Abstract
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a negative regulator of phosphatidylinositol 3-kinase (PI3K) signaling that is frequently inactivated in colorectal cancer through mutation, loss of heterozygosity, or epigenetic mechanisms. The aim of this study was to determine the effect of intestinal-specific PTEN inactivation on intestinal epithelial homeostasis and tumorigenesis. PTEN was deleted specifically in the intestinal epithelium, by crossing PTENLox/Lox mice with villinCre mice. PTEN was robustly expressed in the intestinal epithelium and maximally in the differentiated cell compartment. Targeted inactivation of PTEN in the intestinal epithelium of PTENLox/Lox/villinCre mice was confirmed by genotyping, immunohistochemistry, and qPCR. While intestinal-specific PTEN deletion did not have a major effect on cell fate determination or proliferation in the small intestine, it did increase phosphorylated (p) protein kinase B (AKT) expression in the intestinal epithelium, and 19% of animals developed small intestinal adenomas and adenocarcinomas at 12 mo of age. These tumors demonstrated pAKT and nuclear β-catenin staining, indicating simultaneous activation of the PI3K/AKT and Wnt signaling pathways. These findings demonstrate that, while PTEN inactivation alone has a minimal effect on intestinal homeostasis, it can facilitate tumor promotion upon deregulation of β-catenin/TCF signaling, further establishing PTEN as a bona fide tumor suppressor gene in intestinal cancer.
Keywords: phosphatase and tensin homolog deleted on chromosome ten, intestine, cancer, colon
phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a dual-specificity phosphatase located at 10q23, with both protein and lipid phosphatase activity. PTEN acts as a negative regulator of the phosphatidylinositol 3-kinase (PI3K) signaling pathway by dephosphorylating phosphatidylinositol 3,4,5-triphosphate (PIP3) at the D3 position to generate phosphatidylinositol bisphosphate (PIP2). Individuals with germline mutations in PTEN develop a number of autosomal dominant syndromes, including Cowden's disease where patients are predisposed to developing tumors in the breast, thyroid, endometrium, and kidney (11). Cowden's disease patients also develop multiple hamartomatous (nonmalignant) polyps in the gastrointestinal tract, although occasional hyperplastic, inflammatory, and adenomatous polyps have also been described (5).
The PI3K signaling pathway is constitutively activated in a large number of tumors, including colon cancers, and contributes to tumorigenesis through activation of downstream proproliferative and prosurvival targets, including protein kinase B (AKT), mammalian target of rapamycin (mTOR), forkhead box O, glycogen synthase kinase 3β, p27, and BAD (29). PTEN-mediated conversion of PIP3 to PIP2 antagonizes PIP3-mediated activation of PDK1 and subsequent downstream activation of AKT and mTOR (29). In colon cancer cells, PTEN overexpression inhibits cell growth and promotes apoptosis (23), whereas PTEN knock down promotes cell growth, survival, and epithelial mesenchymal transition (1). PTEN loss has also been reported to facilitate cancer progression in other ways, including increased genomic instability, increased stem cell self renewal, and promotion of cellular senescence and metastasis (29).
Inactivating PTEN mutations are found at high rates in androgen-independent prostate cancer (∼50%), glioblastoma multiform (∼20%), and endometrioid adenocarcinoma (30–80%) and at lower rates in a range of other malignancies (3). Inactivating mutations in PTEN are relatively rare in sporadic colon cancer, with the exception of MSI-High colon cancers where an 18% mutation rate has been reported (9, 25). These mutations occur primarily in two poly(A)6 repeat elements in exons 7 and 8 of the PTEN gene. Loss of PTEN due to allelic loss (2, 30), and epigenetic inactivation of the PTEN promoter (8), has also been described in colon cancer. Notably, loss of PTEN expression and activating mutations in PIK3CA occur in a mutually exclusive manner (7), suggesting that constitutive activation of PI3K signaling is the major mechanism by which PTEN loss facilitates colorectal tumorigenesis. In addition to facilitating tumor cell growth and survival, PTEN loss has also been linked to intrinsic resistance to the epidermal growth factor receptor inhibitors cetuximab and panitumumab (6, 13), used in the treatment of advanced colon cancer.
