Abstract
Mucosal barrier dysfunction contributes to gastrointestinal diseases. Our aims were to validate urine sugar excretion as an in vivo test of small bowel (SB) and colonic permeability and to compare permeability in patients with irritable bowel syndrome-diarrhea (IBS-D) to positive and negative controls. Oral lactulose (L) and mannitol (M) were administered with 99mTc-oral solution, 111In-oral delayed-release capsule, or directly into the ascending colon (only in healthy controls). We compared L and M excretion in urine collections at specific times in 12 patients with IBS-D, 12 healthy controls, and 10 patients with inactive or treated ulcerative or microscopic colitis (UC/MC). Sugars were measured by high-performance liquid chromatography-tandem mass spectrometry. Primary endpoints were cumulative 0–2-h, 2–8-h, and 8–24-h urinary sugars. Radioisotopes in the colon at 2 h and 8 h were measured by scintigraphy. Kruskal-Wallis and Wilcoxon tests were used to assess the overall and pairwise associations, respectively, between group and urinary sugars. The liquid in the colon at 2 h and 8 h was as follows: health, 62 ± 9% and 89 ± 3%; IBS-D, 56 ± 11% and 90 ± 3%; and UC/MC, 35 ± 8% and 78 ± 6%, respectively. Liquid formulation was associated with higher M excretion compared with capsule formulation at 0–2 h (health P = 0.049; IBS-D P < 0.001) but not during 8–24 h. UC/MC was associated with increased urine L and M excretion compared with health (but not to IBS-D) at 8–24 h, not at 0–2 h. There were significant differences between IBS-D and health in urine M excretion at 0–2 h and 2–8 h and L excretion at 8–24 h. Urine sugars at 0–2 h and 8–24 h reflect SB and colonic permeability, respectively. IBS-D is associated with increased SB and colonic mucosal permeability.
Keywords: colon, small intestine, excretion, mannitol, lactulose
disruption of intestinal mucosal barrier function is thought to play a role in the etiopathogenesis of a variety of gastrointestinal diseases including inflammatory bowel disease (21), enteropathy attributable to nonsteroidal anti-inflammatory drugs (2), celiac disease (4, 29), irritable bowel syndrome (IBS) (9), severe acute pancreatitis (16), and chronic liver disease (7, 11). Edematous gut wall secondary to heart failure may also alter intestinal barrier function (27). The intestinal barrier is typically assessed in vivo by permeability tests, which involve oral administration of inert, water-soluble probe molecules such as sugars and radiolabeled EDTA that are absorbed through the intestinal mucosa into the bloodstream and recovered unchanged in the urine. Alternatively, mucosal barrier function is measured using biopsies from the intestinal segment of interest and assessing the passage of probe molecules of different molecular sizes using the Ussing chamber technique in vitro.
Among the molecules used to measure intestinal permeability in vivo, the saccharides lactulose and mannitol have been considered ideal for testing small intestinal permeability. Mannitol is a monosaccharide with a molecular diameter of 6.5 Å (3), which is hypothesized to freely move across the small intestine at the tip of the villi, where there are channels to allow permeability of such small molecules. In contrast, lactulose is a disaccharide, 9.5 Å (3) in molecular diameter, which cannot traverse through these small channels but can move through larger ones found in the villous base and crypt. Because mannitol and lactulose are partially degraded by colonic bacteria, their use for measurement of colonic permeability has been questioned. However, Meddings and Gibbons (20) showed that 25.9 ± 23% (SE) of mannitol and 25.9 ± 10% of lactulose remained after incubation in a simulated rat colon microbial environment; therefore, this bacterial metabolism should not differentially alter cumulative urine excretion of these sugars.
The artificial disaccharide sucralose and chromium-labeled EDTA (51Cr-EDTA) do not undergo fermentation by colonic bacterial fermentation, and they are therefore considered ideal for characterizing colonic permeability. However, patients who have undergone ileostomy pass a considerable proportion of orally ingested sucralose in the urine, suggesting that it is absorbed in the small intestine (1, 23). A recent study assessed the 24-h excretion profile of lactulose, mannitol, and sucralose in adults, and the cumulative recovery of all three molecules was found to be similar over time (19), confirming that sucralose is not selectively absorbed in the colon.
Many previous studies have used only one probe for testing permeability; however, the use of a single probe molecule has traditionally been thought not to account for interindividual differences of transit, which can affect results of permeability measurement. Instead, the use of two different-sized molecules attempts to correct the measurement of permeability for comparisons between groups, assuming that an individual's transit profile affects all molecules in solution similarly. A monosaccharide and disaccharide, such as mannitol and lactulose, respectively, are recommended to test intestinal permeability.
Several studies have used the urinary saccharide excretion (USE) test to document altered permeability in IBS. However, the previous studies (see Table 1) used nonstandardized, highly variable time intervals for the requisite urinary collections, rendering it unclear whether small intestinal and/or colonic permeability were being measured.
Table 1.
