Abstract
The Menkes copper ATPase (Atp7a) and metallothionein (Mt1a) are induced in the duodenum of iron-deficient rats, and serum and hepatic copper levels increase. Induction of a multi-copper ferroxidase (ceruloplasmin; Cp) has also been documented. These findings hint at an important role for Cu during iron deficiency. The intestinal divalent metal transporter 1 (Dmt1) is also induced during iron deficiency. The hypothesis that Dmt1 is involved in the copper-related compensatory response during iron deficiency was tested, utilizing a mutant Dmt1 rat model, namely the Belgrade (b/b) rat. Data from b/b rats were compared with phenotypically normal, heterozygous +/b rats. Intestinal Atp7a and Dmt1 expression was increased in b/b rats, whereas Mt1a expression was unchanged. Serum and liver copper levels did not increase in the Belgrades nor did Cp protein or activity. The lack of fully functional Dmt1 may thus partially blunt the compensatory response to iron deficiency by 1) decreasing copper levels in enterocytes, as exemplified by a lack of Mt1a induction and a lesser induction of Atp7a, 2) abolishing the frequently described increase in liver and serum copper, and 3) attenuating the documented increase in Cp expression and activity.
Keywords: Slc11a2, divalent metal transporter 1, Menkes copper ATPase, ceruloplasmin, metallothionein
the exploration of copper/iron interactions dates back to the early 19th century (29, 30). Copper was thought to influence iron metabolism at the levels of intestinal absorption and release from body storage sites and by being required for cellular Fe utilization during erythropoiesis (56). Reciprocally, iron status can influence copper metabolism (17, 55, 58). Recent findings have identified two multi-copper ferroxidases (FOXs), ceruloplasmin (Cp) and hephaestin (Heph) that represent likely links between iron and copper homeostasis. Heph is required for intestinal iron transport (54), and Cp is necessary for iron release from body storage sites (e.g., hepatocytes, macrophages) (15, 54). Without a functional multi-copper FOX, Fe2+ cannot be oxidized to Fe3+, the transferrin binding form, and Fe2+ will be trapped inside cells (14, 41). Because Cp and Heph are the best known connections between copper and iron metabolism, many previous studies have investigated the expression/regulation of these proteins in various model systems in which iron or copper levels were manipulated. The results of some studies found alterations in copper metabolism in mammals suffering from diet-induced iron deficiency anemia, suggesting that copper might partially mediate the compensatory response to low iron intake (6, 19, 23, 25, 53–55).
Recent studies identified other genes/proteins that exemplify additional links between copper and iron homeostasis, including the Menkes copper ATPase (Atp7a; a copper exporter) and divalent metal transporter 1 (Dmt1) (10, 11). Previous investigations have shown that Atp7a and Dmt1 mRNA and protein levels are increased during iron deficiency (11, 16, 43, 59). Moreover, some studies have shown that Dmt1 can transport copper (2, 52) although the physiological significance of this is unknown. Given the uncertainty of the role of Dmt1 in copper homeostasis, the present studies were undertaken to assess the effect of an inactivating mutation in Dmt1 on copper metabolism during iron deficiency. The model chosen was the Belgrade (b) rat, which resulted from irradiation of 8-day-old female rats, eventually producing a strain with heritable microcytic, hypochromic anemia (48, 49). Early studies on this genetic model of iron deficiency demonstrated a defect in iron uptake into reticulocytes (18) and enterocytes (24, 39). Later, genetic approaches led to the discovery that Nramp2 (now termed Dmt1) was the mutated gene responsible for the Belgrade anemia (21). Surprisingly, the point mutation in the Belgrade rat is identical to the mutation in the microcytic (mk) anemia mouse (22). The same protein (i.e., Dmt1) was also identified almost simultaneously by an expression-cloning technique, and it was shown to function as a transporter of divalent metal ions, including iron (26). It is now clear that the Belgrade and mk mutation in Dmt1 decrease iron absorption from the diet and iron uptake into other cells of the body as a result of a defect in the transferrin cycle, demonstrating an important physiological role for Dmt1 in overall body iron homeostasis.
The present studies analyzed various genetic and biochemical parameters of iron and copper homeostasis in the b/b rat compared with +/b rats, which are phenotypically normal. Although several of the measured parameters were identical between the homozygous mutants and the heterozygotes, key differences were noted. Perhaps of most interest are the facts that 1) the b/b rats did not show an increase in serum and liver copper content, which has been shown to occur in many mammalian species during anemia, and 2) that serum FOX activity (i.e., ceruloplasmin activity) did not increase in the b/b rat, which is in contrast to what has been documented in wild-type rats with dietary iron deficiency anemia (31, 42), in iron-deficient humans (53), and in many species during iron deficiency attributable to hemorrhage (23).
MATERIALS AND METHODS
Chemicals, Reagents, and PCR Primers
Chemicals were obtained mostly from Sigma Aldrich and Fisher Scientific, and were of analytical grade or high purity. Sequencing primers were from IDT. Other sources are mentioned as appropriate.
Experimental Animals
All animal studies were approved by the University of Florida IACUC committee before commencing the investigation. Belgrade rats used in the present studies were adult males and females between the ages of 3.5 and 22 mo, obtained from a breeding colony at the University at Buffalo maintained by Dr. Laura Garrick. The different groups of rats obtained and a description of how they were utilized in the current studies are shown in Table 1. The use of different sexes and ages was necessary because of the expense of maintaining the breeding colony and the smaller litter sizes (24). The +/b rats were fed a normal chow (∼200 ppm Fe), whereas the b/b rats were fed a high-iron-containing chow (∼300 ppm Fe), per the usual husbandry routine. In some experiments, control and iron-deficient, wild-type Sprague Dawley (SD) rats were utilized for comparison. These animals were male rats obtained at weaning from Harlan Laboratories, which were housed in overhanging cages and fed either a semipurified AIN93G-based diet containing 198 ppm (Ctrl) or 3 ppm (FeD) iron for 35 days. This feeding regimen leads to development of iron deficiency anemia, with significantly decreased iron-related hematological parameters (11). All rats were anesthetized by CO2 exposure and killed by cervical dislocation. Blood was collected by cardiac puncture with an 18-gauge stainless-steel needle and transferred into a prechilled Falcon 5 ml polypropylene tube. After 1 h to allow for clotting, the tubes were centrifuged at 1,500 g for 10 min at 4°C. The supernatants (sera) were separated and stored at 4°C for FOX activity assays, which were performed within 48 h. Previous studies demonstrated that the Cp enzyme was stable under these conditions, with no significant decrease in activity noted with storage at 4°C for up to 72 h (42).