To determine the role of PTEN in intestinal homeostasis and tumorigenesis in vivo, we used the Cre-Lox strategy to specifically inactivate PTEN in the intestinal epithelium. While PTEN deletion had minimal effects on intestinal homeostasis, 19% of mice developed intestinal tumors, including adenocarcinomas, albeit with a long latency. These tumors displayed robust expression of phosphorylated (p) AKT and nuclear β-catenin, indicating simultaneous activation of PI3K/AKT and Wnt signaling. The long latency of tumor onset, and the presence of nuclear β-catenin in tumors derived from PTEN-deficient animals, implies that PTEN loss facilitates tumor progression upon stochastic activation of Wnt signaling.
METHODS
Generation of PTENLox/Lox/villinCre mice.
PTENLox/Lox mice were generously provided to us by Dr. Hong Wu and have been described previously (15). Mice were on a mixed 129/Balb/c background. VillinCre mice on the Bl6 background, which express Cre recombinase under the control of the intestinal-specific villin promoter, were obtained from Jackson Laboratories. PTENLox/Lox mice were crossed to villinCre mice to obtain PTENLox/Lox/villinCre and PTENLox/Lox/villinWT mice. Primers used for determining PTEN genotype were forward, TCCCAGAGTTCATACCAGGA, reverse 1: GCAATGGCCAGTACTAGTGAA, and reverse 2: AATCTGTGCATGAAGGGAAC. Primers used for genotyping villinCre transgenic mice were forward: GTGTGGGACAGAGAACAAACC and reverse: ACATCTTCAGGTTCTGCGGG.
Isolation of intestinal epithelial cells.
Epithelial cells from the jejunum and colon of wild-type (WT) and PTEN-deleted mice were extracted by opening the jejunum longitudinally, washing in PBS, and incubating for 15 min in 15 mM EDTA. Sequential isolation of cells along the crypt-villus axis was performed as previously described (18).
qPCR analysis.
RNA isolation was performed using the High Pure RNA isolation kit from Roche Diagnostics (Indianapolis, IN). Reverse Transcription was performed using the Superscript III cDNA synthesis kit from Invitrogen (Carlsbad, CA) using 1 μg of total RNA. Quantitative real-time PCR was performed using the Fast Start Universal SYBR Green master mix from Roche, on a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Primers used were as follows: mouse PTEN (exon 5 specific) forward: TGGGGAAGTAAGGACCAGAG and reverse: GGCAGACCACAAACTGAGGA; mouse β-tubulin forward: AAACAGCACAGCCATCCAG and reverse: GCTTCGGTGAACTCCATCTC; human PTEN forward: ACGACGGGAAGACAAGTTCA and reverse: AGGTTTCCTCTGGTCCTGGT; and human actin forward: CACCTTCACCGTTCCAGTTT and reverse: GATGAGATTGGCATGGCTTT.
Protein preparation and Western blot.
Protein isolation, Western blotting, and signal detection were performed as previously described (17). Anti-PTEN antibody was obtained from Cell Signaling (Danvers, MA), anti-KRT8 from Novus Biologicals (Littleton, CO), anti-actin from Sigma-Aldrich (St. Louis, MO), and anti-GAPDH from Abcam (Cambridge, UK).
5′-Bromo-2′-deoxyuridine staining.
The rate of intestinal cell proliferation was determined by injecting the mice with a mixture of 5′-fluoro-2′-deoxyuridine (12 mg/kg) and 5′-bromo-2′-deoxyuridine (BrdU, 120 mg/kg), in PBS, 2 h before death. The jejunum and distal colon were fixed in 10% buffered formalin and paraffin embedded. BrdU positivity was determined immunohistochemically using a BrdU staining kit (Calbiochem) and quantified by computing the percentage of BrdU-positive cells relative to the total number of cells per crypt in 10 crypts per mouse.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (27). Anti-PTEN and anti-pAKT antibodies were obtained from Cell Signaling, and anti-β-catenin was obtained from BD Transduction Laboratories (Lexington, KY). Anti-chromagranin antibody was obtained from Abcam and anti-lysozyme from Thermo Scientific (Rockford, IL). Monoclonal isotype control antibodies were from Dako (Glostrup, Denmark).
Alkaline phosphatase activity.