Past studies evaluating permeability differences in IBS
| Reference | IBS Group | Probe | In Vivo/In Vitro | Urine Collection Timing, hr | Permeability |
|---|---|---|---|---|---|
| Spiller et al. (30) | PI-IBS | Lactulose, mannitol | In vivo | 0–6 | Increased |
| Marshall et al. (18) | IBS | Sucrose, lactulose, mannitol | In vivo | Overnight | Increased |
| Dunlop et al. (9) | IBS-D (PI and non-PI), IBS-C | 51Cr-EDTA | In vivo | 0–3, 3–5, 5–24 | Increased |
| Shulman et al. (28) | Children with IBS and functional abdominal pain | Sucrose, lactulose, mannitol, sucralose | In vivo | 0–3 | Increased (sucrose/lactulose; sucralose/lactulose only) |
| Gecse et al. (10) | IBS fecal supernatant exposed to mouse colon | FITC-labeled dextran | In vitro | N/A | Increased |
| Kerckhoffs et al. (13) | IBS (all subtypes) | PEG, lactulose, mannitol | In vivo | 0–24, 0–6 | No difference |
| Piche et al. (24) | IBS (all subtypes) colonic biopsies | FITC-sulfonic acid | In vitro | N/A | Increased |
| Zhou et al. (34) | IBS-D | Lactulose, mannitol | In vivo | 0–24 | Increased in 39% of patients |
| Lee et al. (15) | IBS-D rectal biopsies | Horseradish peroxidase | In vitro | N/A | Increased |
| Zhou et al. (33) | IBS-D | Lactulose, mannitol | In vivo | 0–5, 6–24 | Increased in 42% of patients |
IBS, irritable bowel syndrome; PI, postinfectious; IBS-D, IBS with diarrhea; IBS-C, IBS with constipation; PEG, polyethylene glycol.
There have been no methodological enhancements in the in vivo measurement of permeability since the adoption of two or three sugar excretion tests and using the excretion ratios among the sugars to correct for potential differences in the site of absorption of the probes. In a prior preliminary study, the importance of timing urine collections for measurement of permeability was identified; we showed that more than 50% of orally ingested saccharides in solution had reached the colon in just 2 h (5), far more rapidly than the 3, 5, or 6 h typically used to estimate small bowel permeability. This same study also showed that the use of excretion ratios among sugars needs to be interpreted carefully.
We performed two studies to address the following aims: 1) to validate the USE test as a measurement of small bowel and colonic mucosal permeability, and specifically to identify the optimal time for USE to measure small bowel permeability in healthy patients and patients with IBS-diarrhea (IBS-D); and 2) to compare small intestinal and colonic permeability in patients with IBS-D with healthy volunteers and a positive control group of patients with ulcerative or microscopic colitis (disease states known to disrupt the intestinal epithelial barrier). The inclusion of the positive control group was intended to confirm that the USE test is sensitive enough to detect permeability changes in patients with presumed disruption of the intestinal barrier.
MATERIALS AND METHODS
Design and Participants
In the first study, we compared USE after oral ingestion of liquid and delayed-release capsule formulations vs. intracolonic administration of lactulose and mannitol dissolved in radiolabeled solution to confirm the location of saccharides at standard time points by radioscintigraphy in healthy volunteers. Thus 12 healthy volunteers participated in a three-way crossover trial evaluating the urinary excretion of lactulose and mannitol after oral liquid formulation, oral delayed-release capsule, or intracolonic administration. All participants underwent all three study arms on separate occasions, the order of testing was randomized, and laboratory personnel conducting the urine assays were blinded.
In the second study, 12 patients with IBS-D per Rome III criteria and 10 patients with biopsy-proven diagnoses of either treated/inactive microscopic or ulcerative colitis were recruited and underwent urinary excretion measurements of lactulose and mannitol after ingestion of both oral liquid and delayed-release capsule formulations on separate occasions; they did not undergo intracolonic saccharide administration. Order of testing was again randomized, and the personnel conducting the urine assays were blinded. Data from the 12 healthy controls in the first study were used as a negative control for comparison with the two disease groups. An ancillary analysis that utilized the data from the first and second studies evaluated the optimal time for USE after liquid formulation to measure small bowel permeability in the conditions of greatest interest in our laboratory, that is, healthy controls and patients with IBS-D.
The studies were all approved by the Mayo Clinic Institutional Review Board, and all participants signed informed consent before screening. Subjects were excluded if they were outside the age range of 18–75 yr, were females who were pregnant or trying to become pregnant, had used tobacco products within the prior 6 mo, had used oral or topical corticosteroids within the previous 6 wk (except for patients with microscopic or ulcerative colitis who required steroid therapy), or had used nonsteroidal anti-inflammatory drugs within the previous week, or any other prescription, over-the-counter, or herbal medications that could alter gastrointestinal function 2 days before starting the study. They were required to refrain from ingestion of all artificial sweeteners 2 days before the study began until the end of all trials.
Saccharides and Radioisotopes
Lactulose (1,000 mg) (L7877, Sigma-Aldrich) and mannitol (200 mg) (M8429, Sigma-Aldrich) were utilized for all arms of the two studies. For the liquid formulation, these sugars were administered with 99mTc- diethylenetriamine pentaacetic acid (DTPA) in 250 ml of water. In the capsule arm, the same amounts of the saccharides were delivered in a methacrylate-coated gelatin capsule swallowed with 250 ml of water. 111In-DTPA in 100 μl was applied by impregnating cellulose filter paper, and the capsule was then coated with the pH-sensitive polymer, methacrylate, which dissolves in the more neutral pH of the distal ileum to ensure delivery of the contents of the capsule into the ileocolonic region. Radioisotope was used to determine amount and location of the saccharides at standardized time points.