Table 1.
Genotype, sex, and age of all rats used in this study
| Genotype | Sex | Age, Mo | Number | Procedure |
|---|---|---|---|---|
| +/b | M | 22 | 1 | A |
| +/b | M | 19 | 1 | A |
| +/b | M | 12 | 2 | A, B, C, D |
| +/b | M | 9 | 3 | A |
| +/b | M | 6 | 1 | A, B, C, D |
| +/b | M | 3.5 | 4 | A, B, C, D, G |
| +/b | M | 3 | 3 | A, B, C, E, F, G |
| b/b | M | 9 | 5 | A |
| b/b | M | 6 | 2 | A, B, C, D |
| b/b | M | 3.5 | 2 | A, B, C, D, G |
| b/b | M | 3 | 1 | A, B, C, E, F, G |
| +/b | F | 4 | 3 | A, B, C, D |
| +/b | F | 3.5 | 2 | A, B, C, D |
| +/b | F | 3 | 3 | A, B, C, E, F, G |
| b/b | F | 4 | 3 | A, B, C, D |
| b/b | F | 3.5 | 1 | A, B, C, D |
| b/b | F | 3 | 3 | A, B, C, E, F, G |
A, hemoglobin and hematocrit, liver qRT-PCR; B, enterocyte qRT-PCR; C, immunoblots; D, para-Phenylenediamine assay; E, ferrozine assay; F, mineral analysis; G, immunohistochemistry analysis.
Enterocyte Isolation and Subcellular Fractionation
Enterocytes were isolated from rat duodenum and proximal jejunum as previously described (9), with modifications. Briefly, an ∼15-cm segment of the rat proximal intestine was removed immediately after death and flushed with ice-cold 1× PBS. It was then cut into three 5-cm-long pieces and everted on wooden sticks. After a brief rinse in 1× PBS, gut segments were incubated on ice in 1× PBS containing 1.5 mM EDTA for 20 min with occasional gentle rotation to release enterocytes, which were subsequently pelleted at 500 g for 5 min. Enterocytes were washed with 1× PBS three additional times and were then used for FOX enzyme analysis, qRT-PCR, and mineral and protein analysis. Enterocytes and portions of livers were snap frozen in liquid N2, then stored at −80°C for RNA isolation, or stored at −20°C for mineral analysis. Cytosol and solubilized particulate membrane fractions were prepared as previously described (13). Briefly, cells were homogenized in buffer 1 (0.05 M Tris·HCl, pH 7.4 at 4°C, 0.05 M NaCl, + protease inhibitor cocktail) and centrifuged at 16,000 g for 15 min. The supernatant was recentrifuged at 110,000 g for 1 h. The resulting supernatant was termed “cytosol”, and the pellet was resuspended in buffer 2 [buffer 1 plus 0.25% (vol/vol) Tween-20]. The suspension was then sonicated for 10 s in an ice water slurry twice, with 5 s chilling in between, at an output power of 5 watts, followed by centrifugation at 16,000 g for 30 min. This supernatant was the solubilized particulate membrane fraction, which was subsequently used for immunoblotting experiments and FOX/amine oxidase activity assays. Activity assays were performed immediately after purification because of the propensity of the membrane fractions to precipitate out of solution (likely attributable to the presence of the detergent).
Elemental Analyses and Hemoglobin and Hematocrit Measurements
Rat liver and serum samples were submitted to the Diagnostic Center for Population and Animal Health (DCPAH) at Michigan State University for mineral analysis. Liver samples were dry-ashed and digested with nitric acid. The digested tissues/serum samples were then diluted and analyzed by Inductively Coupled Plasma-Mass Spectrometry. Hemoglobin (Hb) and Hematocrit (Hct) were measured in house using a HemoCue Hb analyzer (Hemocue AB) and a Readacrit Hct system, respectively, following the manufacturers' instructions.
Quantitative Real-Time PCR
Total RNA was purified from rat liver by TRIzol reagent (Invitrogen), and qRT-PCR was performed as previously described (13). Briefly, 1 μg of RNA was converted to cDNA with the Bio-Rad iScript cDNA synthesis kit, in a 20-μl reaction. One microliter of the cDNA reaction was used with 10 μl of SYBR Green master mix (Bio-Rad), 0.75 μl (0.25 pM) of each forward and reverse gene-specific primer (sequences listed in Table 2), in a 20-μl reaction. Primers were designed to span large introns to minimize the chances of amplification from genomic DNA. Reactions were run in 96-well plates on a Bio-Rad iCycler with the following cycling parameters: 95°C for 3 min, and 39 cycles with 95°C for 10 s and 58°C for 30 s. A melt curve was performed after 39 cycles of amplification; single amplicons were detected in all cases. Preliminary experiments established the validity of each primer pair in that each set was able to linearly amplify each singular transcript across a range of template concentrations. Each RT reaction was analyzed in duplicate for 18S rRNA, Atp7a (Menkes copper ATPase), Dmt1, transferrin receptor 1 (Tfrc), metallothionein 1A (Mt1a), copper transporter 1 (Ctr1), Wilson's copper ATPase (Atp7b), Heph, Cp, and hepcidin (Hamp) in each experiment. Next, the average of 18S was subtracted from the experimental gene average to generate the cycle threshold (Ct) value. Data were analyzed by routine methods. Briefly, ΔΔCt values were calculated for experimental genes and 18S, for b/b vs. +/b groups. Mean fold induction equates to 2−ΔΔCt. Student's t-test was used to determine significance between means. Statistical significance was set at P < 0.05.