Alkaline phosphatase activity was measured according to the method of Young et al. (28) using p-nitrophenyl phosphate (Sigma) as substrate. The reaction was terminated with 0.05 M sodium hydroxide, and alkaline phosphatase activity was measured spectrophotometrically at 400 nM, against a p-nitrophenol (Sigma) standard curve. Results were expressed as milliunits per milligram protein.
RESULTS
Intestinal-specific deletion of PTEN.
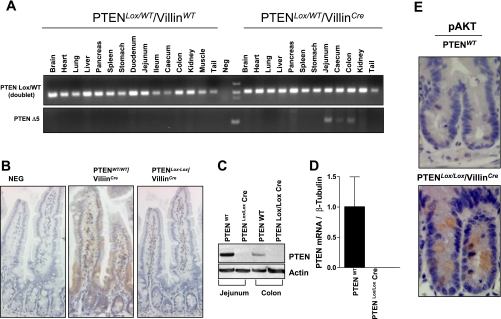
To directly determine the function of PTEN on the maintenance of intestinal epithelial cell homeostasis and tumorigenesis, we used a Cre-Lox strategy to delete exon 5 of the PTEN gene, specifically in intestinal epithelial cells. Deletion of exon 5, which encodes the phosphatase domain of PTEN, results in the generation of an out-of-frame protein product and in a premature stop codon. PTENLox/Lox mice were crossed with villinCre mice, which express Cre recombinase specifically in intestinal epithelial cells, including the stem cell compartment (16). To determine the specificity of PTEN deletion in the intestinal epithelium, DNA was extracted from a number of tissues, and the presence of the deleted PTEN allele was detected by PCR. As shown in Fig. 1A, the presence of the deleted allele was observed in epithelial cells isolated from the jejunum, cecum, and colon but not in other tissues examined. To further confirm PTEN deletion in the intestinal epithelium, we examined PTEN expression in the jejunum of WT and PTENLox/Lox/villinCre mice by immunohistochemistry. In WT mice, PTEN was robustly expressed along the entire length of the crypt-villus axis, with elevated expression at the crypt-villus interface. In contrast, in PTENLox/Lox/villinCre mice, PTEN expression in intestinal epithelial cells was markedly reduced (Fig. 1B). Consistent with the immunohistochemical data, assessment of PTEN protein levels by Western blot analysis and PTEN mRNA levels by qPCR using exon 5-specific primers demonstrated significantly reduced PTEN expression in intestinal epithelial cells isolated from PTENLox/Lox/villinCre mice (Fig. 1, C and D).
Fig. 1.
Targeted inactivation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in the intestinal epithelium in PTENLox/Lox/villinCre mice. A: wild-type (WT) and PTENLox/Lox/villinCre mice were killed at 4 mo of age, and tissue was harvested from various organs. The deleted allele was detected by PCR only in DNA extracted from intestinal epithelial cells isolated from PTENLox/Lox/villinCre animals. B-D: confirmation of PTEN deletion in the intestinal epithelium by immunohistochemistry (B), Western blot (C), and qPCR (D). E: PTEN deletion increases phosphorylated (p) protein kinase B (AKT) expression in cells located in the proliferative crypt compartment.
Because PTEN is a negative regulator of PI3K/AKT signaling, we also determined the effect of intestinal-specific PTEN inactivation on pAKT levels by immunohistochemistry. As shown in Fig. 1E, there was increased pAKT staining in cells located in the proliferative compartment in the crypt base of PTENLox/Lox/villinCre mice, consistent with PTEN deletion.
PTEN expression is induced during intestinal epithelial cell maturation in vitro and in vivo.
