Abstract
We examined the hypothesis that metabolic surgery-induced massive weight loss causes mass-driven and behavioral adaptations in the kinematics and kinetics of obese gait. Gait analyses were performed at three time points over ∼1 yr in initially morbidly obese (mass: 125.7 kg; body mass index: 43.2 kg/m2) but otherwise healthy adults. Ten obese adults lost 27.1% ± 5.1 (34.0 ± 9.4 kg) weight by the first follow-up at 7.0 mo (±0.7) and 6.5 ± 4.2% (8.2 ± 6.0 kg) more by the second follow-up at 12.8 mo (±0.9), with a total weight loss of 33.6 ± 8.1% (42.2 ± 14.1 kg; P = 0.001). Subjects walked at a self-selected and a standard 1.5 m/s speed at the three time points and were also compared with an age- and gender-matched comparison group at the second follow-up. Weight loss increased swing time, stride length, gait speed, hip range of motion, maximal knee flexion, and ankle plantarflexion. Weight loss of 27% led to 3.9% increase in gait speed. An additional 6.5% weight loss led to an additional 7.3% increase in gait speed. Sagittal plane normalized knee torque increased and absolute ankle and frontal plane knee torques decreased after weight loss. We conclude that large weight loss produced mechanical plasticity by modifying ankle and knee torques and gait behavior. There may be a weight loss threshold of 30 kg limiting changes in gait kinematics. Implications for exercise prescription are also discussed.
Keywords: biomechanics, locomotion, obesity, metabolic surgery
human gait exhibits mechanical plasticity because the torques generated by the lower extremity muscles become reorganized according to the mechanical requirements present in a specific condition, including knee osteoarthritis (OA) (2, 27, 31), anterior cruciate ligament insufficiency (13), aging (11, 18), and obesity (6, 12, 43). In addition, the pattern of lower extremity joint torques during gait varies with the rate of obesity: moderate obesity [i.e., body mass index (BMI) of ∼35 kg/m2] uniformly increases lower extremity torques at each joint (6), whereas morbid obesity (BMI of ∼45 kg/m2) induces a shift in mechanical output to the ankle joint from hip and knee joints (12).
Weight loss is a vital method for the treatment of obesity and for its many comorbidities yet one of its most obvious outcomes, adaptations in gait biomechanics, remains uncharacterized. While diet-, exercise-, diet plus exercise, and pharmaceutical interventions produce weight loss, at best, ∼10% (16, 22), metabolic surgery can produce as much as 3.3 kg/mo or in a few cases >60 kg (30–40%) final weight loss (8) and the Lap-Band version may become an option for individuals who have diabetes and a BMI as low as 30 kg/m2 (10). However, the impact of weight loss on the mechanics of gait is virtually unknown in obese individuals who are free of comorbidities such as age and painful knee OA (34). Small weight loss of 7% over 3 mo produced small increases in gait velocity, stride length, stride rate, and swing duration and shortened cycle time, stance, and double support phases in healthy 37-yr-old women with a BMI of 37 kg/m2 (35). After 6 kg or 5% weight loss, 14-yr-old children walked with 5 cm or 4% longer strides, 0.04 Hz or 4% lower stride frequency, and less leg muscle work to raise the center of mass (34). Low weight loss of 3% and high weight loss of 10% increased walking velocity from 1.21 to 1.30 m/s and 1.17 to 1.25 m/s (31), and high vs. low weight loss produced larger reductions in clinically important knee joint forces, estimated with a mathematical model (29). Both studies were conducted in overweight and obese older adults with radiographic evidence for painful knee OA (29, 31). Here we examine the possibility that massive weight loss (>20 kg) produces adaptations of gait in obese but otherwise healthy adults. A simple mass-related adaptation would manifest in changes in joint kinematics and kinetics in proportion to weight loss. For example, one such adaptation would be a reduction in knee extensor torques in the sagittal and frontal planes in proportion to the weight lost. However, based on the concept of mechanical plasticity and the previously observed kinematic changes with low weight loss, behavioral adaptations in gait such as increased stride length and walking velocity are also expected to occur after massive weight loss. For example, an increase in gait velocity after weight loss would concurrently increase joint torques and could counteract the beneficial effects of weight loss on musculoskeletal loading. An elucidation of such mass- and nonmass-dependent adaptations is important because obesity is the primary risk factor for knee OA (15, 39), a condition that afflicts almost 40% of the US population over age 60 (14, 40), but the mechanism of its evolution is not fully understood. Therefore, the purposes of the present study were to quantify the effects of metabolic surgery-induced weight loss on gait biomechanics of obese but otherwise healthy individuals and compare their gait after weight loss to the gait of a lean age- and gender-matched comparison group. We examined the hypothesis that metabolic surgery-induced weight loss causes simple mass-driven changes in gait such as reductions in ground forces and joint torques in proportion to weight loss. We tested these changes at a standard gait speed at three times points, before, midway, and after ∼1 yr surgery. We also hypothesized that weight loss produces nonuniform modifications in torques at the ankle, knee, and hip joints and in gait behavior, providing evidence for mechanical plasticity in gait. We tested this prediction at a self-selected gait speed at three times points during the intervention.
METHODS
Subjects.
Between August 2007 and February 2009, we enrolled 20 individuals cleared for metabolic surgery at Pitt County Memorial Hospital (Greenville, NC). In this weight loss group, we report gait biomechanics data in 10 individuals at baseline before surgery and 7.0 (±SD 0.7) mo and 12.8 (±0.9) mo after surgery intended to produce a substantial weight loss. Despite substantial financial incentives, 10 individuals failed to complete the study not because they had complications from the surgery but because they had scheduling conflicts (n = 4), moved away (n = 2), were involved in a car accident (n = 1), or lost an interest in participation (n = 3). Based on a medical exam, subjects were excluded who were older than age 60 and had one of the following conditions: BMI >60 kg/m2, neurological disorder (stroke, Parkinson's disease), hip or knee joint replacement, knee OA (based on an X-ray), orthopedic injury (ACL reconstruction, meniscal tear), a history of falls, osteoporosis, and medications that cause dizziness. Thus members of the weight loss group were, except for being obese, healthy, mobile, and pain-free throughout the study. The 10 patients who completed the study underwent Roux-en-Y gastric metabolic surgery performed by 2 surgeons. Although we did not control for physical activity after surgery, none of the subjects reported that they were involved in any form of regular physical activity or exercise program. All subjects read and signed an informed consent form approved by the University and Medical Center Institutional Review Board at East Carolina University.
Members of a comparison group, matched with the weight loss group for gender, age, and height were selected from our laboratory database and, based on a medical examination by a geriatrician, were extremely healthy. They met all of the above exclusion criteria and had a BMI <25 kg/m2. They were physically active but did not exercise vigorously on a regular basis and none were enrolled in a formal exercise program. Table 1 summarizes the physical characteristics of the two groups.
Table 1.
