress progressed from moderate to more severe heating (28, 35). Based upon these observations, it may be that the increase in MSNA upon progressing from moderate to more severe heat stress would likewise be attenuated, recognizing the limitation that MSNA responses are not necessarily representative of the sympathetic response to splanchnic and renal vascular beds. Counter to that hypothesis, MSNA responses continued to increase throughout the heat stress at a rate comparable to the early phase of the heat stress. The specific mechanism(s) responsible for increases in MSNA during heat stress are unclear. MSNA can be modulated by baroreceptor loading status, where baroreceptor unloading increases MSNA and baroreceptor loading reduces MSNA. Previously we showed that during moderate heat stress reductions in central venous pressure, and presumably cardiopulmonary baroreceptor unloading, were not responsible for accompanying increases in MSNA (4). However, in the cited study the elevation in internal temperature was moderate, and thus it remains unknown whether cardiopulmonary baroreceptor unloading contributes to the elevations in MSNA during more severe heat stress. In contrast, sympathetically mediated elevations in splanchnic vascular resistance during whole body heat stress are not caused by baroreceptor unloading (36), and increases in splanchnic sympathetic nerve activity in hyperthermic rats occur in both baroreceptor-denervated conditions (20). Furthermore, arterial baroreceptor modulation of MSNA remains intact during heat stress (3, 19), suggesting a preserved coupling of arterial baroreceptor control of MSNA.
Heat stress-induced elevations in core temperature cause increases in ventilation that may aid in heat loss (45). Sympathetic nerve activity is subject to respiratory modulation through a within-breath pattern as a result of an inhibitory pulmonary stretch reflex (14, 38) and central (medullary) respiratory oscillators, e.g., central respiratory drive (11), suggesting that thermal hyperpnea could contribute to heat stress-induced sympathoexcitation. There are, however, a number of factors that do not support this hypothesis. First, the profile of the change in respiratory rate with heating does not match the temporal profile of the MSNA response. The change in ventilation during hyperthermia displays an exponential increase in which the majority of the elevation in ventilation occurs later during heat stress; i.e., after a core temperature increase of ∼1°C (45) and well after large increases in MSNA have occurred (see Fig. 2). Second, although the within-breath pattern of MSNA is potentiated during hyperpneic conditions (38) and is reflected in a reduction in MSNA during inspiration and an increase in MSNA during expiration, increases in tidal volume produce no or small decreases in steady-state total MSNA (38, 41). Although ventilation was not measured in the present study, it is unlikely that relatively subtle changes in ventilation in the progression from moderate to more severe heat is responsible for further increases in MSNA. Finally, interventions that exclusively manipulated central respiratory drive (i.e., those that do not induce chemoreceptor activation) do not change MSNA (14, 38). These data suggest that even if central respiratory drive increased with heating at and above elevations in core temperature of ∼1°C, this response is unlikely to contribute to the elevation in MSNA.
One hypothesis for heat stress-induced elevations in MSNA, and likely SSNA, is a central activation of the sympathetic nervous system. Central regulation of sympathetic outflow occurs primarily within the brain stem (medulla), pons, and hypothalamus, which integrate inputs from many different peripheral and central sources and provide major sources of supraspinal sympathoexcitatory outflow to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord (11). The role of the central nervous system in mediating sympathetic nerve responses to heat stress is complex. Previous studies in rodents suggest that hypothalamic, brain stem, and spinal cord sites are involved (22–25). In those models sympathoexcitation is not specifically dependent on intact neural connections between the forebrain and brain stem, while brain stem neural circuits play a key role (25), in particular the rostral ventral lateral medulla (24). Nevertheless, the mechanism(s) that might contribute to the heat stress-related sympathetic activation in the human are unclear.
A central mechanism that may contribute to sympathoexcitation associated with heat stress in humans might revolve around changes in body water content. Sweating is a critical heat loss mechanism during hyperthermic conditions, but it is accompanied with dehydration (40). Dehydration increases MSNA without alterations in blood pressure, presumably via activation of the renin-angiotensin system (31). Increases in renin and angiotensin II occur during whole body hyperthermia (15, 26), supporting the possibility that factors associated with dehydration and the renin-angiotensin system could explain heat stress-induced sympathoexcitation. In support of this hypothesis, blockade of central angiotensin II-mediated neurotransmission attenuates heat stress-induced elevations in splanchnic sympathetic nerve activity and reductions in renal blood flow in rats (2, 27). Further research is warranted to investigate this hypothesis.
Limitations.
In the present study, heat stress induced an average increase in internal temperature of ∼1.3°C, which was approximately double that of previous studies that have assessed sympathetic nerve activity responses to this challenge. Despite this extension of previous investigations, the findings of the present study are only applicable to the temperature range studied. It is thus unknown if MSNA and SSNA would continue to increase or plateau beyond the internal temperature studied in the present investigation.
Vasoconstrictor, sudomotor, and perhaps vasodilator components are contained within the SSNA signal, and it is not possible to separate primary sources of elevations in SSNA among these components. The primary responses observed in the integrative SSNA recording is likely related to the neural signal that is closest, and has the greatest neural fiber recruitment, relative to the recording electrode. It is therefore possible that the magnitude of the elevation in SSNA to the heat stress at a site (i.e., in a given subject) that primarily records from sudomotor fibers may be different from a site whose recording has significant contributions from cutaneous vasoconstrictor fibers. This would result in an increased variability in the elevation in integrative SSNA between subjects during the heat stress (see Fig. 1). However, every subject increased SSNA from normothermic to moderate heat stress as well as from moderate to severe heat stress as sudomotor and perhaps vasodilator fibers were activated.