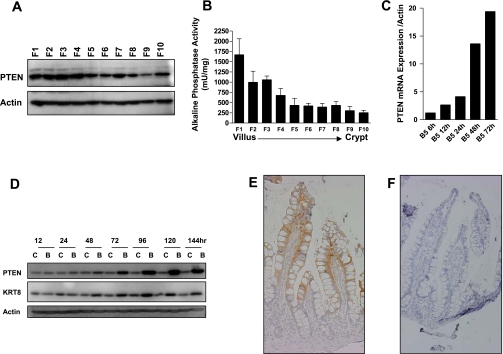
The observation that PTEN expression is robustly induced at the crypt-villus interface suggested a potential role for PTEN in regulating cell maturation in the intestinal epithelium. To confirm this observation, we next examined PTEN protein expression along the crypt villus axis by Western blotting. To do this, small intestinal epithelial cells along the mouse crypt-villus axis were sequentially isolated using the Weiser cell fractionation method (18), where cells collected in fraction 1 correspond to cells at the villus tip, and cells in fraction 10 correspond to cells in the crypt base. As shown in Fig. 2A, PTEN expression in all cell fractions was confirmed, with highest expression in cells isolated from the differentiated compartment (fractions 1–7). Validation of the fractionation method is shown by the decreasing gradient of activity of the cell differentiation marker, alkaline phosphatase, from fractions 1 through 10 (Fig. 2B). Second, intestinal cell differentiation can be modeled in vitro by treatment of colon cancer cells with the HDAC inhibitor sodium butyrate, which induces cell-cycle arrest, differentiation, and apoptosis (19). As shown in Fig. 2, C and D, PTEN mRNA and protein were markedly induced in Caco-2 colon cancer cells treated with sodium butyrate. Finally, we examined PTEN expression along the crypt-villus axis in human small intestine by immunohistochemistry. Consistent with its induction during cell maturation in vitro and in the mouse small intestine, PTEN expression was maximal in the differentiated compartment in human small intestine (Fig. 2E). Collectively, these findings indicate that PTEN expression is induced during intestinal cell differentiation in vitro and in vivo.
Fig. 2.
PTEN expression is induced during intestinal cell differentiation. A: PTEN protein expression in cells sequentially isolated along the mouse crypt-villus axis, with F1 corresponding to cells isolated from the villus tip and F10 corresponding to cells from the crypt base. B: confirmation of the fractionation efficiency as assessed by measurement of activity of the intestinal cell differentiation marker alkaline phosphatase in fractions 1–10. C and D: induction of PTEN mRNA (C) and protein (D) expression in Caco-2 cells treated with 5 mM butyrate. Shown in parallel is induction of the known differentiation marker keratin 8. E: immunohistochemical localization of PTEN in human small intestine. F: negative control (no primary antibody).
Intestinal-specific PTEN deletion does not affect cell proliferation or cell fate determination.
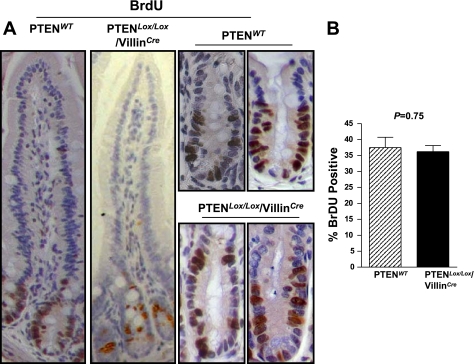
To determine the effect of PTEN deletion on proliferation rates of cells in the crypt base in the small intestine, mice were injected with BrdU 2 h before death, and BrdU positivity was assessed by immunohistochemistry. As shown in Fig. 3, no expansion of the proliferative zone was evident in mice with PTEN inactivation (Fig. 3A), and quantification of the number of BrdU-positive cells per crypt demonstrated no significant difference between PTENWT and PTENLox/Lox/villinCre mice (Fig. 3B). Consistent with this finding, there was no significant difference in crypt-villus height in the jejunum of PTENWT (95.7 ± 9.0 cells/hemi crypt-villus axis, n = 13) and PTENLox/Lox/villinCre mice (94.1 ± 11.1 cells/hemi crypt-villus axis, n = 17, P = 0.68, unpaired t-test).
Fig. 3.
A: proliferation rates in WT and PTENLox/Lox/villinCre mice as assessed by 5′-bromo-2′-deoxyuridine (BrdU) staining. Mice were injected with BrdU 2 h before death. BrdU incorporation was detected by immunohistochemistry. B: quantification of the percentage of BrdU-positive cells per crypt in PTENLox/Lox/villinCre (n = 5) and WT mice (n = 6). Values shown are means + SE.