For the third arm, the healthy participants received 2 l of a standard polyethylene glycol colon cleansing solution the evening before the procedure, and participants remained fasting after midnight. Intravenous midazolam was used for sedation. Participants underwent colonoscopy, and the saccharides dissolved in 250 ml of water containing 99mTc-DTPA were administered into the ascending colon with the use of a through-the-scope flexible catheter. Ten additional milliliters of water were injected to rinse the tube of the solution and isotope. Radioisotope was used to document placement of sugars into the right side of the colon.
Urine Collections, Radioscintigraphic Imaging/Calculations, and Meal Standardization
After oral ingestion of the sugars in liquid or capsule forms, urine was collected every 30 min for the first 2 h (when the participant was able to provide a specimen, and cumulated for the entire 2 h), every 2 h for the next 6 h, and from 8 to 24 h. After intracolonic administration of sugars, urine was collected every 30 min for the first 2 h and every 2 h for the next 6 h. The total volume of each collection was measured, and an aliquot from each collection was obtained to estimate the total content of each sugar for the different time intervals. The urine aliquot was stored at −20°C until it was thawed for analysis.
In studies that involved the liquid formulation, γ-camera images were obtained every 30 min for the first 2 h, every 2 h for the next 6 h, and at 24 h to determine the location of liquid at each time point. In studies using the capsule, images were obtained at the same time points to document time of arrival of the capsule into the right colon, identified by radioopaque markers placed over the right anterior superior iliac spine. For the studies with intracolonic administration, images were obtained every 30 min for the first 2 h and then every 2 h for the next 6 h to document the sugars that remained within the colon.
Participants ingested a standardized meal during the first 8 h to avoid potential variations in gastrointestinal transit times. Specifically, 500 ml of water was given 30 min after sugar and radioisotope administration to aid in the collection of urine. A breakfast of egg, toast, and water was given after the 2-h camera image and a lunch of chicken, potato, and water was offered after the 6-h camera image. Water was allowed ad libitum throughout the day.
Measurement of Urinary Saccharides
Urinary saccharide concentrations were measured by high-performance liquid chromatography-tandem mass spectrometry. Details of this methodology are described elsewhere in the literature (6).
Data and Statistical Analysis
Intestinal permeability was measured using the urinary lactulose and mannitol excretion test in all subjects. The primary endpoints for the study were the cumulative excretion of liquid and encapsulated lactulose and mannitol during three nonoverlapping intervals: 0–2, 2–8, and 8–24 h, and in addition the 0–8-h cumulative excretion of intracolonic lactulose and mannitol: cumulative excretion = [concentration of sugar (μg/ml)]* total urine volume (ml).
The secondary endpoints were the 0–2-h cumulative excretion of liquid and encapsulated lactulose and mannitol and the lactulose: mannitol ratio (L:M ratio): L:M ratio = 0.2 × (cumulative excretion lactulose)/(cumulative excretion mannitol).
In the first study involving only healthy volunteers, the cumulative excretions of the saccharides by the three routes of administration were compared using the paired t-test or nonparametric alternative, the Wilcoxon signed-rank test, as warranted; the 0–8-h excretion after intracolonic administration of sugars was compared with the 2–8-h excretion after ingestion of liquid and encapsulated sugars.
To appraise the optimal time for USE to measure small bowel permeability, data from urine excretion after ingestion of liquid formulation sugars were analyzed for the patients with IBS-D and healthy controls. Because the delayed-release capsule was intended to “protect” the sugars from absorption in the small intestine during transit of the capsule through the small bowel, we considered it best to try to optimize or validate the USE for small bowel permeability following liquid formulation. The time before the arrival of >25% isotope in the colon (TCF25%) was calculated by radioscintigraphy; this was selected as the comparator because it reflected the time when 75% of the sugars were still located in the small bowel. We assessed the agreement between the observed USE for TCF25% and different time periods (0–60, 0–90, 0–120 min). The 0–30-min urine collection was not calculated, as several participants could not provide a urine specimen at 30 min. The (linear) concordance correlation coefficient (CCC) and Bland-Altman plot between each collection period and TCF25% excretion were analyzed.
In the second study, the overall association of subject group (healthy volunteers, patients with IBS-D, patients with ulcerative/microscopic colitis) with the cumulative excretion of the saccharides after oral liquid and separately, delayed-release capsule formulations, was assessed using the Kruskal-Wallis test (using an α level of 0.05). The association of subject group with saccharide excretion for pairs of subject groups was assessed using the Wilcoxon rank-sum test (using an α level of 0.0167, i.e., adjusting for 3 tests).