In conclusion, relative to a moderate level of passive heating, MSNA and SSNA continue to increase during more profound passive heat stress sufficient to elevate internal temperature ∼1.3°C. These results indicate that MSNA and SSNA do not plateau but continue to increase as internal/mean body temperatures increase above previously reported values. The mechanisms causing heat stress-induced elevations in MSNA and SSNA remain to be determined.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-61388 and HL-84072.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of D. A. Low: Autonomic and Neurovascular Medicine Unit, Faculty of Medicine, St Marys Hospital, Imperial College London, London W2 1NY, United Kingdom.
REFERENCES
- 1. Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537–552, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen F, Liu F, Badoer E. AT1 receptors in the paraventricular nucleus mediate the hyperthermia-induced reflex reduction of renal blood flow in rats. Am J Physiol Regul Integr Comp Physiol 300: R479–R485, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Crandall CG, Cui J, Wilson TE. Effects of heat stress on baroreflex function in humans. Acta Physiol Scand 177: 321–328, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol 86: 605–610, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL, Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 290: H1601–H1609, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 282: R252–R258, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol 96: 2103–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol 91: 1679–1686, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972 [DOI] [PubMed] [Google Scholar]
- 14. Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 130: 3–20, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Escourrou P, Freund PR, Rowell LB, Johnson DG. Splanchnic vasoconstriction in heat-stressed men: role of renin-angiotensin system. J Appl Physiol 52: 1438–1443, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972 [DOI] [PubMed] [Google Scholar]
- 17. Johnson JM. Nonthermoregulatory control of human skin blood flow. J Appl Physiol 61: 1613–1622, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology: Adaptations to the Environment. Bethesda, MD: Am. Physiol. Soc., 1996, p. 215–243 [Google Scholar]
- 19. Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. J Appl Physiol 78: 881–889, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Kenney MJ, Claassen DE, Bishop MR, Fels RJ. Regulation of the sympathetic nerve discharge bursting pattern during heat stress. Am J Physiol Regul Integr Comp Physiol 275: R1992–R2001, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Kenney MJ, Fels RJ. Forebrain and brain stem neural circuits contribute to altered sympathetic responses to heating in senescent rats. J Appl Physiol 95: 1986–1993, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kenney MJ, Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am J Physiol Regul Integr Comp Physiol 283: R513–R520, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kenney MJ, Meyer CN, Hosking KG, Fels RJ. Is visceral sympathoexcitation to heat stress dependent on activation of ionotropic excitatory amino acid receptors in the rostral ventral lateral medulla? Am J Physiol Regul Integr Comp Physiol 301: R548–R557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenney MJ, Pickar JG, Weiss ML, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol 278: R1329–R1338, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Kosunen KJ, Pakarinen AJ, Kuoppasalmi K, Adlercreutz H. Plasma renin activity, angiotensin II, and aldosterone during intense heat stress. J Appl Physiol 41: 323–327, 1976 [DOI] [PubMed] [Google Scholar]
- 27. Kregel KC, Stauss H, Unger T. Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol Regul Integr Comp Physiol 266: R1985–R1991, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst 63: 61–67, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Normell LA, Wallin BG. Sympathetic skin nerve activity and skin temperature changes in man. Acta Physiol Scand 91: 417–426, 1974 [DOI] [PubMed] [Google Scholar]
- 31. Rabbitts JA, Strom NA, Sawyer JR, Curry TB, Dietz NM, Roberts SK, Kingsley-Berg SM, Charkoudian N. Influence of endogenous angiotensin II on control of sympathetic nerve activity in human dehydration. J Physiol 587: 5441–5449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974 [DOI] [PubMed] [Google Scholar]
- 33. Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension 15: 505–507, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Rowell LB. Thermal Stress In: Human Circulation Regulation During Physical Stress. New York: Oxford Univ. Press, 1986, p. 174–212 [Google Scholar]
- 35. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 36. Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man–role of falling blood pressure. J Appl Physiol 31: 864–869, 1971 [DOI] [PubMed] [Google Scholar]
- 37. Rowell LB, Johnson DG, Chase PB, Comess KA, Seals DR. Hypoxemia raises muscle sympathetic activity but not norepinephrine in resting humans. J Appl Physiol 66: 1736–1743, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res 72: 440–454, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 19: 313–349, 1991 [PubMed] [Google Scholar]
- 40. Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol 100: 1692–1701, 2006 [DOI] [PubMed] [Google Scholar]
- 41. St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984 [DOI] [PubMed] [Google Scholar]
- 43. Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 44. Vissing SF, Scherrer U, Victor RG. Increase of sympathetic discharge to skeletal muscle but not to skin during mild lower body negative pressure in humans. J Physiol 481: 233–241, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. White MD. Components and mechanisms of thermal hyperpnea. J Appl Physiol 101: 655–663, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole body heating in humans. J Physiol 536: 615–623, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]