Table 2.
qRT-PCR primer sequences
| Gene Symbol | Direction | Sequence (5′ to 3′) |
|---|---|---|
| 18S | FOR | TCCAAGGAAGGCAGCAGGC |
| 18S | REV | TACCTGGTTGATCCTGCCA |
| Atp7a | FOR | TGAACAGTCATCACCTTCATCGTC |
| Atp7a | REV | GCGATCAAGCCACACAGTTCA |
| Atp7b | FOR | TTAGCATCCTGGGCATGACTTG |
| Atp7b | REV | TTGGTGTGTGAGGAGTCCTCTAGTGT |
| Ctr1 | FOR | CTACTTTGGCTTTAAGAATGTGGACC |
| Ctr1 | REV | AACATTGCTAGTAAAAACACTGCCAC |
| Cp | FOR | ACTTATGCAGATCCTGTGTGCCTATC |
| Cp | REV | TGCATCTTGTTGGACTCCTGAAAG |
| Hamp | FOR | GCAAGATGGCACTAAGCACTC |
| Hamp | REV | GCAACAGAGACCACAGGAGGAAT |
| Heph | FOR | ACACTCTACAGCTTCAGGGCATGA |
| Heph | REV | CTGTCAGGGCAATAATCCCATTCT |
| Mt1a | FOR | CTTCTTGTCGCTTACACCGTTG |
| Mt1a | REV | CAGCAGCACTGTTCGTCACTTC |
| Tfrc | FOR | ATTGCGGACTGAGGAGGTGC |
| Tfrc | REV | CCATCATTCTCAGTTGTACAAGGGAG |
FOR, forward; REV, reverse; Atp7a, Menkes copper transporting ATPase; Atp7b, Wilson's copper transporting ATPase; Cp, ceruloplasmin; Ctr1, copper transporter 1; Hamp, hepcidin; Heph, hephaestin; Mt1a, metallothionein 1A; Tfrc, transferrin receptor 1.
Western Blot Analysis.
For Western blot experiments, four validated antibodies against Atp7a, Dmt1, Heph, and Cp were utilized. The Atp7a antiserum (called 54–10) has been extensively validated (13, 43). The Dmt1 antibody was a kind gift from Dr. Michael Garrick, University at Buffalo. This affinity-purified reagent is a polyclonal antibody raised in rabbits against a NH2-terminal peptide, which was modeled after a recent publication (20). Validation in the Garrick laboratory includes studies in HEK293 cells overexpressing Dmt1 (or not), experiments with brain-specific Dmt1 knockout mice, as well as studies performed in control and iron-deficient rats, and in b/b vs. +/b rats (personal communication with Dr. Michael Garrick). All indications are that this reagent specifically recognizes the rat Dmt1 protein. The thoroughly characterized Heph antibody (called D4) (8) was a kind gift from Dr. Chris Vulpe, University of California, Berkeley. A well-established chicken anti-Cp antibody and a peroxidase-coupled secondary antibody were kind gifts from Sigma-Aldrich (cat. nos. GW20074F and A9046, respectively).
Identical quantities of enterocyte membrane or serum proteins from +/b and b/b rats were electrophoresed, blotted, and blocked following published methods (12, 42) with the following variations: 1:4,000 dilution of primary antisera (except 1:5,000 for Cp) and 1:6,000 dilution of horseradish peroxidase-conjugated anti-rabbit (secondary) antibody. Blots were stained with Ponceau S solution to confirm equal sample loading and efficient transfer. The optical density of immunoreactive bands on film and proteins on stained blots were quantified using the digitizing software UN-SCAN-IT (Silk Scientific), and the average pixel numbers were used for normalization and comparison. The intensity of immunoreactive bands on film was normalized to the intensity of total proteins on stained blots.
Immunohistochemical Analysis of Intestinal Tissue.
Transverse sections of intestinal tissues were harvested from the duodenum of +/b and b/b and of control and iron-deficient SD rats. Tissues were fixed overnight in 10% buffered formalin and then transferred to 70% ethanol. Samples were then embedded in paraffin, and slices were cut and affixed to slides. Tissue sections on slides were subsequently deparaffinized with xylene and a series of ethanol washes. Sections were then blocked for 30–45 min with immunofluorescence blocking solution (Bethyl Laboratories) followed by a 10-min wash in PBS. The Atp7a and Dmt1 polyclonal antisera were then applied at a 1:1,000 and 1: 6,000 dilutions, respectively, overnight in a humidified chamber, followed by a 10-min wash in PBS. A secondary antibody (Alexa Fluor 647 goat antirabbit IgG; Invitrogen Molecular Probes) was then applied for 30 min at a 1:1,000 dilution. After another brief wash in PBS, coverslips were mounted with a fluorescent mounting medium. Slides were visualized in the Confocal and Flow Cytometry Facility in the McKnight Brain Institute at the University of Florida with an Olympus IX81-DSU confocal microscope. A 633-nm line from a green HeNe laser was used for sample excitation along with a Cy5 emission filter set. The confocal settings were kept identical across the different samples, so direct comparison of fluorescence intensity was possible.
Oxidase Activity Assays
Principal.
Heph and Cp activity can be measured by in gel assays or with a spectrophotometer; both approaches were utilized in the present investigation. For FOX activity assays, the spectrophotometric approach was utilized. After samples were reacted with ferrous iron at 37°C, they were incubated with ferrozine (Fz); when Fz interacts with ferric iron, a color change ensues that can be quantified on a spectrophotometer. Heph and Cp, in addition to having FOX activity, possess amine oxidase activity, which serves as a surrogate for FOX activity. In this assay, the substrate para-Phenylenediamine (pPD), a six-carbon phenyl derivative, is oxidized to a fused-ring, aromatic compound (C18H18N6), with an absorption maxima at ∼530 nm, which was either visualized as a dark band in a gel or measured quantitatively in a spectrophotometer (44).
In-gel pPD assay.
One milligram of serum proteins per lane was electrophoresed through a native 7.5% polyacrylamide gel at a constant 80 V in 1× native running buffer (0.12 M Tris and 0.04 M glycine) in an ice-water bath. The gel was then briefly rinsed in water, incubated in 30 ml 0.1% pPD in 0.1 M Na2CO3-CH3COOH buffer, pH 5.0, for 2–3 h in the dark with gentle shaking, rinsed again, and air-dried overnight, as previously described in detail (42).
Spectrophotometric pPD assay.
The reaction mix contained 500 μg of serum (15–20 μl) or 60 μg of membrane proteins isolated from duodenal enterocytes with 0.1% pPD in 0.1 M Na2CO3-CH3COOH buffer, pH 5.0, which was incubated at 37°C. After consideration of the kinetic properties of the enzyme and based on results of extensive pilot experiments, a 1-h reaction time, end-point assay was chosen (42). The reaction was stopped by addition of NaN3 to a final concentration, of 10 mM, the sample was mixed, and absorbance was read at 530 nm in a Beckman DU 640 spectrophotometer. Blank (complete reaction buffer devoid of serum, i.e., enzyme source) readings were subtracted from sample readings.
Spectrophotometric Fz assay.