Statistical Power
Previous data in the literature that used a 0–6-h urinary excretion profile indicated an intersubject standard deviation in L:M excretion ratios of 0.0021 with a mean of 0.0088 [coefficient of variation = 24% (30)]. Because we did not have an estimate of the variation in (within subject) Δs for capsule vs. liquid formulation of L:M ratios before initiation of the study, we developed our study sample size assuming the variation would be greater and analogous to that between rather than within subjects. This precaution was taken to ensure that we did not underpower our proposed crossover study. Using an SD = 0.0021, there was ∼91% power to detect a difference of 0.0022 (vs. a zero difference in 0–6-h urine L:M ratios) between liquid and capsule formulations with n = 12 using a paired t-test. Note that this detectable difference in L:M ratio values between liquid and capsule formulations is 25% of the difference reported the literature between IBS and health using a liquid formulation (30), indicating that the study had sufficient power to identify differences in L:M ratio.
RESULTS
Participant Demographics
Table 2 lists demographic information for each group. All 12 healthy participants completed the three arms of the study. Ten of twelve patients with IBS-D completed both liquid and capsule arms of the study; one patient was only able to participate in the capsule arm and another patient only in the liquid arm of the study. Seven patients with microscopic colitis and three patients with ulcerative colitis with treated or inactive disease participated in both arms of the study and were combined into one group for analyses.
Table 2.
Demographics of participants
| Health (n = 12) | IBS-D (n = 12) | Microscopic/ Ulcerative Colitis (n = 10) | |
|---|---|---|---|
| Age, yr | 37.4 ± 2.6 | 42.4 ± 3.0 | 58.0 ± 4.5 |
| Female sex (n) | 11 | 12 | 6 |
| BMI, kg/m2 | 24.4 ± 0.8 | 30.9 ± 1.8 | 30.6 ± 1.9 |
| Tobacco (n) | 0 | 0 | 0 |
Applicable values shown are means ± SE or frequencies.
BMI, body mass index.
Identification of Optimal Time for Evaluation of Small Bowel Permeability
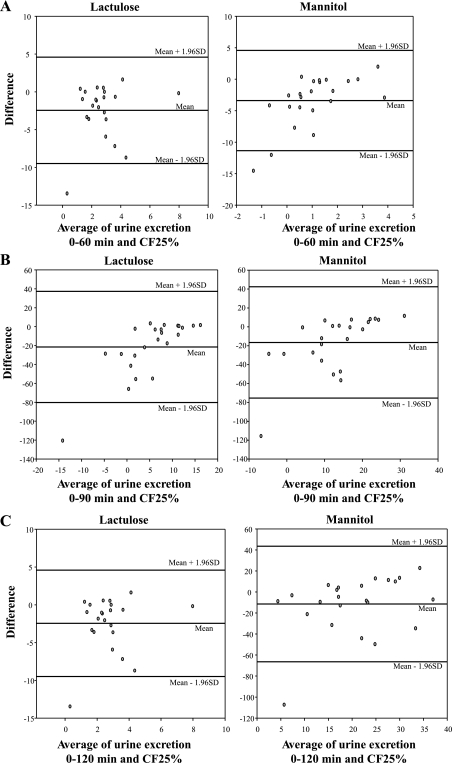
The median time for CF25% was 52 min [IQR 32, 82 (range 17–146)] for health (n = 12) and 71 min [48, 146 (37–195)] for IBS-D. Bland-Altman plots (Fig. 1) exhibited similar differences across the entire range of mean USE for each time interval and TCF25%. The CCCs for the 0–60-, 0–90-, and 0–120-min excretions were not significantly different for either lactulose or mannitol (Table 3). We concluded that the 0–120-min collection best reflects small bowel residence of the saccharides. Therefore, in the remainder of the studies performed here, we used the 0–120-min collection to assess small bowel permeability.
Fig. 1.
Bland-Altman plots evaluating the differences in excretion for 0–60 min and time before the arrival of >25% isotope in the colon (TCF25%) across different mean sugar excretion rates (A). Note that the difference is similar across different mean excretion rates and that a similar pattern of the differences is observed with the 0–90-min (B) and 0–120-min (C) collections. Also note that the size of the differences appears smaller with 0–120-min than for the 0–90-min collections.
Table 3.
CCCs comparing urine sugar excretions at different times
| Response | Lactulose (CCC, 95% CI) | Mannitol (CCC, 95%CI) |
|---|---|---|
| TCF25% | — | — |
| 0-60 min | 0.64 (0.32, 0.83) | 0.26 (−0.16, 0.60) |
| 0-90 min | 0.59 (0.29, 0.79) | 0.20 (−0.08, 0.45) |
| 0-120 min | 0.48 (0.23, 0.68) | 0.16 (−0.03, 0.33) |
Excretion is during the time before arrival of >25% isotope in the colon (TCF25%).
CCC, concordance correlation coefficient; CI, confidence interval.
Study 1: USE after Oral Liquid and Delayed-Release vs. Intracolonic Administration in Healthy Controls
Proportion of radioisotope in the colon at prespecified times.
Two hours after ingestion of liquid, the mean (± SE) proportion of radioisotope in the colon was 62 ± 9% (median 66%). The percentage of isotope in the colon at 8 h was 89 ± 3% (median 89.5%).
The mean time of arrival of the radiolabeled capsule in the colon was 345 ± 37 min (median 300 min). Scintiscans demonstrated that the intracolonic administration was correctly delivered to the right colon. The capsule did not reach the colon within 2 h postingestion in any participant. It was located in the colon by 8 h in all participants.