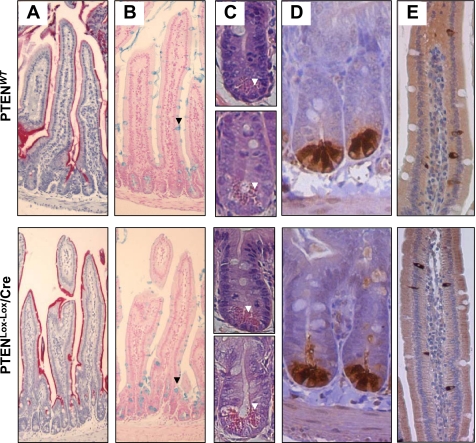
The effect of intestinal-specific PTEN deletion on the presence of the four major intestinal epithelial lineages was also examined. No gross differences in alkaline phosphatase or alcian blue staining were evident between WT and PTEN-deficient mice, indicating maturation of the absorptive and goblet cell lineages, respectively, were not altered by PTEN inactivation (Fig. 4, A and B). Likewise, hematoxylin and eosin staining demonstrated no major difference in either the morphology or frequency of Paneth cells located at the base of the crypts in the small intestine, which was confirmed by staining with the Paneth cell marker lysozyme (Fig. 4, C and D). Finally, no difference in the number of enteroendocrine cells was observed between PTEN WT and deficient mice, as demonstrated by staining and quantification of the number of cells staining positive for the neuroendocrine marker chromagranin A (Fig. 4E).
Fig. 4.
Assessment of markers of lineage-specific differentiation in PTENWT and PTENLox/Lox/villinCre mice. A: alkaline phosphatase staining (absorptive cell lineage). B: alcian blue staining (goblet cell lineage, back arrowheads). C: hematoxylin and eosin (H&E) staining showing Paneth cells with the characteristic eosinophilic granules at the crypt base (white arrows) in duodenum (top) and jejunum (bottom). D: lysozyme staining (Paneth cell lineage). E: chromogranin A staining (enteroendocrine cell lineage).
PTEN-deficient mice develop invasive tumors in the small intestine at 12 mo of age.
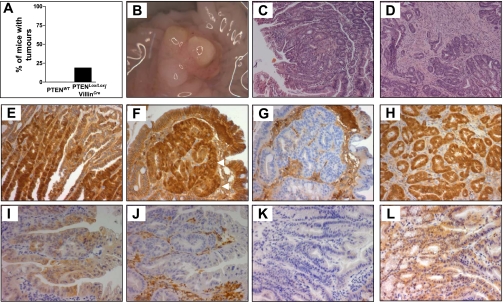
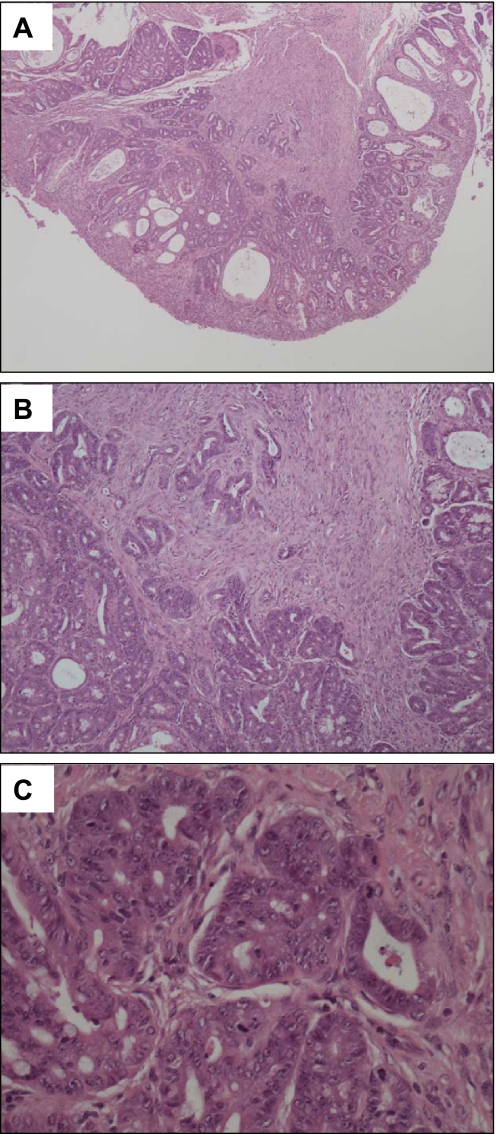
To determine the effect of PTEN deletion on intestinal tumorigenesis, an additional cohort of mice was maintained for 12 mo, and the intestinal tracts were examined for the presence of tumors at this time point. While no tumors were observed in any of the PTENWT mice (0/10), 3 out of 16 PTENLox/Lox/villinCre mice developed intestinal tumors (19%, P = 0.145 Chi square test) (Fig. 5A). Two of the three mice with tumors developed tumors in the small intestine (duodenum and jejunum), and one mouse developed a cecal tumor. Histopathological examination of these tumors demonstrated that the small intestinal tumors were tubulovillous adenomas (Fig. 5, C and D), and the cecal tumor was a moderately differentiated adenocarcinoma arising within a tubulovillous adenoma (Fig. 6).