Subject characteristics
| Variable | Weight Loss Group | Comparison Group |
|---|---|---|
| Age, yr | 42.8 ± 10.6 | 43.6 ± 10.7 |
| Height, m | 1.70 ± 0.09 | 1.68 ± 0.09 |
| Mass, kg | 125.7 ± 26.6 | 62.3 ± 11.7 |
| BMI, kg/m2 | 43.2 ± 6.5 | 21.8 ± 2.8 |
| WOMAC | ||
| Pain (scale 0 to 20) | 3.3 ± 2.4 | 2.3 ± 1.83 |
| Stiffness (0 to 12) | 2.3 ± 1.7 | 2.1 ± 1.57 |
| Mobility difficulty (0 to 68) | 10.0 ± 9.4 | 8.7 ± 6.78 |
| Weight loss at 7.0 mo, kg | 34.0 ± 9.3* | |
| Weight loss at 12.8 mo, kg | 42.2 ± 14.1† |
Values are mean ±SD. WOMAC, Western Ontario and McMaster Universities Arthritis Index.
P < 0.05, relative to before metabolic surgery.
P < 0.05, relative to before and 7.0 mo after metabolic surgery.
Experimental set-up.
Subjects walked on a 15-m level walkway fitted with a force platform (LG6–4-2000; AMTI, Newton, MA) in the middle of the walkway. The force plate data collection system measured three-dimensional (3D) ground forces and the moments around the center of the plate at 960 Hz and stored the data on a computer. The vertical force channels were calibrated with known weights ranging from 0 to 2,100 N. The voltage outputs were highly linear throughout the tested range and the coefficient of determination between force and voltage was R2 = 0.999. The system was thus appropriate even for the heaviest subject whose mass was 168.7 kg. The 3D kinematics were recorded at 120 Hz during gait using a digital, 8-camera system (Qualisys MacReflex 240, Gothenburg, Sweden). A Brower infrared timing system (model IRD-T175; Salt Lake City, UT) was used to measure gait speed in the self-selected condition and to constrain walking velocity to a nominal value of 1.50 m/s.
Testing protocol.
Participants reported to the laboratory for one, 2-h-long session before and, as originally planned, ∼6 mo and 12 mo after metabolic surgery. Members of the comparison group were tested one time. Subjects read and signed an informed consent and answered questions in the Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire. This survey is a valid and reliable inventory to determine pain, stiffness, and mobility difficulty (4). We used the WOMAC to exclude individuals with pain and disability associated with knee OA.
Participants wore black spandex bicycle shorts, a tight fitting T-shirt, and athletic shoes. Standing height and mass were measured at each test session. It is difficult to accurately place anatomical markers on morbidly obese people, particularly in the pelvic region. We attempted to get the most accurate marker positions using the following methods. Reflective markers were placed using a modified Helen Hayes model with marker placements over the anterior superior iliac spines, the posterior superior iliac spines and the right posterior heel. The anterior superior iliac spines and posterior superior iliac spines markers were placed over the estimated location of the bony surfaces using participant feedback as a guide during palpation. Rigid arrays of four markers were placed on the lateral aspects of the right thigh and leg and on the superior surface of the right foot. Additionally, calibration markers were placed on the right foot first and fifth metatarsal heads, right lateral and medial malleoli, the lateral and medial right femoral condyles, and both greater trochanters. Calibration markers were used by Visual 3D to locate the joint centers relative to the other markers. Knee and ankle joint centers were located at the midpoint on the line between the calibration markers, which corresponded with the radius of the segment cylinder at the particular joint. Hip joint centers were calculated using a measured radius that was determined as 25% of the distance between the right and left greater trochanters at the hip for joint center depth, and the greater trochanter marker for vertical and anterior/posterior positioning. We found through pilot testing that calibration marker placements changed ∼1–2 cm with weight loss due to a reduction in soft tissue and further that joint torque calculations were affected by differences in marker positions of this magnitude. Joint angular position varied between 1–2° different with these different marker positions; however, differences in joint torques could be as large as 15% (∼5 Nm). If the markers were placed too high and too anterior on the greater trochanter, for example, there would be changes in kinematics with less hip flexion and more hip extension and a reduction in hip flexor torque and an increase in hip extensor torque. We therefore recorded the absolute vertical and anterior/posterior positions of the calibration markers and replicated these positions in the tests at follow-ups 1 and 2 using digitized coordinates from the motion capture system and consistent positioning of the subject for the static calibration. Consistent positioning was accomplished using a solid, wooden frame that was always located in the same position relative to the origin of the laboratory coordinate system. The subjects would place their feet in the standardized frame each time they were recorded, and the current calibration marker positions relative to the laboratory origin would be adjusted to the original calibration marker positions. This method ensured that all comparisons would use identical joint centers for inverse dynamic calculations.
Participants then walked on the walkway for several minutes until they were relaxed and comfortable. A starting point was selected so that the right foot would contact the force platform in a normal stride. Subjects in the weight loss group completed two conditions: self-selected speed and standard speed at 1.5 m/s, whereas lean participants were tested in only the standard speed condition. The order of testing the two conditions was counterbalanced among the obese subjects. Five successful trials were collected as a minimum for each subject and gait condition. Trials were discarded if the subject's velocity was >5% different than the 1.5 m/s target speed in the standard speed condition, if the foot was not completely on the force platform, or if the subject made visually obvious stride alterations to contact the force platform.
We wished to distinguish between two general types of adaptations to weight loss. “Mass driven” refers to simple, direct, and intuitive reductions in ground reactions forces and joint torques with weight loss. For example, the comparisons between obese and lean individuals in numerous Browning articles (e.g., Ref. 6), showing lower ground reaction forces and torques in the lean adults conceptually demonstrate this idea. We used the standard speed to determine the unadulterated effects of weight loss on gait biomechanics and compare the data with a comparison group that was tested at the standard speed only. “Behavioral” refers to gait adaptations that subjects exhibit in response to weight loss but are not necessarily simple, direct, and intuitive. For example, increased walking velocity and step length are not necessary outcomes of weight loss. Individuals can certainly select to walk at the same speed and with the same step length before and after weight loss. We consider behavioral adaptations as those over which the individuals had some available options or choices, whereas mass-driven would be outcomes directly caused by reduced weight. Therefore, we also collected data at a self-selected speed to determine if weight loss would produce behavioral changes in gait such as a change in stride length and walking velocity. No participants reported fatigue or required rest during the test session.
Data analysis.
Gait data were processed with Visual 3D software (C-Motion, Rockville, MD). The digitized Cartesian coordinates of the reflective markers describing the stance phase on the force platform were processed bidirectionally through a second order low-pass digital filter with a 6-Hz cut-off frequency. Ground reaction force data were filtered using a second order low-pass digital filter with a 45-Hz cut-off frequency. Swing time, stride length, cadence, and gait velocity were used to characterize the stride pattern and overall gait behavior. Joint kinematics analysis focused on the range of motion in the swing phase and average angular position in the stance phase at the hip, maximal knee flexion and average knee angular position in the stance phase, and average ankle angular position in the stance phase in the sagittal plane. We estimated changes in abdominal girth using the left and right iliac crest and left and right greater trochanter markers′ coordinate data to calculate a rough circumference of the person using the standard circumference = (diameter * π) calculation, where diameter equaled the distance between the markers.