Urinary excretion profile with three routes of administration of sugars.
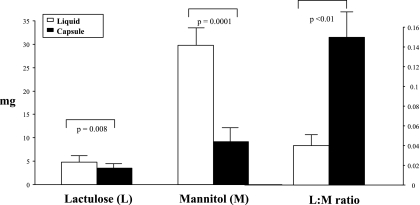
Figures 2–4 show the cumulative urinary saccharide excretion, summarized as the mean across all 12 healthy volunteers, and the calculated L:M ratio from 0–2, 2–8, and 8–24 h, respectively. For the intracolonic infusion arm, we cumulated the entire 8-h collection and compared it with the 2–8-h collections by the two other formulations (Fig. 3). Urinary excretion of mannitol and lactulose during 0–2 h was greater with the oral liquid formulation than with the capsule; this is consistent with the intended protection of the sugars from absorption in the small bowel during this time.
Fig. 2.
0–2-h urinary saccharide excretion (mg) and lactulose:mannitol (L:M) ratio after administration of sugars in liquid and capsule formulations. Note there was a 5:1 intake of lactulose to mannitol. Values shown are means (± SE).
Fig. 3.
0–8-h (intracolonic formulation) and 2–8-h (liquid and capsule formulations) urinary saccharide excretion (mg) and L:M ratio after administration of sugars. Note there was a 5:1 intake of lactulose to mannitol. Values shown are means (± SE).
Fig. 4.
8–24-h urinary saccharide excretion (mg) and L:M ratio with liquid and capsule formulations. Note there was a 5:1 intake of lactulose to mannitol. Values shown are means (± SE).
We observed that the 2–8-h urinary excretion of mannitol after oral capsule formulation was very similar to the 0–8-h mannitol excretion after intracolonic infusion of the sugar. In contrast, it was higher with the oral liquid formulation. It is worth noting that, on average, there was still ∼34% of the liquid formulation in the small intestine at 2 h, and the higher urine mannitol excretion at 2–8 h might reflect, in part, ongoing small bowel permeability, whereas the capsule delivered the sugars predominantly to the colon. There was no difference in lactulose excretion by oral liquid and capsule formulations, suggesting that the two methods of administration are similar in measurement of permeability of the disaccharide during the 2–8-h period. The 2–8-h urinary excretions of lactulose with liquid or capsule formulation were, however, much lower than the 0–8-h excretion after intracolonic infusion of the sugar, suggesting that absorption of this disaccharide may be altered by bowel preparation.
The 0–8/2–8-h L:M urinary excretion ratios via the three formulations were significantly different because of the observed differences in cumulative excretion of mannitol and lactulose outlined above.
The 8–24-h urinary excretions of both liquid and capsule lactulose and mannitol are similar, consistent with the observation that ∼90% (median) of the sugars had entered the colon by 8 h. Thus the 8–24-h L:M ratios by the two formulations were also found to be similar, as they reflected the presence of 90% of the sugars in the colon.
It is worth noting that even though five times as much lactulose as mannitol was given (Fig. 4), there was a higher amount [total mass and hourly fractional excretion rate (not shown)] in excretion of mannitol than lactulose with the three forms of administration in all the timed urine collections: 0–2, 0–8/2–8, and 8–24 h.
Study 2: Comparison of USE after Oral Liquid and Delayed-Release Administration in Patients with Health, IBS-D, and Microscopic/Ulcerative Colitis
Timed proportion of radioisotope into the colon.
Two hours after ingestion of liquid, the mean (± SE) proportion of radioisotope in the colon was 56 ± 11% in the patients with IBS-D and 35 ± 8% for the combined colitis patients, compared with 62 ± 9% in health. Eight hours after ingestion of liquid formulation, the mean proportion of radioisotope in the colon was 90 ± 3% in the patients with IBS-D and 78 ± 6% for the patients with combined colitis, compared with 89 ± 3% in health. The mean (± SE) time of arrival of the radiolabeled capsule in the colon was 322 ± 36 min for the IBS-D group and 313 ± 53 min for the patients with colitis, compared with 345 ± 37 min in health. The capsule had reached the colon at 60 min in one patient with IBS-D and at 120 min in one patient with IBS-D and one with colitis. Eight hours after ingestion, the capsule was located in the colon in all participants.
Urinary saccharide excretion and L:M ratios after oral liquid and delayed-release capsule administration.
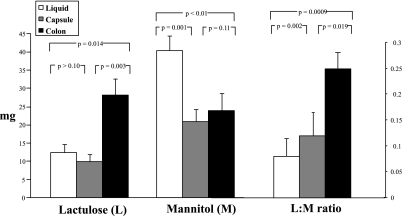
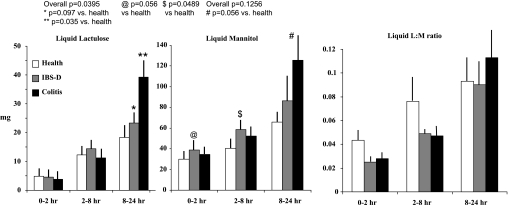
Figure 5 shows the comparative urinary lactulose and mannitol excretion and L:M ratio after oral liquid administration. Increased urinary excretion of both sugars at 8–24 h after oral liquid formulation (mannitol, P = 0.056; lactulose, P = 0.035) was observed in the colitis group compared with health, not at 0–2 h. There were no significant associations of sugar excretion with group for the colitis and IBS-D groups.