All Fz assays were initial velocity assays at a 1-min time point. The reaction conditions were as mentioned above except the reaction was stopped by addition of Fz to a final concentration of 3 mM. The sample was then mixed, and absorbance was read at 570 nm in a Beckman DU 640 spectrophotometer. Blank readings were subtracted from sample readings. In both spectrophotometric methods, sera were also assayed in the presence of 10 mM NaN3, a well-studied FOX inhibitor, to confirm the identity of the enzyme.
RESULTS
Hematological Status as a Function of Diet
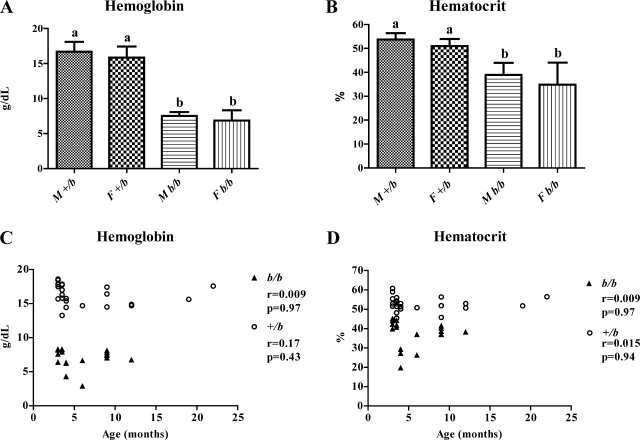
Hb (Fig. 1A) and Hct (Fig. 1B) values were followed as markers of hematological status. Because the Belgrade rats used for this study were mixed sex and age, appropriate statistical analysis was chosen to remove bias and justify combining the data from all rats of each genotype for each parameter studied. Because there was no a priori reason to expect interaction between sex and genotype, the rats were divided into four independent groups (M +/b, M b/b, F +/b, and F b/b; M = male, F = female) and data were analyzed by one-way ANOVA followed by Tukey analysis to test the difference between each group. As expected, Hb and Hct levels in the b/b rats were significantly lower than in the +/b rats (Fig. 1, A and B). No differences were noted between males and females. Thus the b/b rats showed a significant iron-deficient phenotype, whereas +/b rats had normal hematological parameters. Next, to test whether different ages could affect hematological status, correlation analysis was applied between age and Hb/Hct levels (Fig. 1, C and D). As results show, there was no correlation, demonstrating that age had no affect on hematological parameters. On the basis of these statistical approaches showing no effect of sex or age on hematological status, it was concluded that it was reasonable to group data from all animals of each individual genotype.
Fig. 1.
Hematological status of experimental animals. Hemoglobin (Hb) (A) and hematocrit (Hct) (B) levels are shown graphically. M, male; F, female. a,bStatistically different from one another (P < 0.05). Means ± SD are shown. Correlation analysis was performed to compare Hb and Hct to age, as seen in C and D, respectively. r, Pearson Correlation coefficient. n = 24 for +/bs; n = 18 for b/bs.
Serum Copper Levels and Hepatic Iron and Copper Levels
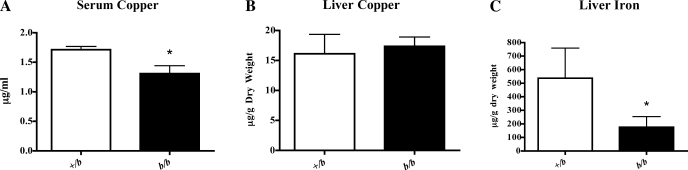
Genetic iron deficiency attributable to Dmt1 mutation slightly reduced serum copper in b/b rats (Fig. 2A), whereas hepatic copper was the same in both genotypes (Fig. 2B). These results showed a different pattern from what has been documented in multiple mammalian species including rats, namely that serum and hepatic copper levels increase during moderate to severe iron deficiency (23, 28, 43, 53). Moreover, liver iron was reduced by ∼70% in the b/b rats compared with the +/bs.
Fig. 2.
Copper content in rat serum and hepatic iron and copper content. A: serum copper level. B: liver copper content. Serum, n = 3 for +/b and n = 4 for b/b; Liver, n = 6 for +/b and n = 4 for b/b. C: hepatic iron content (n = 6 for +/b and n = 4 for b/b). Means ± SD are shown. *Statistically significant differences between genotypes (P < 0.05).
Expression of Cu and Fe Homeostasis-Related Genes
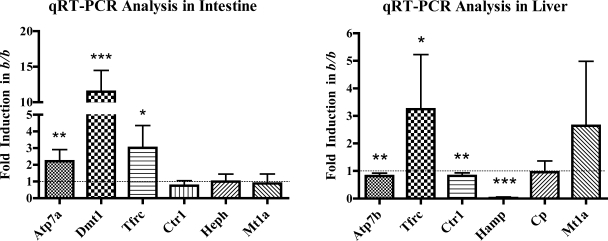
qRT-PCR analysis of intestinal and hepatic genes related to iron and copper homeostasis was performed in all rats to determine whether a lack of fully functional Dmt1 caused altered gene expression. Expression of genes quantified in intestine encoded potential copper importers (Dmt1; Ctr1), an intracellular copper binding protein (Mt1a), and a copper exporter (Atp7a). Expression of genes encoding proteins related to iron transport were also quantified, including Dmt1 and Tfrc, as well as a basolateral iron oxidase (Heph) and the hepatic iron regulatory hormone Hamp. In liver, expression of the copper importer Ctr1 and the copper exporter, Atp7b, were also quantified. In enterocytes from the proximal small bowel, Dmt1 (∼11.5-fold), Tfrc (∼3-fold), and Atp7a (∼2-fold) were all induced in b/bs compared with +/bs (Fig. 3A). There was no significant change, however, in the expression of Heph, Ctr1, and Mt1a mRNAs. In the liver, Hamp was significantly decreased (>40-fold), whereas Tfrc was induced (∼3-fold) in b/bs compared with +/bs (Fig. 3B). Mt1a and Cp mRNA levels in liver were not different between genotypes. Other hepatic Cu homeostasis-related genes, Atp7b and Ctr1, showed a slight reduction in the b/bs.