The inverse dynamics functions in Visual 3D software (C-Motion) were used to calculate the joint reaction forces and internal torques at each right lower extremity joint throughout the gait cycle. Proprietary laboratory software was then used to determine selected variables from the ground reaction- and joint torque-by-time curves. These variables included the rate and maximal value of the vertical ground reaction force and the maximal horizontal braking force. We computed the extensor angular impulses at the hip, knee, and ankle, and we also calculated the maximal knee joint extensor torque in the sagittal plane (12) and the first maximal abduction torque in the frontal plane (2).
Statistical analyses.
Kinematic- and kinetic-dependent variables as well as weight loss from baseline and at the two follow-ups were compared with a one-way ANOVA with repeated measures. In case of a significant F value, a Tukey's post hoc contrast was used to determine the means that were different and these differences are in Tables 1–5 (denoted by symbols). Using data at the second follow-up, we compared gait variables between the weight loss and comparison groups using an independent two-tailed t-test. Pearson product moment correlation coefficients were computed to determine the association between selected variables. The level of significance was set at P < 0.05.
Table 2.
Changes in stride characteristics after metabolic surgery-induced weight loss
| Self-Selected Speed |
Standard Speed |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | T0 | T1 | T2 | F, P | T0 | T1 | T2 | F, P |
| Swing time, % | 35.3 ± 1.9 | 36.6 ± 1.6* | 37.8 ± 2.0† | 16.5, 0.001 | 36.3 ± 1.1 | 37.5 ± 1.4* | 38.0 ± 1.5* | 6.3, 0.008 |
| Stride length, m | 1.38 ± 0.11 | 1.44 ± 0.15* | 1.49 ± 0.18† | 9.1, 0.002 | 1.48 ± 0.12 | 1.52 ± 0.12* | 1.53 ± 0.14* | 6.5, 0.007 |
| Velocity, m/s | 1.30 ± 0.14 | 1.35 ± 0.18* | 1.45 ± 0.17† | 6.8, 0.006 | 1.49 ± 0.09 | 1.49 ± 0.04 | 1.51 ± 0.04 | 1.2, 0.338 |
| Cadence, step/min | 112 ± 8.9 | 111 ± 8.6 | 116 ± 7.5 | 2.8, 0.086 | 121 ± 7.5 | 117 ± 8.2* | 119 ± 8.6* | 6.8, 0.006 |
Values are means ± SD. Standard speed, walking speed at 1.5 m/s. T0, baseline; T1, follow-up 1 at 7.0 mo after baseline; T2, follow-up 2 at 12.8 mo after baseline; F, P, one-way ANOVA F and P values. F value has 2,18° of freedom. Italics denote P < 0.05.
P < 0.05, different relative to baseline.
P < 0.05, different relative to baseline and follow-up 1.
Table 3.
Changes in kinematics after metabolic surgery-induced weight loss
| Self-Selected Speed |
Standard Speed |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | T0 | T1 | T2 | F, P | T0 | T1 | T2 | F, P |
| Hip ROM, ° | 30.2 ± 4.1 | 32.9 ± 3.2 | 34.1 ± 7.2* | 4.4, 0.020 | 32.8 ± 4.0 | 35.5 ± 4.4 | 34.4 ± 7.7 | 1.5, 0.252 |
| Hip AP, ° | −4.1 ± 2.2 | −5.3 ± 4.2 | −5.6 ± 2.2 | 1.1, 0.384 | −4.4 ± 2.6 | −5.0 ± 4.4 | −6.1 ± 2.6 | 0.9, 0.424 |
| Maximal KF, ° | −11.2 ± 4.2 | −12.3 ± 4.6 | −14.7 ± 3.9* | 6.9, 0.006 | −13.29 ± 5.5 | −12.8 ± 5.0 | −14.8 ± 5.6 | 2.2, 0.144 |
| Knee AP, ° | −9.04 ± 3.9 | −9.0 ± 3.9 | −9.6 ± 3.56 | 0.9, 0.583 | −9.14 ± 4.5 | −8.5 ± 3.6 | −9.4 ± 3.9 | 0.6, 0.570 |
| Ankle AP, ° | −3.6 ± 2.8 | −1.5 ± 2.6* | −2.1 ± 2.4* | 42.1, 0.001 | −3.6 ± 2.3 | −1.3 ± 2.6* | −1.8 ± 2.2* | 4.8, 0.021 |
Values are means ± SD. Negative values denote joint flexion and ankle dorsiflexion. Hip ROM, difference between most extended and flexed positions of the hip joint in the swing phase; Hip AP, average hip joint angular position in the stance phase; Maximal KF, maximal knee flexion in early stance phase; Knee AP, average knee joint angular position in the stance phase; Ankle AP, average ankle angular position in the stance phase. Italics denote P < 0.05.
P < 0.05, different relative to baseline.
Table 4.
Changes in ground forces during gait after metabolic surgery-induced weight loss
| Self-Selected Speed |
Standard Speed |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | T0 | T1 | T2 | F, P | T0 | T1 | T2 | F, P |
| VGRF, N | 1,320 ± 309 | 964 ± 250* | 924 ± 264* | 52.4, 0.001 | 1,398 ± 309 | 1,012 ± 221* | 926 ± 223† | 68.7, 0.001 |
| VGRF, N/kg | 10.5 ± 0.8 | 10.5 ± 0.8 | 11.0 ± 0.7* | 6.2, 0.009 | 11.1 ± 0.6 | 11.1 ± 0.5 | 11.1 ± 0.4 | 0.9, 0.907 |
| Slope, N/s | 7,660 ± 3,044 | 5,693 ± 2,143* | 6,090 ± 2,031* | 4.0, 0.037 | 9,021 ± 2,391 | 6,655 ± 1,170* | 6,250 ± 1,094* | 19.7, 0.001 |
| Slope, N·s−1·kg−1 | 61.5 ± 23.1 | 62.0 ± 15.1 | 72.9 ± 12.3 | 2.4, 0.117 | 72.1 ± 13.1 | 73.5 ± 6.3 | 76.2 ± 7.0 | 0.8, 0.447 |
| Braking force, N | −239 ± 56 | −190 ± 59* | −188 ± 67* | 9.2, 0.002 | −277 ± 80 | −211 ± 60* | −192 ± 51† | 21.9, 0.001 |
| Braking force, N/kg | −1.90 ± 0.26 | −2.0 ± 0.25 | −2.2 ± 0.33† | 5.2, 0.016 | −2.2 ± 0.35 | −2.3 ± 0.28 | −2.3 ± 0.23 | 0.7, 0.500 |
Values are means ± SD. VGRF, first peak of the vertical ground reaction force; slope, rate of rise of first peak of the vertical ground reaction force; braking force, maximum posterior ground reaction force. Italics denote P < 0.05.