Fig. 5.
Cumulative excretion (mg) and L:M ratio for liquid formulation saccharides in health, irritable bowel syndrome with diarrhea (IBS-D), and ulcerative/microscopic colitis. Values shown are means (± SE).
With the liquid formulation, comparison of health and IBS-D (Fig. 5) showed an association of urine mannitol excretion at 0–2 h (P = 0.056) and 2–8 h (P = 0.0489) and a numerical but nonsignificant difference in lactulose excretion (P = 0.097) at 8–24 h.
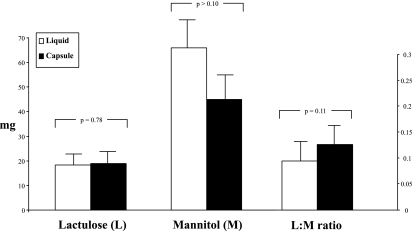
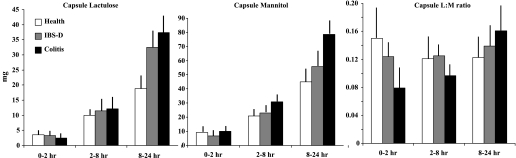
Figure 6 shows the excretion data and L:M ratios after the delayed-release capsule formulation in the health, IBS-D, and colitis groups. Although the data trends are similar to those observed with the liquid formulation, no significant associations with group were observed.
Fig. 6.
Cumulative excretion (mg) and L:M ratio for capsule formulation saccharides in health, IBS-D, and ulcerative/microscopic colitis. Values shown are means (± SE).
DISCUSSION
This study provides information on the optimal time to collect urine in the USE test for assessing small intestinal and colonic permeability. It also provides more convincing evidence of increases in both small bowel and colonic permeability in IBS-D, on the basis of our validated times for urine collections.
Validation of Optimal Urinary Excretion Profiles for Small Bowel and Colonic Permeability
As shown in Table 1, the prior literature documents the use of several different time periods to assess small bowel permeability including 0–2, 0–3, 0–5, and 0–6 h. In addition, colonic permeability with 51Cr-EDTA typically used three collections, 0–3, 3–5, and 5–24 h after ingestion of the probe molecule. Dunlop et al. (9) identified a higher excretion during 0–3 h in postinfectious IBS than in controls; however, no differences were identified in the 3–5- or 5–24-h collections, suggesting that the higher excretion from 0–3 h reflected predominantly small bowel permeability. The lack of difference in the 5–24-h collections suggests that these patients with postenteritis IBS did not have increased colonic mucosal permeability. Given the molecular weight (292 Daltons) and diameter [∼10Å (3)] of EDTA and the fact that small intestinal permeability is greater than colonic permeability, it is conceivable that this measurement of permeability by 51Cr-EDTA is more sensitive to identify changes in small bowel than colonic permeability in IBS.
We used the simultaneous abdominal imaging approach to assess when the isotope was located in the colon, as a means of identifying the time when the urinary excretion reflected mostly small bowel permeability. Overall, our data suggest that 0–2 h is the most appropriate collection period to assess sugar excretion as a measure of small bowel permeability and that the liquid formulation is most useful for this assessment. When we protected the sugars from proximal small intestinal delivery by means of a delayed-release capsule, we observed the expected difference in sugar excretion during 0–2 h between liquid and capsule formulations, as the latter had not released most of the sugars to allow their absorption.
Despite the fact that a small proportion of the sugars was identified in the colon by 2 h, we found that the CCCs between 0–60-, 0–90-, and 0–120-min excretions and TCF25% excretion of each saccharide were not significantly different. In our study, almost 70% of patients with IBS-D had TCF25% of >120 min. Because we are interested in studies of small bowel permeability in human diseases such as IBS, our data suggest that the 0–120-min urine collection reflects small bowel residence of the saccharides. This collection period is most practical, allowing sufficient contact time with small bowel mucosa and adequate time for urine excretion to occur. We therefore propose that 0–2 h be used as the standard collection time for studies of small bowel permeability in vivo in humans, especially if an assessment of time to arrival of saccharides into the colon by simultaneous imaging is not possible.
The oral liquid and capsule formulations were associated with similar urinary excretion profiles between 8 and 24 h when the Scintiscans showed that most (median ∼90%) of the isotopes were in the colon, thus reflecting colonic mucosal permeability. To further validate the observation that urine excretion by liquid formula beyond 2 h reflected (in part) colonic mucosal permeability, we compared the excretion of sugars 0–8 h after intracolonic administration with excretion 2–8 h after oral liquid and capsule formulation. As noted in Fig. 3, mannitol excretion is not different by capsule and intracolonic administration, but both are lower than oral liquid formulation. This may reflect the fact that at 2 h there was still about a third of the liquid in the small bowel by scintigraphy, and therefore the mannitol excretion at 2–8 h after liquid formulation is attributable to both small bowel as well as colonic permeability. We also found higher lactulose excretion 0–8 h after intracolonic administration compared with oral ingestion of both liquid or capsule formulations, suggesting that colon cleansing with polyethylene glycol-containing solution may increase absorption and subsequent excretion of the disaccharide.