Fig. 3.
qRT-PCR analysis of intestinal and hepatic gene expression. qRT-PCR was performed with RNA samples extracted from isolated enterocytes (left) and liver (right) of +/b and b/b rats. Experimental repetitions utilizing different groups of +/b or b/b animals were as follows: +/b, n = 19 and b/b, n = 13 for intestine; +/b, n = 24 and b/b, n = 18 for liver. Y-axis shows fold change in b/bs compared with +/bs. The dashed line corresponding to 1.0-fold change (i.e., no change) on the y-axis is shown in both panels; bars below 1.0 indicate decreases, and bars above indicate increases in the b/b compared with the +/bs. *P < 0.05, **P < 0.01, ***P < 0.001; all indicating significant differences between genotypes. Means ± SD are shown. Atp7a, Menkes copper transporting ATPase; Atp7b, Wilson's copper transporting ATPase; Cp, ceruloplasmin; Ctr1, copper transporter 1; Dmt1, divalent metal transporter 1; Hamp, hepcidin; Heph, hephaestin; Mt1a, metallothionein 1A; Tfrc, transferrin receptor 1.
Western Blot Analysis of Cu and Fe Homeostasis-Related Proteins
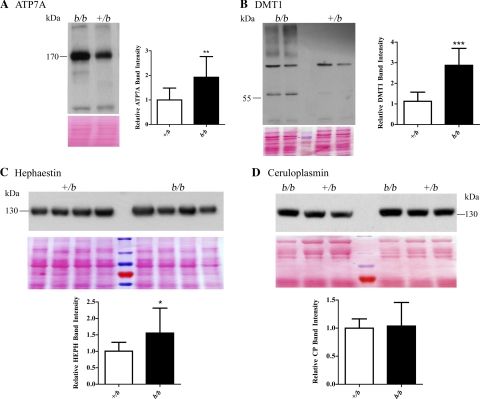
Immunoblot analyses of membrane proteins isolated from enterocytes showed increased expression of Atp7a and Dmt1 (∼2- fold) in b/b rats (Fig. 4, A and B). Heph protein expression in enterocyte membrane preps was slightly increased (<2.0-fold) in the b/b rats (Fig. 4C) compared with +/bs. Finally, there was no difference in serum Cp protein levels between genotypes (Fig. 4D).
Fig. 4.
Western blot analysis of iron/copper-related proteins. In each panel, a representative Western blot is shown along with quantitative data from all rats studied. Numbers beside the Western blots indicate the placement of the closest molecular weight marker. Band intensities were normalized vs. total protein on the stained blots (shown below each lane of the Western blots). In A, B and C, membrane proteins extracted from enterocytes were reacted with antibodies against the respective proteins. A: anti-ATP7A antibody (+/b, n = 12 and b/b, n = 7). B: anti-DMT1 antibody (+/b, n = 10 and b/b, n = 6). The band just above 55 kDa was quantified (See discussion for explanation). C: anti-HEPH antibody (+/b, n = 15 and b/b, n = 8). D: serum proteins were reacted with anti-Cp antibody (+/b, n = 13 and b/b, n = 7). *P < 0.05, **P < 0.01, ***P < 0.001; all indicating significant differences between genotypes. Means ± SD are shown.
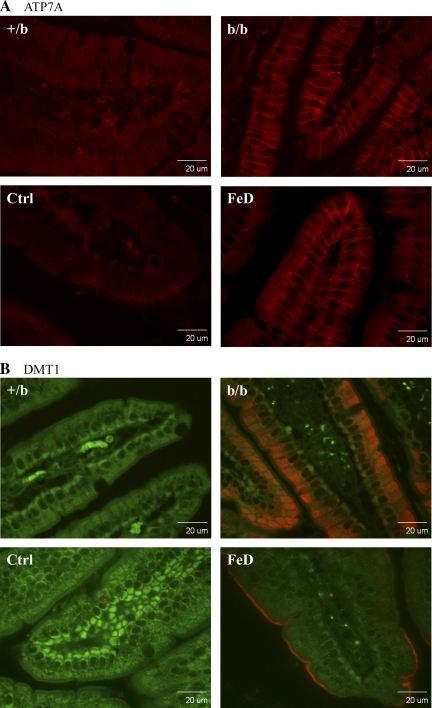
Immunohistochemical Analyses of Atp7a and Dmt1 Protein Expression in Rat Duodenum
Because the subcellular location is also important for protein function, immunolocalization studies were performed in fixed intestinal tissue samples from proximal small intestine, utilizing anti-Atp7a and -Dmt1 antibodies. Control (Ctrl) and iron-deficient (FeD) SD rat samples were used for comparison. Confocal microscopic imaging revealed robust Atp7a protein expression along the basolateral membrane of enterocytes in FeD and b/b rats with little expression observed in Ctrl or +/b rats (Fig. 5A). Dmt1 expression was very low in Ctrl and +/b rats and was much higher in the FeD rats and b/bs. Robust Dmt1 expression was noted along the apical surface of villus enterocytes in iron-deficient rats, whereas the protein was detected intracellularly and on the apical surface in the b/b rats.
Fig. 5.
Immunohistochemical analysis of Atp7a and Dmt1 protein expression in rat duodenum. Fixed tissue sections were reacted with the anti-Atp7a- or -Dmt1-specific antiserum followed by a fluorescent-tagged secondary antibody and imaged with a confocal microscope. A: Atp7a protein is depicted by the red color. B: autofluorescence is shown (green) along with the specific signal (red color) depicting the Dmt1 protein. The confocal settings remained constant across all images. Images are typical of several experiments. Ctrl, control Sprague Dawley (SD) rat; FeD, iron-deficient SD rat.
Activity Assays for Cp and Heph
Serum and enterocyte FOX and amine oxidase activity assays were performed to determine whether enzyme function was altered by genetic iron deficiency. No differences in activity between genotypes were noted in serum representing Cp activity (data not shown; spectrophotometric assays: pPD, +/b n = 6, b/b n = 4; Fz, +/b n = 13, b/b n = 9) or enterocyte membrane representing Heph activity (data not shown; spectrophotometric assays: pPD, +/b n = 6, b/b n = 4; Fz, +/b n = 9, b/b n = 5). Furthermore, rat serum FOX/amine oxidase activity was almost completely abolished in the presence of 10 mM NaN3 (not shown), consistent with a recent publication (42), whereas inhibition of enterocyte membrane Heph activity was consistently ∼75% (data not shown).