P < 0.05, different relative to baseline.
P < 0.05, different relative to baseline and follow-up 1.
Table 5.
Changes in joint torques during gait after metabolic surgery-induced weight loss
| Self-Selected Speed |
Standard Speed |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | T0 | T1 | T2 | F, P | T0 | T1 | T2 | F, P |
| Sagittal plane | ||||||||
| Hip, Nm·s | 9.8 ± 5.3 | 8.7 ± 7.5 | 8.3 ± 3.1 | 0.5, 0.644 | 11.3 ± 4.9 | 9.1 ± 7.3 | 8.4 ± 3.2 | 3.1, 0.068 |
| Hip, Norm. | 0.49 ± 0.26 | 0.57 ± 0.43 | 0.61 ± 0.21 | 0.9, 0.429 | 0.56 ± 0.24 | 0.60 ± 0.42 | 0.62 ± 0.21 | 0.3, 0.723 |
| Knee1, Nm·s | 7.0 ± 5.5 | 6.5 ± 4.3 | 6.9 ± 3.8 | 0.1, 0.931 | 8.8 ± 7.0 | 6.7 ± 4.0 | 7.6 ± 3.7 | 0.9, 0.421 |
| Knee1, Norm. | 0.31 ± 0.19 | 0.43 ± 0.25 | 0.49 ± 0.19 | 3.3, 0.062 | 0.39 ± 0.23 | 0.44 ± 0.24 | 0.53 ± 0.18 | 1.7, 0.212 |
| Knee2, Nm | 41.2 ± 25.9 | 42.0 ± 25.1 | 46.2 ± 23.2 | 0.4, 0.661 | 62.0 ± 36.4 | 45.9 ± 19.5 | 51.1 ± 18.7 | 1.7, 0.212 |
| Knee2, Norm. | 1.8 ± 0.83 | 2.8 ± 1.37* | 3.3 ± 1.08† | 7.7, 0.004 | 2.6 ± 1.09 | 3.1 ± 1.28 | 3.7 ± 1.03 | 2.9, 0.083 |
| Ankle, Nm·s | 42.2 ± 14.4 | 30.8 ± 10.2* | 28.2 ± 9.5* | 19.8, 0.001 | 39.0 ± 13.2 | 29.8 ± 10.8* | 28.3 ± 10.6* | 18.2, 0.001 |
| Ankle, Norm. | 2.0 ± 0.31 | 2.0 ± 0.25 | 2.0 ± 0.22 | 0.1, 0.992 | 1.8 ± 0.20 | 1.9 ± 0.23 | 2.0 ± 0.25 | 1.9, 0.186 |
| Frontal plane | ||||||||
| Knee3, Nm·s | −47.8 ± 19.9 | −36.1 ± 16.8* | −37.3 ± 18.7* | 6.4, 0.007 | −50.7 ± 18.6 | −40.3 ± 18.8* | −37.0 ± 19.3* | 9.5, 0.002 |
| Knee3, Norm. | −2.3 ± 0.72 | −2.2 ± 0.62 | −2.5 ± 0.71 | 1.4, 0.267 | −2.4 ± 0.66 | −2.5 ± 0.74 | −2.5 ± 0.84 | 0.2, 0.832 |
Values are means ± SD. Norm., torque normalized to (%body mass * height). Knee1, extensor angular impulse in early stance; Knee2, peak extensor torque in early stance; Knee3, internal knee abductor torque in the frontal plane in early stance. Italics denote P < 0.05.
P < 0.05, different relative to baseline.
P < 0.05, different relative to baseline and follow-up 1.
RESULTS
Table 1 shows that the weight loss and comparison groups were similar in age, height, and WOMAC scores. At baseline, the weight loss group was 63.4 kg heavier and had nearly twice greater BMI relative to the comparison group. Weight loss participants returned to the laboratory for the first follow-up 7.0 mo (±0.7) after baseline and for the second follow-up 5.8 mo (±1.14) later or a total of 12.8 mo (±0.9) after surgery. Table 1 shows that patients lost significant weight of 27.1 ± 5.1% (34.0 ± 9.3 kg) by follow-up 1 and significantly more weight of 6.5 ± 4.2% (8.2 ± 6.0 kg) by follow-up 2 (both P < 0.05). The surgery resulted in a significant final weight loss of 33.6 (±9.4) or 42.2% kg [±14.1; time main effect, F(2,18)= 92.9; P = 0.001]. The largest and smallest individual weight loss, respectively, was 69.0 and 24.0 kg. None of the subjects gained weight between follow-ups. Final weight loss correlated with initial body wt r = 0.66 (P = 0.010). Initial abdominal girth of 1.58 m (±0.16) decreased by 25.7% (±12.6) to a final girth of 0.41 m (±0.22; P = 0.001).
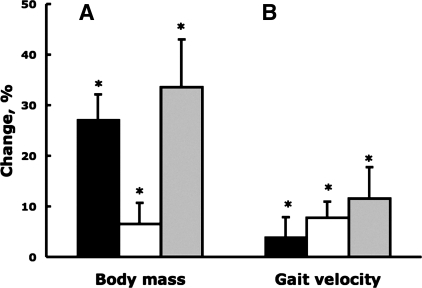
Table 2 shows that when walking at a self-selected pace, weight loss subjects significantly increased swing time 7.1% (3.7 and 3.4%, respectively, between baseline and follow-up 1 and between follow-ups 1 and 2), made 7.9% longer strides (4.2 and 3.7%), and walked 11.6% faster (3.9 and 7.7%; all P < 0.05). When subjects walked at the standard speed of 1.5 m/s, there were significant increases in swing time (4.7%) and stride length (3.2%) and decreases in cadence (−1.2%; all P < 0.05). Figure 1 summarizes the changes in weight loss and gait velocity.
Fig. 1.
Percent changes in body mass (A) and self-selected gait velocity (B) between baseline and follow-up 1 (black), between follow-ups 1 and 2 (white), and between baseline and follow-up 2 (gray). Vertical bars indicate +1SD. *P < 0.05.
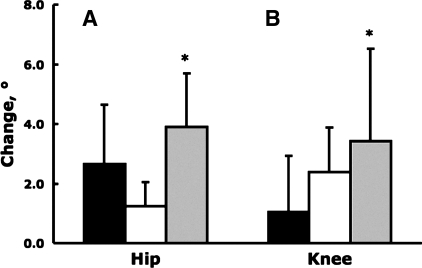
Table 3 shows that at the self-selected speed, hip range of motion in the swing phase increased significantly 12.9%, maximal knee flexion in early stance increased 30.4%, and ankle joint function shifted 40.5% toward plantarflexion (all P < 0.05; Fig. 2). At the standard speed, there was a significant change of 1.8° at only the ankle joint becoming more plantarflexed (P < 0.05). Significant reductions in abdominal girth and increases in hip range of motion in the swing phase correlated r = −0.74 (P = 0.014).