Overall, these observations suggest that the 0–2-h urinary mannitol excretion after oral liquid formulation provides the best estimation of small bowel permeability, on the basis of location of the sugars and the much higher absorption and excretion of the monosaccharide mannitol than the disaccharide lactulose. The 8–24-h excretion of mannitol and lactulose after either oral liquid or capsule formulation provides similar summaries of colonic permeability. On the other hand, the 2–8-h collections reflect both small bowel and colonic permeability. Therefore, urine collections beyond 2 h cannot be used to selectively assess small bowel permeability. Data utilizing urine collections from 0–6 h after ingestion of sugars or other probes used in prior studies in the literature (Table 1) to characterize small bowel permeability are likely to include components of both small bowel and colonic permeability.
Interpretation of L:M Ratios
Similar to our observations in a prior study (5), we noted a discrepancy between cumulative urinary excretion of mannitol and the calculated L:M ratio after liquid formulation in the 0–2-, 2–8-, and 8–24-h collections. Traditionally, a higher L:M ratio is interpreted as representing “increased” permeability and vice versa. However, we found that the L:M ratio after liquid formulation increased in value over time, i.e., the L:M ratio in healthy volunteers was 0.04 (mean) at 0–2 h and 0.09 (mean) at 8–24 h, suggesting that the colon is more permeable than the small bowel. This is inconsistent with studies of transepithelial resistance in different regions of the intestine and colon in the literature, which suggest greater resistance in the colon than in the small intestine (17). Furthermore, the L:M ratio with direct colonic infusion was 0.25 (mean), whereas the liquid formulation L:M ratio at 0–2 h was 0.04 (mean), again incorrectly suggesting increased permeability in the colon compared with the small bowel.
Despite the fivefold higher mass of lactulose ingested compared with mannitol, we detected greater cumulative urine excretion by mass of liquid mannitol than lactulose in the 0–2-, 2–8-, and 8–24-h collections in IBS-D and both control groups, suggesting greater absorption of the monosaccharide than the disaccharide. This consequently produced a decrease in the value of the L:M ratio over time, leading to an erroneous impression of decreased permeability in the small intestine.
We also found that the mean L:M ratio in IBS-D was smaller than in health, suggesting lower permeability in IBS-D. This contrasts with the finding of significantly increased cumulative urinary mannitol excretion 0–2 and 2–8 h after ingestion of the liquid formulation sugar, compared with health.
Another example of the L:M ratio leading to misinterpretation is seen in the positive control group of patients with colitis. The expected increase in colonic permeability was identified in this group by the cumulative excretion of each individual sugar; however, the L:M ratio was not significantly increased relative to controls. Utilizing the L:M ratio alone in this group would have failed to detect the increased colonic permeability. It appears clear that increased urinary mannitol excretion during all three timed collections decreases the value of the L:M ratio, leading to an inaccurate interpretation of permeability measurements. Because of our findings, we have come to the conclusion that the L:M ratio needs to be interpreted with caution and that there does not appear to be any advantage or additional information provided by L:M ratios over that from the excretion profile of the individual sugars.
Comparison of Permeability in IBS-D and Positive and Negative Control Groups
The expected increase in urinary saccharide excretion at 8–24 h was observed in the positive control group of treated or inactive ulcerative/microscopic colitis, which are illnesses associated with increased colonic permeability (26). The patterns of urine sugar excretion after both liquid and capsule formulations are similar; however, the liquid formulation was associated with significant differences even with the relatively small sample sizes in this study, and, given the ease of preparation and convenience, the liquid formulation appears to be the more convenient. Compared with healthy controls, 8–24-h urinary lactulose excretion was increased in IBS-D, reflecting higher colonic mucosal permeability. Although there was higher 8–24-h urine mannitol excretion in IBS-D than in healthy controls, this did not reach significance and may represent a type II error.
Several prior studies in the literature have also documented increased permeability (based on the timing of the urine collection), presumably in the small bowel (9) and in the colon (33, 34) in IBS, including IBS-D. The increased urinary mannitol excretion at 0–2 h reflects greater small bowel permeability in IBS-D, on the basis of our simultaneous scintigraphic data documenting location of the sugars. We also observed increased urine mannitol excretion at 2–8 h in IBS after liquid formulation, possibly reflecting the increased small bowel mucosal permeability in IBS-D.
Keita and Soderholm summarized the neuroimmune interactions with the epithelial cells in the regulation of barrier function (12). Neural impulses from extrinsic vagal and/or sympathetic efferent fibers or intrinsic enteric nerves influence mucosal barrier function via direct effects on epithelial cells or via interaction with immune cells. Nerve-mediated activation of the barrier includes corticotropin-releasing hormone or cholinergic pathways. In addition, mucosal mast cells (32), which are implicated in IBS animal models and in some patients with IBS, release a variety of mediators such as tryptase, tumor necrosis factor-α, nerve growth factor, and interleukins with affect transcellular and/or paracellular permeability (12). These neurohormonal and inflammatory influences on permeability suggest that optimizing in vivo permeability measurements is important, as some of these influences may be lost when permeability is tested in vitro in mucosal biopsies, which may also be susceptible to sampling bias.