In-Gel Serum Amine Oxidase Activity Assays
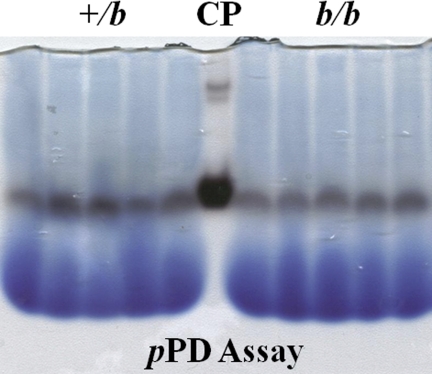
To further confirm enzymatic activity utilizing a complementary experimental approach, in-gel assay was performed under native conditions. Results showed the product of the reaction catalyzed by serum protein using pPD as a substrate (Fig. 6). As seen, activity in the b/bs was almost identical to that in the +/bs. Purified human Cp (Sigma; cat no. C4519), used as a positive control, showed a strong band.
Fig. 6.
In-gel serum Cp activity assays. Serum samples were separated by native gel electrophoresis and subsequently reacted with the substrate (para-Phenylenediamine, pPD) to estimate enzyme activity levels. The site of enzyme activity is represented by the dark bands just below the midpoint of the gel. The blue, diffuse bands at the bottom of the gel are loading dye. Each genotype is represented by samples from 5 individual rats. Purified human Cp protein was used as positive control (CP).
DISCUSSION
Previous studies documented induction of the Atp7a and Mt1a genes in the duodenum of iron-deficient rats (11). This observation, combined with observed increases in serum and hepatic copper levels in several mammalian species during iron deficiency, leads to speculation that increased copper export from enterocytes via Atp7a may be involved in this phenomenon. Moreover, it seems likely that alterations in copper homeostasis reflect some aspect of the compensatory response to maximize iron absorption from the diet and release from body stores to support normal erythropoiesis. In this scenario, one would predict that there would also be increases in the copper import machinery, with Dmt1 being a potential player in this process. The possibility that iron deficiency enhances intestinal copper absorption, which contributes to hepatic copper loading, has in fact been previously proposed (51), and increased copper levels in duodenal tissue were reported in iron-deficient rats (43); however, the role of Dmt1 is not clear. The present studies were designed to examine the role of Dmt1 in copper homeostasis during iron deficiency by taking advantage of a naturally occurring Dmt1 mutation in the Belgrade rat. Part of the approach was to compare/contrast with an extensive body of data obtained in a wild-type rat (SD) dietary iron deficiency model (11, 13).
The Belgrade rats used in this study were highly iron deficient as exemplified by significant reductions in blood Hb and Hct and hepatic iron levels. Unexpectedly, copper levels were not increased in the liver or serum of the b/b rats, findings that are inconsistent with a host of studies done in many mammalian species (e.g., humans, dogs, rats) documenting increased body copper during moderate to severe iron deficiency (7, 31, 35, 42, 46, 50, 53, 58) and hemorrhagic anemia (5, 32, 40, 45). One might thus speculate that Dmt1 plays a role in hepatic copper loading and the subsequent increase in blood copper, which is likely the result of increased production and secretion of holo-ceruloplasmin (42).
Gene expression studies revealed many similar alterations in mRNA expression between the b/b rats and iron-deficient SD rats, including induction of Dmt1, Atp7a, and Tfrc in duodenum and strong downregulation of Hamp in liver. Of note was a lack of induction of Mt1a in duodenum and liver, an observation that varies from studies in SD rats in which there was a strong upregulation of Mt1a mRNA expression in both tissues (∼50-fold in intestine and ∼10-fold in liver; L. Jiang, J. Collins, unpublished observation) (10, 11). The lack of copper accumulation and a lesser induction of Atp7a in the Belgrades (∼2-fold vs. 6–8-fold in iron-deficient SD rats), suggests that Mt1a induction may be linked to copper accumulation in these tissues, which does not occur in the Belgrades. Mt1a is in fact strongly induced by copper and is thought to be involved in intestinal (33) and liver (3) copper homeostasis.
Immunoblot analyses exemplified the physiological relevance of increases in transcript expression in that the Atp7a and Dmt1 proteins also increased significantly in the Belgrades. Again, the induction of Atp7a was much less than the increases noted in iron-deficient SD rats (43). In the present investigation, the Dmt1 band, which was much stronger in the b/b rats, was noted at slightly >55 kDa; other bands on blots were of similar intensity in +/bs and b/bs, likely representing nonspecific signals. A survey of the primary literature reveals a wide discrepancy in the molecular weight of Dmt1 in Western blot analyses, with variations in rodent and human intestine noted from ∼43 to 85 kDa (34, 36, 37, 57, 59). The molecular weight of the Dmt1 detected in the present studies thus falls within ranges reported elsewhere, and its strong induction in the b/b rats lends confidence to it being specific. Consistent with the immunoblots, Atp7a and Dmt1 protein expression was noted to increase in intestinal tissue sections derived from b/b rats, which is consistent with the increase observed in iron-deficient SD rats. Atp7a protein was detected intracellularly, most likely in the trans-Golgi, and also on the basolateral membrane of enterocytes in the iron-deficient condition. Dmt1 expression was observed along the apical surface of villus enterocytes in iron-deficient SD rats, but the protein was abnormally distributed in the b/b rats. This finding is consistent with a previous publication that showed mislocalization of the mutant Dmt1 protein in the mk mouse (harboring the exact same point mutation as in the Belgrade rat) (4). It should be noted that, although the cellular machinery that mediates the induction of Dmt1 mRNA and protein expression during iron deficiency is obviously intact in the Belgrade rat, it is without functional consequence, as the mutant protein is mislocalized in the intestine and has a greatly diminished capacity to transport iron, as exemplified by the iron-deficient phenotype.
Given the role of the multi-copper FOXs Cp and Heph in body iron homeostasis and based on the fact that they are copper-dependent enzymes, it was important to consider the expression/activity of these proteins in the present investigation. Heph is a membrane-bound protein found on the basolateral surface of enterocytes, which is considered necessary for optimal iron absorption (9, 54). Previous reports have suggested that Heph, while not being strongly regulated, is upregulated during iron deficiency (<2-fold) (1). Consistent with this observation, in the present studies, a moderate increase in enterocyte membrane Heph protein was detected on Western blots; however, no change in membrane FOX or amine oxidase activity was noted. This finding is consistent with observations made by the authors in iron-deficient SD rats (P. Ranganathan, Y. Lu, J. Collins, unpublished observation). Dmt1 thus does not seem to play a role in regulating the expression or activity of intestinal Heph during iron deficiency.