Fig. 2.
Changes in hip range of motion of the in swing phase (A) and maximal knee flexion in stance phase (B) measured at self-selected gait speed between baseline and follow-up 1 (black), between follow-ups 1 and 2 (open), and between baseline and follow-up 2 (gray). Vertical bars indicate +1SD. *P < 0.05.
Table 4 shows that from baseline to the second follow-up the first peak of the vertical ground reaction force decreased significantly 30.0% in absolute units and increased 5.0% when normalized to mass at the self-selected pace. The rate of rise of first peak of the vertical ground reaction force decreased significantly 20.5%, and the maximum posterior ground reaction (braking) force per unit mass increased 16% (all P < 0.05). When subjects walked at the standard speed, the first peak of the vertical ground reaction force revealed a final significant decrease of 33.8% (27.6 and 8.2%, respectively, between baseline and follow-up 1 and between follow-ups 1 and 2), the rate of rise of this force significantly decreased 31.2%, and braking force revealed a final decrease of 30.6% (23.6 and 7.0%; all P < 0.05). Changes in mass and ground forces correlated r = 0.89 (self-selected speed) and 0.92 (standard speed; both P = 0.001).
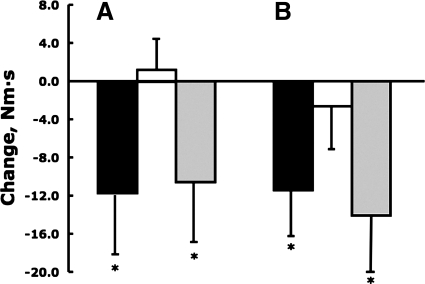
Table 5 shows that from baseline to second follow-up normalized peak extensor knee torque in early stance increased significantly 77.4% (49.9 and 18.3%, respectively, between baseline and follow-up 1 and between follow-ups 1 and 2) and ankle angular plantarflexion impulse decreased significantly 33.2% (27.0 and 8.5%) while walking at a self-selected speed (all P < 0.05; Figs. 3 and 4). When subjects walked even at the standard speed, the ankle plantarflexion impulse decreased significantly 27.3% after weight loss (Fig. 4). At self-selected and standard speeds, internal knee abductor torque decreased significantly 22.0 and 27.3%, respectively (both P < 0.05; Fig. 3).
Fig. 3.
Changes in angular impulse in the knee in the frontal plane (A) and in the ankle in the sagittal plane (B) measured at self-selected gait speed between baseline and follow-up 1 (black), between follow-ups 1 and 2 (white), and between baseline and follow-up 2 (gray). Vertical bars indicate ±1 SD. *P < 0.05.
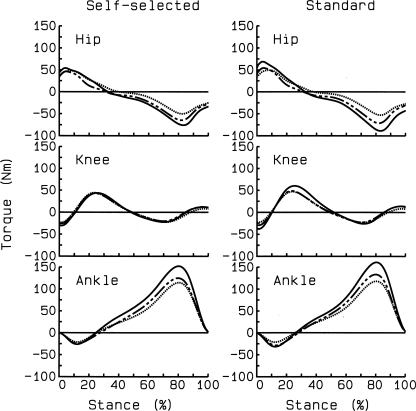
Fig. 4.
Mean joint torque curves at baseline (solid line), follow-up 1 (dashed line), and follow-up 2 (dotted line) for self-selected and standard speeds. Positive values are internal extensor torques. Despite massive weight loss, extensor angular impulses at hip and knee joints remained statistically unchanged whereas there were significant reductions in ankle plantarflexor angular impulse from baseline to follow-up 1.
Table 6 shows that although the weight loss group relative to the comparison group was still significantly 34.0% or 21.2 kg heavier at the second follow-up, eight of the nine stride and joint kinematics variables were statistically similar between the two groups. The first peak of the vertical ground reaction force and maximum posterior ground reaction (braking) force were significantly 26.3% higher in the weight loss than in the comparison group. The normalized first peak of the vertical ground reaction force and the slope of this force each became significantly 10.3% smaller in the weight loss than in the comparison group. Knee extensor angular impulse and peak extensor torque in early stance were significantly 63% higher, and normalized hip torques were 31.0% lower in the weight loss than in the comparison group.
Table 6.
Comparison of body mass and gait between initially obese individuals who lost 42.2 kg of body mass and a lean age- and gender-matched comparison group
| Variable | Weight Loss Group at T2 (means ± SD) | Comparison Group (means ± SD) | Δ | t-Test P Value |
|---|---|---|---|---|
| Mass, kg | 83.5 ± 20.3 | 62.3 ± 11.7 | 21.2 | 0.001 |
| Stride analysis | ||||
| Swing time, % | 38.0 ± 1.6 | 40.1 ± 0.9 | −2.1 | 0.001 |
| Step length, m | 1.53 ± 0.14 | 1.53 ± 0.10 | 0.0 | 0.963 |
| Velocity, m/s | 1.51 ± 0.04 | 1.52 ± 0.01 | −0.01 | 0.114 |
| Cadence, steps/min | 119.7 ± 8 | 122.2 ± 7 | −2.5 | 0.487 |
| Joint kinematics | ||||
| Hip ROM, ° | 34.4 ± 7.7 | 37.1 ± 4.01 | −2.7 | 0.323 |
| Hip AP, ° | −6.1 ± 2.6 | −4.3 ± 2.3 | −1.8 | 0.117 |
| Maximal KF, ° | −14.8 ± 5.6 | −18.4 ± 4.8 | 3.6 | 0.136 |
| Knee AP, ° | −9.4 ± 3.9 | −11.6 ± 3.1 | 2.2 | 0.174 |
| Ankle AP, ° | −1.8 ± 2.2 | 0.1 ± 3.3 | −1.9 | 0.151 |
| Ground forces | ||||
| VGRF, N | 926.3 ± 223 | 744.1 ± 131 | 182.2 | 0.039 |
| VGRF, N/kg | 11.1 ± 0.4 | 12.0 ± 0.9 | −0.9 | 0.011 |
| Slope, N·s | 6,250 ± 1,094 | 5,362 ± 917 | 888 | 0.065 |
| Slope, N·s−1·kg−1 | 76.2 ± 7.0 | 87.0 ± 11.7 | −10.8 | 0.022 |
| Braking force, N | −192 ± 51 | −151 ± 27.0 | −41.0 | 0.039 |
| Braking force, N/kg | −2.3 ± 0.23 | −2.5 ± 0.37 | 0.2 | 0.258 |
| Joint torques | ||||
| Hip, Nm·s | 8.4 ± 3.2 | 8.9 ± 2.2 | −0.5 | 0.680 |
| Hip, Normalized | 0.62 ± .21 | 0.90 ± 0.29 | −0.3 | 0.026 |
| Knee1, Nm·s | 7.6 ± 3.7 | 4.3 ± 2.4 | 3.3 | 0.030 |
| Knee1, Normalized | 0.53 ± 0.18 | 0.43 ± 0.26 | 0.1 | 0.329 |
| Knee2, Nm | 51.1 ± 18.7 | 34.0 ± 16.3 | 17.1 | 0.042 |
| Knee2, Normalized | 3.70 ± 1.03 | 3.30 ± 1.74 | 0.4 | 0.716 |
| Ankle, Nms·s | 28.3 ± 10.6 | 22.0 ± 10.6 | 6.3 | 0.199 |
| Ankle, Normalized | 2.0 ± .22 | 2.0 ± 0.40 | 0.0 | 0.545 |
| Knee3, Nms·s | −37.0 ± 19.3 | −27.8 ± 6.5 | −9.2 | 0.170 |
| Knee3, Normalized | −2.5 ± 0.84 | −2.7 ± 0.65 | 0.2 | 0.631 |
T2, follow-up 2 at 12.4 mo after surgery. Δ, Absolute difference between the 2 groups, expressed as weight loss minus comparison group. Italics denote P < 0.05.