We did not include sucralose in our sugar cocktail despite the potential advantage that this sugar is not metabolized by colonic bacteria. Sucralose is absorbed in the small intestine [as shown in experiments conducted in ileostomates (1)], and recent evidence suggests its absorption and excretion profile is similar to that of mannitol. The performance on repeat testing of the lactulose and mannitol USE test has been reported in the literature (31).
The noninvasive and relatively simple measurements using two sugars of different molecular sizes add to the potential usefulness of the method. We have demonstrated the expected increase in colonic permeability in inactive ulcerative and microscopic colitis and identified both small bowel and colonic permeability increase in patients with IBS-D. The method can be applied to study mucosal function in patients with IBS, food hypersensitivity including gluten intolerance, and the effects of treatments directed at mucosal pathophysiology such as anti-inflammatory agents, mast cell stabilizers, and modulation of the microbial flora with nonabsorbed antibiotic or probiotics (8, 14, 22, 25).
In conclusion, we have validated the optimal design and interpretation of the two-sugar USE test to measure small bowel and colonic permeability in humans. The liquid formulation appears to be the most sensitive and has the additional appeal of simplicity and convenience. Measurements of L:M ratios do not appear to add relevant information to the absolute excretion of sugars at 0–2 and 8–24 h after oral administration. Patients with IBS-D have increased small bowel and colonic permeability.
GRANTS
Dr. Camilleri's research in IBS is supported by NIH grants R01-DK079866 and 1RC1-DK086182.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). A. Rao was a fellow coinvestigator and conducted participant screening and writing. M. Camilleri contributed to PI, concept, writing, and colonic intubations. D. Eckert was a study coordinator and participant recruiter. I. Busciglio was a study coordinator and technologist. D. Burton and M. Ryks were technologists and worked with transit measurements and assays. B. Wong was a coinvestigator and worked with the writing. J. Lamsam was a technologist and worked with biochemical assays. R. Singh worked with biochemical assays. A. R. Zinsmeister was a biostatistician and coinvestigator and worked with concept and writing.
ACKNOWLEDGMENTS
The excellent secretarial support of Mrs. Cindy Stanislav is gratefully acknowledged.
REFERENCES
- 1. Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand 182: 171–177, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104: 1832–1847, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 108: 1566–1581, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bjarnason I, Peters TJ, Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet 1: 323–325, 1983 [DOI] [PubMed] [Google Scholar]
- 5. Camilleri M, Nadeau A, Lamsam J, Linker-Nord S, Ryks M, Burton D, Sweetser S, Zinsmeister AR, Singh R. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 22: e15–e26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R. Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil 21: e734–e743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G, Martorelli L, Esposito P, Carteni M, Del Vecchio Blanco C, Loguercio C. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis 42: 200–204, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Corinaldesi R, Stanghellini V, Cremon C, Gargano L, Cogliandro RF, De Giorgio R, Bartesaghi G, Canovi B, Barbara G. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Aliment Pharmacol Ther 30: 245–252, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101: 1288–1294, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57: 591–599, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Kalaitzakis E, Johansson JE, Bjarnason I, Björnsson E. Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol 41: 326–330, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 22: 718–733, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Kerckhoffs AP, Akkermans LM, de Smet MB, Besselink MG, Hietbrink F, Bartelink IH, Busschers WB, Samsom M, Renooij W. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci 55: 716–723, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59: 1213–1221, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Lee JW, Park JH, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci 55: 2922–2928, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 36: 192–196, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B 180: 591–598, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SMInvestigators WEL Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkteron, Ontario. Aliment Pharmacol Ther 20: 1317–1322, 2004 [DOI] [PubMed] [Google Scholar]
- 19. McOmber ME, Ou CN, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr 50: 269–275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 114: 83–92, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Miki K, Moore DJ, Butler RN, Southcott E, Couper RT, Davidson GP. The sugar permeability test reflects disease activity in children and adolescents with inflammatory bowel disease. J Pediatr 133: 750–754, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59: 325–332, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Münch A, Söderholm JD, Wallon C, Ost A, Olaison G, Ström M. Dynamics of mucosal permeability and inflammation in collagenous colitis before, during, and after loop ileostomy. Gut 54: 1126–1128, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP, Study Group TARGET Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 364: 22–32, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 17: 362–381, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, Kalra PR, Buhner S, Hermann R, Springer J, Doehner W, von Haehling S, Anker SD, Rauchhaus M. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol 2010. [Epub ahead of print] doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28. Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 153: 646–650, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Maurino E, Meddings JB. Gastrointestinal permeability in celiac disease. Gastroenterology 112: 1129–1136, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47: 804–811, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Elburg RM, Uil JJ, Kokke FT, Mulder AM, van de Broek WG, Mulder CJ, Heymans HS. Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars. J Pediatr Gastroenterol Nutr 20: 184–188, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Wallon C, Söderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann NY Acad Sci 1165: 206–210, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59: 775–784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146: 41–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]