The second FOX, Cp, is a liver-derived, circulating protein involved in iron release from sites of storage (38). Serum Cp activity increases during iron deficiency in humans (53), and a similar increase in immunoreactive protein and activity was recently documented in iron-deficient SD rats (42). However, in the Belgrades, no change in Cp protein expression or serum FOX or amine oxidase activity (both presumably contributed by circulating Cp) was observed, compared with the phenotypically normal +/b rats. The most likely explanation for this observation relates to the lack of hepatic copper loading in the Belgrades, as discussed above, again implicating Dmt1 in this process. Another possible interpretation of these data relates to the degree of iron deficiency. The Belgrades used in these studies exhibited significant iron deficiency as indicated by an ∼70% reduction in liver iron, whereas iron-deficient SD rats in which previous studies were performed showed slightly greater decreases in liver iron (∼83%) (42). It is, however, important to point out that various degrees of iron deficiency in a host of mammalian species have been shown to lead to increases in liver and serum copper, including moderate iron deficiency in rats with an ∼18% reduction in Hb levels (11, 43) and with a 56% reduction in liver iron but no change in Hb levels (47). Additional examples of such exist in moderately iron-deficient rats (46, 50). Furthermore, iron deficiency anemia has been extensively associated with increased serum copper levels in humans (17, 27); many other examples have also been documented. The key point here is that iron deficiency anemia in humans is a moderate iron deficiency, with Hbs reduced by perhaps 50%, similar to the reduction seen in the Belgrade rats used in the present studies. On the basis of these facts, we feel that the most likely explanation for there being no increases in body copper levels in the Belgrades, despite a level of iron deficiency that alters copper levels in wild-type rats and other mammalian species, is the lack of fully functional Dmt1.
In summary, data presented in this article reveal aspects of Dmt1 function that are necessary for the normal, copper-dependent compensatory response to iron deficiency. Lack of Mt1a induction in the intestine and liver, lesser induction of duodenal Atp7a, and a lack of hepatic copper loading demonstrate that alterations in copper homeostasis during iron deficiency are less pronounced in the absence of fully functional Dmt1. Furthermore, increases in holo-Cp levels do not occur in the b/b rats, a finding that may also be explained by a previously unrecognized property of Dmt1 that permits hepatic copper loading to occur during iron deficiency. Overall, this investigation has revealed potentially novel physiological aspects of Dmt1 function and has provided additional evidence of the utility of the Belgrade rat model of iron deficiency to reveal novel aspects of mammalian iron homeostasis.
GRANTS
This study was supported by NIH grant R01 DK074867 (J. Collins).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Anderson GJ, Frazer DM, McKie AT, Vulpe CD. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol Dis 29: 367–375, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 284: C1525–C1530, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bremner I. Involvement of metallothionein in the hepatic metabolism of copper. J Nutr 117: 19–29, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Canonne-Hergaux F, Fleming MD, Levy JE, Gauthier S, Ralph T, Picard V, Andrews NC, Gros P. The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood 96: 3964–3970, 2000 [PubMed] [Google Scholar]
- 5. Cartwright GE, Huguley CM, Jr, Aschenbrucker H, Fay J, Wintrobe MM. Studies on free erythrocyte protoporphyrin, plasma iron and plasma copper in normal and anemic subjects. Blood 3: 501–525, 1948. [PubMed] [Google Scholar]
- 6. Cartwright GE, Lauritsen MA, Humphreys S, Jones PJ, Merrill IM, Wintrobe MM. The anemia of infection. II. The experimental production of hypoferremia and anemia in dogs. J Clin Invest 25: 81–86, 1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartwright GE, Wintrobe MM. Studies on free erythrocyte protoporphyrin, plasma copper, and plasma iron in normal and in pyridoxine-deficient swine. J Biol Chem 172: 557–565, 1948 [PubMed] [Google Scholar]
- 8. Chen H, Attieh ZK, Dang T, Huang G, van der Hee RM, Vulpe C. Decreased hephaestin expression and activity leads to decreased iron efflux from differentiated Caco2 cells. J Cell Biochem 107: 803–808, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Chen H, Su T, Attieh ZK, Fox TC, McKie AT, Anderson GJ, Vulpe CD. Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood 102: 1893–1899, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Collins JF. Gene chip analyses reveal differential genetic responses to iron deficiency in rat duodenum and jejunum. Biol Res 39: 25–37, 2006 [PMC free article] [PubMed] [Google Scholar]
- 11. Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol 288: G964–G971, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Collins JF, Hu Z, Ranganathan PN, Feng D, Garrick LM, Garrick MD, Browne RW. Induction of arachidonate 12-lipoxygenase (Alox15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am J Physiol Gastrointest Liver Physiol 294: G948–G962, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Collins JF, Hua P, Lu Y, Ranganathan PN. Alternative splicing of the Menkes copper ATPase (Atp7a) transcript in the rat intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 297: G695–G707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curzon G. Some properties of coupled iron-caeruloplasmin oxidation systems. Biochem J 79: 656–663, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curzon GORS. A coupled iron-ceruloplasmin oxidation system. Biochem Biophys Res Commun 284–286, 1960 [Google Scholar]
- 16. Dupic F, Fruchon S, Bensaid M, Loreal O, Brissot P, Borot N, Roth MP, Coppin H. Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut 51: 648–653, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ece A, Uyanik BS, Iscan A, Ertan P, Yigitoglu MR. Increased serum copper and decreased serum zinc levels in children with iron deficiency anemia. Biol Trace Elem Res 59: 31–39, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Edwards JA, Garrick LM, Hoke JE. Defective iron uptake and globin synthesis by erythroid cells in the anemia of the Belgrade laboratory rat. Blood 51: 347–357, 1978 [PubMed] [Google Scholar]