DISCUSSION
Nature of weight loss.
To the best of our knowledge, the present work is the first one to determine the effects of massive weight loss on gait biomechanics. Obesity is the primary risk factor for knee OA (15, 39, 40). Thus it is of great interest to determine if large weight loss produces adaptations in gait due to mass loss alone or whether behavioral changes would occur in gait. This is because a previous study, against predictions, reported that obesity was not associated with increased knee joint torque and power during level walking, suggesting a nonmass-related reconfiguration of torques in the lower extremity (12). We found that massive weight loss in morbidly obese individuals produced both simple mass-related, “linear” adaptations in gait mechanics (tested at a standard speed) and mechanical plasticity in the form of a reorganization of lower extremity joint torques (tested at the self-selected speed). We further propose that weight loss in morbidly obese individuals induces behavioral changes including longer stride length and faster walking velocity but the musculoskeletal benefits associated with large weight loss are partially counteracted by these behavioral adaptations (9). We emphasize that the present sample included patients who were extremely healthy, were free of pain, and had no detectable clinical conditions other than being obese (Table 1).
Changes in stride characteristics and joint kinematics after weight loss.
Walking at a self-selected pace before and after weight loss allowed us to determine if weight loss had brought about changes in gait behavior. Because 81% of the total weight loss occurred in 7 mo, only 7 out of 25 variables examined in Tables 2–5 revealed additional changes between follow-ups 1 to 2 (denoted by symbols). By follow-up 1, swing time, stride length, and gait velocity all increased and then increased more by follow-up 2. Before surgery, obese participants walked somewhat faster (1.30 m/s) than the 1.18 (24, 26) or the 1.09 m/s (41) but slower than the 1.40 m/s speed (7). Patients in the present study were extremely healthy, while in one of these studies (26) subjects must have had mobility limitations because the authors decided not to conduct a maximal or a submaximal treadmill test due patients reporting joint pain. Abdominal dimensions decreased 25% and allowed subjects to perform greater hip flexion and swing the legs for a longer time, which in turn produced longer strides. The combination of these factors, without changes in cadence at the self-selected speed, resulted in a functionally meaningful increase of 0.15 m/s in gait velocity, reaching nearly 1.5 m/s at the end point of the study. Although 27% initial weight loss was coupled with 3.9% increase in gait velocity, only 6.5% additional weight loss was coupled with 7.3% more increase in gait velocity, suggesting an interaction between magnitude of weight loss and gait behavior (21) (Fig. 1). That is, adaptations in gait velocity take longer to develop than the period of weight loss because there is a level of weight loss needed for gait velocity to increase. Supporting our hypothesis, and in general the concept of mechanical plasticity of human gait (11, 12), this interaction between weight loss and gain in gait velocity suggests that weight loss may produce not only mass-driven linear adaptations but also adaptive changes in gait behavior.
Weight loss modified spatiotemporal (stride) characteristics of gait even at the standard speed of 1.5 m/s. After weight loss, a combination of longer swing time, stride length, and a lower cadence produced the 1.5 m/s speed (Table 2, Standard Speed). In a previous study (34), 14-yr-old boys and girls with a BMI of ∼34 kg/m2 walked at a standard speed of 1.25 m/s before and after 6 kg or 5% weight loss and again, despite the fixed speed, walked with significantly 5 cm or 4% longer stride length and 0.04 Hz or 4% lower stride frequency after weight loss but, unlike in the present study, without changes in stance or single support duration. The longer swing time suggests a reduction in time needed to support the smaller body weight after weight loss. Most likely, these changes in spatiotemporal characteristics measured after weight loss at a fixed speed are mediated by more ankle plantarflexion, observed in the present study (Table 3, Standard Speed, Cadence).
Weight loss had a strong effect on joint kinematics of the hip, knee, and ankle joints (31% overall change) at the self-selected gait speed (Fig. 2). A lack of change in joint kinematics after the initial 27.1-kg weight loss but large changes after the 33.6-kg total weight loss suggest, like for gait velocity, the existence of a weight loss threshold ∼30 kg. Obese gait became more dynamic because the hip joint range of motion increased during swing, knee flexion increased in early stance, and ankle function shifted to a more plantarflexed foot. We (12) previously showed that morbidly obese adults walk with a more erect posture compared with lean adults, and we presently show in similarly obese individuals that when such subjects lose weight gait becomes more dynamic due to increased hip range of motion, knee flexion, and ankle function. The 25.7% reduction in abdominal girth reduced obstruction at the pelvis, allowing subjects to flex their hips more, suggested by the r = −0.74 correlation between the changes in these two variables.
Weight loss also increased knee flexion in stance phase of gait. The 30% increase in knee flexion means greater vertical acceleration of the center of mass especially as subjects walked faster after weight loss. Although we did not measure maximal quadriceps strength in our subjects, previous studies (33, 42) reported no changes in maximal voluntary quadriceps force and muscle contractile characteristics after metabolic surgery. Thus the increased knee flexion during stance can be the result of neuromuscular adaptation in quadriceps function, including improved muscle activation and reduced antagonist coactivation. The 77.4% increase in normalized knee extensor torque after weight loss also attests to initially obese adults' maintained capability to stop the vertical deceleration of the body during a larger knee flexion after weight loss (Table 5). Indeed, stride characteristics and joint kinematics after weight loss were similar to the gait of lean subjects although subjects in the weight loss group were still ∼20 kg heavier (Table 6). This difference in mass between the two groups and the 28.6 (±4.1) BMI after weight loss suggest that gait kinematics become indistinguishable between two individuals who have a difference in mass of 20 kg. Despite the large weight loss, walking at the standard speed produced changes in kinematics only at the ankle joint being more plantarflexed (as discussed in the previous paragraph) without changes at the hip or knee joint (Table 3, Standard Speed), suggesting that the 1.5 m/s speed limited changes in hip and knee joint excursions despite the weight loss.