Fig. 5.
A: frequency of adenoma formation in PTENWT and PTENLox/Lox/villinCre mice at 12 mo of age. B: macroscopic image of a cecal tumor in a PTENLox/Lox/villinCre mouse. C and D: H&E staining demonstrating a tubulovillous adenoma (C) and an adenocarcinoma (D) derived from PTENLox/Lox/villinCre mice. E and F: tumors derived from PTENLox/Lox/villinCre mice show evidence of nuclear β-catenin. Tumors from PTENLox/Lox/villinCre mice were stained with β-catenin as indicated in methods. Nuclear β-catenin is indicated by arrows in F. G: negative control. Nonspecific staining is due to the use of an anti-mouse secondary antibody. Shown for comparison in H is nuclear β-catenin in an adenoma derived from an Apc1638 mouse that harbors an inactivating mutation at codon 1638 of the Apc gene. I and K: pAKT staining in adenomas derived from PTENLox/Lox/villinCre and Apc1638 mice, respectively. J and L: PTEN staining in adenomas derived from PTENLox/Lox/villinCre and Apc1638 mice, respectively. J: PTEN staining in stromal tissue shows specificity of PTEN deletion to epithelial cells.
Fig. 6.
PTENLox/Lox/villinCre mice develop adenocarcinomas in the small intestine. Shown is a representative invasive adenocarcinoma at ×10 (A), ×100 (B), and ×400 (C) magnification.
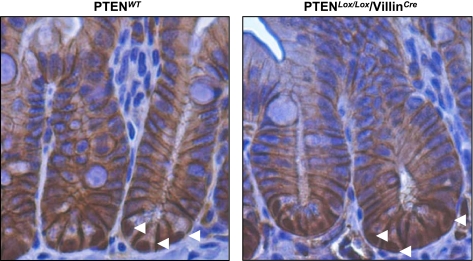
To determine the mechanistic basis for tumor formation in PTENLox/Lox/villinCre mice, we stained these tumors with an anti-β-catenin antibody to determine whether aberrant activation of Wnt signaling was involved. As shown in Fig. 5, E and F, clear nuclear β-catenin staining was evident in the adenomas derived from PTENLox/Lox/villinCre mice, which was comparable to the β-catenin staining pattern observed in adenomas derived from Apc1638 mice (Fig. 5H). This finding indicates that activation of Wnt signaling is a component of intestinal tumorigenesis driven by loss of PTEN. To determine whether the presence of nuclear β-catenin in these tumors was induced by PTEN loss, or the consequence of random deregulation of the Wnt pathway, we examined the frequency of nuclear β-catenin staining in the normal mucosa of PTENWT and PTENLox/Lox/villinCre mice. In the normal mucosa, no difference in the frequency of nuclear β-catenin staining was observed between WT and PTEN-deficient mice, which is consistent with previous reports (26). Moreover, as shown previously, nuclear β-catenin staining was predominantly in Paneth cells located in the base of the crypts (Fig. 7). These findings imply that the nuclear β-catenin staining observed in tumors derived from PTEN-deficient animals likely arose from stochastic activation of Wnt signaling, such as mutation or epigenetic alteration of the Apc or CTNNB1 (β-catenin) genes.
Fig. 7.
Nuclear β-catenin staining (white arrowheads) in the small intestine of PTENLox/Lox/villinCre and WT mice.