- 19. Fay JCG, Wintrobe MM. Studies on free erythrocyte protopotphyrin, serum iron, serum iron-binding capacity and plasma copper during normal pregnancy. J Clin Invest 487–491, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferguson CJ, Wareing M, Ward DT, Green R, Smith CP, Riccardi D. Cellular localization of divalent metal transporter DMT-1 in rat kidney. Am J Physiol Renal Physiol 280: F803–F814, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95: 1148–1153, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16: 383–386, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals 16: 9–40, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Garrick M, Scott D, Walpole S, Finkelstein E, Whitbred J, Chopra S, Trivikram L, Mayes D, Rhodes D, Cabbagestalk K, Oklu R, Sadiq A, Mascia B, Hoke J, Garrick L. Iron supplementation moderates but does not cure the Belgrade anemia. Biometals 10: 65–76, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Gubler CJ, Lahey ME, Cartwright GE, Wintrobe MM. Studies on copper metabolism. IX. The transportation of copper in blood. J Clin Invest 32: 405–414, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Gurgoze MK, Olcucu A, Aygun AD, Taskin E, Kilic M. Serum and hair levels of zinc, selenium, iron, and copper in children with iron-deficiency anemia. Biol Trace Elem Res 111: 23–29, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Hahn PF, Whipple GH. Iron metabolism in experimental anemia: “Availability of iron”. J Exp Med 67: 259–265, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hart EB, Steenbock H, Elvehjem CA, Waddell J. Nutritional anemia on whole milk diets and the utilization of inorganic iron in hemoglobin building. J Biol Chem 65:67–80, 1925 [Google Scholar]
- 30. Hart EB, Steenbock H, Waddell J, Elvehjem CA. Iron in nutrition. VII. Copper as a supplement to iron for hemoglobin building in the rat. J Biol Chem 277: 797–812, 1928 [PubMed] [Google Scholar]
- 31. Iwanska SSD. Copper metabolism in different states of erythropoiesis activity. Acta Physiol Pol 465–474, 1978 [PubMed] [Google Scholar]
- 32. Johnson DA, Osaki S, Frieden E. A micromethod for the determination of ferroxidase (ceruloplasmin) in human serums. Clin Chem 13: 142–150, 1967 [PubMed] [Google Scholar]
- 33. Kelly EJ, Palmiter RD. A murine model of Menkes disease reveals a physiological function of metallothionein. Nat Genet 13: 219–222, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Knopfel M, Zhao L, Garrick MD. Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry 44: 3454–3465, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Lahey ME, Gubler CJ, Cartwright GE, Wintrobe MM. Studies on copper metabolism. VII. Blood copper in pregnancy and various pathologic states. J Clin Invest 32: 329–339, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu YQ, Duan XL, Chang YZ, Wang HT, Qian ZM. Molecular analysis of increased iron status in moderately exercised rats. Mol Cell Biochem 282: 117–123, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut 53: 655–660, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyajima H, Nishimura Y, Mizoguchi K, Sakamoto M, Shimizu T, Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology 37: 761–767, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Oates PS, Morgan EH. Defective iron uptake by the duodenum of Belgrade rats fed diets of different iron contents. Am J Physiol Gastrointest Liver Physiol 270: G826–G832, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Pagliardi E, Giangrandi E, Vinti A. Behavior of plasmatic & erythrocytic copper in morbid conditions. Rass Fisiopatol Clin Ter 29: 907–921, 1957 [PubMed] [Google Scholar]
- 41. Pinkerton PH, Bannerman RM. Hereditary defect in iron absorption in mice. Nature 216: 482–483, 1967 [DOI] [PubMed] [Google Scholar]
- 42. Ranganathan P, Lu Y, Jiang L, Kim C, Collins JF. Serum ceruloplasmin (CP) protein expression and activity increases in iron deficient rats and is further enhanced by higher dietary copper intake. Blood 2011, July 18. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes Copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem 280: 36221–36227, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roeser HP, Lee GR, Nacht S, Cartwright GE. The role of ceruloplasmin in iron metabolism. J Clin Invest 49: 2408–2417, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sachs A. The effect of bleeding ulcers and hemmoraghic anemia upon whole blood copper and iron. Am J Digest Dis Nutr 4: 803–804, 1938 [Google Scholar]
- 46. Sherman AR, Tissue NT. Tissue iron, copper and zinc levels in offspring of iron-sufficient and iron-deficient rats. J Nutr 111: 266–275, 1981 [DOI] [PubMed] [Google Scholar]
- 47. Shukla A, Agarwal KN, Shukla GS. Effect of latent iron deficiency on the levels of iron, calcium, zinc, copper, manganese, cadmium and lead in liver, kidney and spleen of growing rats. Experientia 46: 751–752, 1990 [DOI] [PubMed] [Google Scholar]
- 48. Sladic-Simic D, Martinovitch PN, Zivkovic N, Pavic D, Martinovic J, Kahn M, Ranney HM. A thalassemia-like disorder in Belgrade laboratory rats. Ann NY Acad Sci 165: 93–99, 1969 [DOI] [PubMed] [Google Scholar]
- 49. Sladic-Simic D, Zivkovic N, Pavic D, Marinkovic D, Martinovic J, Martinovitch PN. Hereditary hypochromic microcytic anemia in the laboratory rat. Genetics 53: 1079–1089, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stangl GI, Kirchgessner M. Effect of different degrees of moderate iron deficiency on the activities of tricarboxylic acid cycle enzymes, and the cytochrome oxidase, and the iron, copper, and zinc concentrations in rat tissues. Z Ernahrungswiss 37: 260–268, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Sugawara N, Sugawara C. An iron-deficient diet stimulates the onset of the hepatitis due to hepatic copper deposition in the Long-Evans Cinnamon (LEC) rat. Arch Toxicol 73: 353–358, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Tennant J, Stansfield M, Yamaji S, Srai SK, Sharp P. Effects of copper on the expression of metal transporters in human intestinal Caco-2 cells. FEBS Lett 527: 239–244, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Venakteshwara Rao M, Khanijo SK, Chande RD, Chouhan SS, Bisarya BN. Serum ceruloplasmin in iron deficiency anaemia. J Assoc Physicians India 23: 571–576, 1975 [PubMed] [Google Scholar]
- 54. Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21: 195–199, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Warburg OKH. Uber locker gebundenes Kupfer und Eisen im Blutserum. Biochem Z: 143–149, 1927 [Google Scholar]
- 56. Wintrobe MM, Cartwright GE, Lahey ME, Gubler CJ. The role of copper in hemopoiesis. Trans Assoc Am Physicians 310–315, 1951 [PubMed] [Google Scholar]
- 57. Yeh KY, Yeh M, Watkins JA, Rodriguez-Paris J, Glass J. Dietary iron induces rapid changes in rat intestinal divalent metal transporter expression. Am J Physiol Gastrointest Liver Physiol 279: G1070–G1079, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Yokoi K, Kimura M, Itokawa Y. Effect of dietary iron deficiency on mineral levels in tissues of rats. Biol Trace Elem Res 29: 257–265, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 120: 1412–1419, 2001 [DOI] [PubMed] [Google Scholar]