Changes in ground forces and joint kinetics after weight loss.
It is well established that obese compared with lean individuals can have as much as ∼60% higher vertical ground forces (6). In addition, there also is a strong association (r = 0.76) between levels of obesity and ground forces (28). We observed that the first peak of the vertical ground reaction force in obese compared with lean adults was 1.88- and 1.24-fold greater, respectively, before and after weight loss. In addition, the changes in mass and ground forces correlated r = 0.89 (self-selected speed) and 0.92 (standard speed; both P = 0.001). An intriguing observation of clinical relevance was that while vertical ground forces decreased with weight loss, when walking at a self-selected pace, normalized vertical ground forces significantly increased (Table 4, Self-Selected Speed, variable 2). Because weight loss subjects were still ∼20 kg heavier than subjects in the comparison group after a total weight loss of 42.2 kg but increased their spontaneous walking speed, ground forces per unit mass increased. The same pattern emerged for maximum posterior ground reaction forces. One interpretation of the data is that caution is needed in prescribing walking as an exercise at increasingly faster speeds in step with weight loss for overweight individuals because increased gait speed counteracts the effect weight loss would have in reducing ground forces (23, 31), hence, impact associated with foot strike (6) and subsequent knee joint forces (19). This is especially critical for overweight patients in the preknee OA state (40). Exercise prescription for the period immediately after massive weight loss (>20 kg) should include walking at a self-selected (or at a slower) pace (24) in combination with other low-impact activities such as cycling and swimming, a recommendation consistent with a previous study (4). Table 6 (Ground forces) shows that after weight loss, weight loss subjects still exhibited statistically significant differences in ground forces in four of six variables, underscoring the functional impact of 20-kg mass difference on forces affecting the legs in stance phase of gait. Therefore, after weight loss gait kinematics were similar between the two groups but weight loss patients still had much larger ground forces, as they were still overweight.
Weight loss produced no changes in the absolute sagittal plane hip and knee torques but substantial changes in ankle torques (Table 5 and Figs. 3 and 4). In a previous study (Table 2 in Ref. 30), a weight loss of ∼3% also failed to produce changes in several variables describing knee joint moments, but in that study subjects were obese and also had a diagnosis knee OA. Therefore, it was surprising that 33.6% weight loss had no effect on hip extensor angular impulse. Hip kinematics suggested a more dynamic hip function through increased swing time and range of motion, contributing to the increased stride length but hip flexor torques also remained unchanged (data not shown). Weight loss also had no effect on knee extensor impulse. However, there was 77.4% increase in normalized peak knee extensor torque in early stance. This behavioral adaptation was, as discussed in kinematics above, probably related to the interaction between weight loss and increased gait speed and knee flexion in early stance. The low peak knee torque in the obese state agrees with our (12) previous finding, showing similar knee effort, as a result of adaptation to increased mass, in obese and lean adults. While others did not observe such a similarity, the level of obesity was also much lower (e.g., Ref. 6) than in the present work, and the increasing normalized peak knee torques with weight loss validate this argument. We (12) previously reported a proximal to distal shift in lower extremity muscle effort to the plantarflexor muscles in obese adults, and weight loss produced a substantial, 33.2%, reduction in plantarflexor torques. Considering a significant increase in gait velocity after weight loss, this reduction in plantarflexion torque is thus a hallmark of weight change in obese gait and signifies a key behavioral adaptation to weight loss (Table 5). It thus seems that morbidly obese subjects' gait is driven by ankle function because after weight loss more flexion in the knee joint increases lever arms for external forces (5), producing knee angular impulse values that are unchanged after a large weight loss (Table 5). This supports our previous data that ankle torques drive morbidly obese gait. The role of ankle torques becomes less with weight loss and also less in individuals who are less obese (6).
Similar to a previous study (30), we also observed a 24.5% initial reduction in nonnormalized internal knee abductor torque but additional weight loss of 6.5% did not produce additional reduction in this variable (Fig. 3). Frontal plane adductor torque, as a surrogate measure of the medial-lateral load distribution in the tibiofemoral joint, is associated with body mass (37) and with the onset, severity, and progression of knee OA (1, 2, 17, 32, 38). Obesity-related increase in internal abductor torque is implicated in the development of medial compartment knee OA (1), and its reduction after weight loss is thought to reduce the stress placed on the knee joint (6). Compared with lean subjects (Table 6), initially obese adults who lost 42.2 kg of their body mass were still 34% heavier and thus, predictably, revealed still significantly greater ground forces at the standard speed. Although there is evidence that increased impact forces represent a part of the stimulus for cartilage health (20), there is also evidence that such high forces are precursors to degenerative joint disease (40).
Limitations.
We recognize the limitation of the small sample size used in the present study; hence, the inability to test a gender effect even though gender may pay a role in gait adaptations to weight loss in the preknee OA state (36). Although we allocated substantial resources to recruit and retain patients, the dropout rate was still 50% by the end of the study. Highlighting the difficulties in retaining patients in the study, considering the study's context, we encountered odd behavior from subjects who appeared for follow-ups with McDonald's food trays. An additional limitation is that we did not test a nonweight-loss control group that would have allowed us to assess the reliability of the dependent variables. The study focuses primarily on sagittal plane analyses, although there are important conceptual and clinical implications of weight loss for frontal plane gait biomechanics (30). Using the greater trochanter as representative of sagittal plane is an accepted measure of hip joint center location; however, frontal plane approximations can vary up to 3 cm away from the true joint center with the absence of bone scan data (3). Given this finding, we chose to only report sagittal plane kinetics and kinematics at the hip joint. Weight loss as small as 14 vs. the 34% in the present study can change body segmental inertial parameters (segmental masses, center of mass, and radii of gyration) to a different extent, potentially affecting the magnitude of changes in joint torques (25). Since the inertial forces and torques in walking are typically quite low, however, we do not think these changes substantially affected the results (5). This study also did not evaluate measures of functional outcomes and leg strength, but we decided to exclude these measurements to reduce testing time and keep more subjects in the study. Finally, we must point out that although we were extremely cautious with our motion analyses procedures and devised a new and careful calibration procedure for consistent marker placement, it is a limitation of this study that X-rays of the lower extremities were not available to achieve even more accurate marker placements. We also recognize that the hip joint center location used in our analysis may not have been accurate for the frontal plane due to subcutaneous tissue between the skin and the actual greater trochanter. However, our procedure did ensure accurate sagittal plane hip joint center location and is therefore appropriate for sagittal plane kinematic and kinetic calculations.