In addition, we also examined whether the tumors arising in PTEN-deficient animals showed evidence of activated AKT signaling. As shown in Fig. 5I, tumors arising in PTEN-deficient animals showed robust cytoplasmic pAKT staining that was not evident in tumors derived from Apc1638 mutant mice (Fig. 5K). These findings indicate that aberrant activation of both Wnt and AKT signaling is a feature of tumors arising in PTEN-deficient mice.
pH2AX expression is not altered in PTEN-deficient mice.
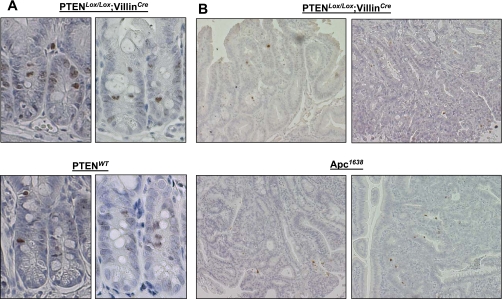
Independent of its role as a negative regulator of PI3K/AKT signaling, PTEN has also been shown to play a role in the maintenance of chromosomal integrity through facilitating DNA double-strand break (DSB) repair (24). To determine whether the frequency of DSBs was increased in the normal intestinal epithelium upon PTEN inactivation, we examined pH2AX expression in the jejunum of WT and PTENLox/Lox/villinCre mice, and in tumors derived from PTEN-deficient mice (Fig. 8). While the mean number of pH2AX-positive loci per crypt was higher in PTENLox/Lox/villinCre mice (4.4 ± 2.8) compared with WT animals (3.1 ± 1.6), this difference was not statistically significant (P = 0.39). Furthermore, the frequency of pH2AX staining in tumors derived from PTENLox/Lox/villinCre was comparable to that observed in tumors derived from Apc1638 animals, indicating PTEN deletion does not markedly alter the frequency of DSBs in the mouse intestine.
Fig. 8.
A: pH2AX staining in the normal small intestinal epithelium of PTENLox/Lox/villinCre and PTENWT mice. B: pH2AX staining in tumors derived from PTENLox/Lox/villinCre mice. Shown for comparison is pH2AX staining in tumors derived from Apc1638 mice (bottom).
DISCUSSION
The PI3K/AKT signaling pathway is among the most frequently activated pathways in human cancers, and ∼30–40% of colon tumors harbor mutations that result in constitutive activation of this signaling pathway (21). Activation of PI3K signaling in colon cancers is mediated by gain of function mutations in the kinase and helical domains of the PIK3CA gene or loss of function of the PTEN gene. Mutations are also observed in other regulators of the pathway, including the regulatory subunit of PI3K, p85 (12), as well as in downstream signaling mediators PDK1, AKT2, and PAK4 (21), although the latter are detected at lower frequencies.
A number of mouse models of PTEN inactivation have been reported previously (4, 10, 22). Constitutive inactivation of PTEN results in embryonic lethality, whereas PTEN heterozygous animals are prone to the development of a wide range of tumors, including in the intestinal tract (4, 22). However, the majority of these intestinal tumors are lymphoid polyps or lymphoid polyps with epithelial hyperplasia (22). Likewise, He et al. (10) used Mx1-Cre to inactivate PTEN in the intestinal epithelium and a variety of other cell types, including stromal cells, and observed the formation of hamartomatous intestinal polyps. Recently, two groups reported that PTEN inactivation specifically in the intestinal epithelium enhances Apc-initiated intestinal tumorgenesis (14, 20). However, neither group reported the onset of intestinal adenomas following inactivation of PTEN alone.
In the current study, we demonstrate efficient intestinal-specific deletion of PTEN using a Cre/Lox strategy. Consistent with PTEN's role as a major inhibitor of the proproliferation/prosurvival PI3K/AKT signaling pathway, pAKT levels were increased in the normal crypts of PTEN-deficient mice; however, no significant differences in the rate of cell proliferation were evident. These finding are consistent with those recently reported by Marsh et al. (20) but differ somewhat from Langlois et al. (14), who observed a small but statistically significant increase in cell proliferation upon PTEN inactivation.