In conclusion, 33.6% or 42.2 kg weight loss increased swing time, stride length, gait speed, hip range of motion, maximal knee flexion, and ankle plantarflexion. In particular, 27% initial weight loss led to 3.9% increase in gait speed and an additional 6.5% weight loss resulted in an additional 7.3% increase in gait speed. Sagittal plane normalized knee torques increased and absolute ankle and frontal plane knee torques decreased after weight loss. There may be a weight loss threshold of 30 kg limiting changes in gait kinematics. Large weight loss produced mechanical plasticity by modifying ankle and knee torques and gait behavior. Future studies should use a larger sample size, use imaging-guided marker placement, and examine potential interactions between gait mechanics and, respectively, gender, physical activity, and clinical state of the knee joint after surgery-induced large weight loss.
GRANTS
Support for this study was provided in part by National Institute on Aging Grant AG-024161.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Beth White and Jonathan Patterson (undergraduate); Michael McNally, Ben Long, and Caitlin Pearl (graduate students); and Kelly Jernigan and Patrick Sams (staff members at Pitt County Memorial Hospital) for assistance in data collection, analysis, and patient recruitment. We also thank the reviewers for extremely constructive comments that have vastly improved manuscript.
REFERENCES
- 1. Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am 25: 395–403, 1994 [PubMed] [Google Scholar]
- 2. Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol 18: 514–518, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bell AL, Pedersen DR, Brand RA. A comparison of the accuracy of several hip center location prediction methods. J Biomech 23: 617–621, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15: 1833–1840, 1988 [PubMed] [Google Scholar]
- 5. Biewener AA, Farley CT, Roberts TJ, Temaner M. Muscle mechanical advantage of human walking and running: implications for energy cost. J Appl Physiol 97: 2266–2274, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Browning RC, Kram R. Effects of obesity on the biomechanics of walking at different speeds. Med Sci Sports Exerc 39: 1632–1641, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res 13: 891–899, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 31: 743–750, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chung MJ, Wang MJ. The change of gait parameters during walking at different percentage of preferred walking speed for healthy adults aged 20–60 years. Gait Posture 31: 131–135, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Department of Health and Human Services, Food and Drug Administration. Gastroenterology and Urology Devices Panel Meeting Announcement. [Online]. Dept. Health and Human Services. http://www.fda.gov/AdvisoryCommittees/Calendar/ucm226970.htm [cited 2–3 Dec 2010]
- 11. DeVita P, Hortobágyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol 88: 1804–1811, 2000 [DOI] [PubMed] [Google Scholar]
- 12. DeVita P, Hortobágyi T. Obesity is not associated with increased knee joint torque and power during level walking. J Biomech 36: 1355–1362, 2003 [DOI] [PubMed] [Google Scholar]
- 13. DeVita P, Hortobágyi T, Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc 30: 1481–1488, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol 33: 2271–2279, 2006 [PubMed] [Google Scholar]
- 15. Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am 42: 1–9, v, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 107: 1755–1767, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res 20: 101–107, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil 79: 317–322, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Fernandez JW, Akbarshahi M, Walter JP, Fregly BJ, Pandy MG. Evaluation of predicted knee-joint muscle forces during gait using an instrumented knee implant. J Orthop Res 27: 1326–1331, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Krampla WW, Newrkla SP, Kroener AH, Hruby WF. Changes on magnetic resonance tomography in the knee joints of marathon runners: a 10-year longitudinal study. Skeletal Radiol 37: 619–626, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Larsson UE, Mattsson E. Influence of weight loss programmes on walking speed and relative oxygen cost (%VO2max) in obese women during walking. J Rehabil Med 35: 91–97, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 176: S1–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu MQ, Anderson FC, Schwartz MH, Delp SL. Muscle contributions to support and progression over a range of walking speeds. J Biomech 41: 3243–3252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malatesta D, Vismara L, Menegoni F, Galli M, Romei M, Capodaglio P. Mechanical external work and recovery at preferred walking speed in obese subjects. Med Sci Sports Exerc 41: 426–434, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Matrangola SL, Madigan ML, Nussbaum MA, Ross R, Davy KP. Changes in body segment inertial parameters of obese individuals with weight loss. J Biomech 41: 3278–3281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord 21: 380–386, 1997 [DOI] [PubMed] [Google Scholar]
- 27. McGibbon CA, Krebs DE. Compensatory gait mechanics in patients with unilateral knee arthritis. J Rheumatol 29: 2410–2419, 2002 [PubMed] [Google Scholar]
- 28. Messier S, Ettinger W, Doyle T, Morgan T, James M, O'Toole M, Burns R. Obesity: effects of gait in an osteoarthritic population. J Appl Biomech 12: 161–172, 1996 [Google Scholar]
- 29. Messier SP, DeVita P, Cowan RE, Seay J, Young HC, Marsh AP. Do older adults with knee osteoarthritis place greater loads on the knee during gait? A preliminary study. Arch Phys Med Rehabil 86: 703–709, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum 52: 2026–2032, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Messier SP, Legault C, Loeser RF, Van Arsdale SJ, Davis C, Ettinger WH, Devita P. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis Cartilage 19: 272–280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum 52: 2835–2844, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Newham DJ, Harrison RA, Tomkins AM, Clark CG. The strength, contractile properties and radiological density of skeletal muscle before and 1 year after gastroplasty. Clin Sci (Lond) 74: 79–83, 1988 [DOI] [PubMed] [Google Scholar]
- 34. Peyrot N, Morin JB, Thivel D, Isacco L, Taillardat M, Belli A, Duche P. Mechanical work and metabolic cost of walking after weight loss in obese adolescents. Med Sci Sports Exerc 42: 1914–1922, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Plewa M, Cieslinska-Swider J, Bacik B, Zahorska-Markiewicz B, Markiewicz A, Błaszczyk JW. Effects of weight loss treatment on selected kinematic gait parameters in obese women. J Human Kinetics 18: 3–14, 2007 [Google Scholar]
- 36. Segal NA, Yack HJ, Brubaker M, Torner JC, Wallace R. Association of dynamic joint power with functional limitations in older adults with symptomatic knee osteoarthritis. Arch Phys Med Rehabil 90: 1821–1828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segal NA, Yack HJ, Khole P. Weight, rather than obesity distribution, explains peak external knee adduction moment during level gait. Am J Phys Med Rehabil 88: 180–188; quiz 189–191, 246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma L. The role of varus and valgus alignment in knee osteoarthritis. Arthritis Rheum 56: 1044–1047, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol 18: 147–156, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol 22: 533–537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns MA, Simon SR. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil 72: 1065–1070, 1991 [PubMed] [Google Scholar]
- 42. Wadstrom C, Larsson L, Knutsson E, Edstrom L. The effect of excessive weight loss on skeletal muscle in man. A study of obese patients following gastroplasty. Eur J Surg 157: 347–354, 1991 [PubMed] [Google Scholar]
- 43. Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev 7: 239–250, 2006 [DOI] [PubMed] [Google Scholar]