We also observed that PTEN expression is induced during differentiation of intestinal epithelial cells, in both human and mouse, using a variety of approaches. Despite this, PTEN deletion had no discernable effect on lineage-specific cell differentiation, with cells comprising the four major cell lineages present at levels similar to those in WT animals. It remains possible that PTEN deletion may result in more subtle effects on terminal cell differentiation, as recently suggested by Langlois et al. (14), who observed decreased expression of markers of the secretory cell lineages upon intestinal-specific deletion of PTEN, which could be sufficient to perturb mucosal homeostasis.
Most notably, we observed that PTEN inactivation alone is sufficient to initiate intestinal tumorigenesis, with 19% of mice in our cohort developing intestinal tumors, including adenocarcinomas. There is, however, a lengthy lag period, with tumors only observed in animals at 12 mo of age, suggesting that tumor development requires the accumulation of other stochastic events. To determine the mechanism of tumor development in PTEN-deficient mice, we explored a number of mechanisms. First, PTEN has previously been shown to play a role in DSB DNA repair (24), suggesting PTEN inactivation may facilitate tumor formation through increased genomic instability. However, no clear evidence of increased DSBs, as assessed by pH2AX staining, in either the normal mucosa or in tumors derived from PTENLox/Lox/villinCre mice was observed, suggesting enhanced DNA damage is unlikely to be a key mechanism of intestinal tumor formation in PTEN-deleted mice.
In contrast, and consistent with PTEN's role as a negative regulator of the proproliferative/prosurvival PI3K/AKT signaling pathway, tumors derived from PTEN-deficient mice demonstrated strong pAKT expression, indicating constitutive activation of the PI3K/AKT signaling pathway. In addition, tumors arising in PTEN-deficient mice demonstrated strong nuclear β-catenin staining, indicating activation of Wnt signaling, a key pathway in the initiation of the vast majority of colon cancers. Notably, PTEN deletion had no effect on nuclear β-catenin levels in the normal mucosa, suggesting the nuclear β-catenin observed in tumors derived from PTEN-deficient mice likely arose from random activation of the Wnt pathway. These findings, combined with the long latency of tumor onset and the formation of adenocarcinomas, which typically require the accumulation of multiple mutational events, support a model of tumorigenesis whereby constitutive activation of PI3K/AKT signaling induced by PTEN inactivation promotes rapid clonal expansion of rare cells that stochastically acquire constitutive activation of Wnt signaling.
In summary, we demonstrate that intestinal-specific PTEN deletion has no major effect on intestinal homeostasis but does result in an increased incidence of tumor formation. These findings further confirm PTEN's role as a bona fide tumor suppressor gene in intestinal cancer.
GRANTS
These studies were supported by National Cancer Institute Grants CA-123316, CA-100823, and CA-88104 and National Health and Medical Research Council of Australia Project Grant ID 566679. J. M. Mariadason is supported by a Future Fellowship from the Australian Research Council.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of the late Dr. D.-S. Byun to this paper.
REFERENCES
- 1. Bowen KA, Doan HQ, Zhou BP, Wang Q, Zhou Y, Rychahou PG, Evers BM. PTEN loss induces epithelial–mesenchymal transition in human colon cancer cells. Anticancer Res 29: 4439–4449, 2009 [PMC free article] [PubMed] [Google Scholar]
- 2. Chang JG, Chen YJ, Perng LI, Wang NM, Kao MC, Yang TY, Chang CP, Tsai CH. Mutation analysis of the PTEN/MMAC1 gene in cancers of the digestive tract. Eur J Cancer 35: 647–651, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit 10: RA235–241, 2004 [PubMed] [Google Scholar]
- 4. Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet 19: 348–355, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Farooq A, Walker LJ, Bowling J, Audisio RA. Cowden syndrome. Cancer Treat Rev 36: 577–583, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, Camponovo A, Etienne LL, Cavalli F, Mazzucchelli L. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 97: 1139–1145, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frattini M, Signoroni S, Pilotti S, Bertario L, Benvenuti S, Zanon C, Bardelli A, Pierotti MA. Phosphatase protein homologue to tensin expression and phosphatidylinositol-3 phosphate kinase mutations in colorectal cancer (Abstract). Cancer Res 65: 11227, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Goel A, Arnold CN, Niedzwiecki D, Carethers JM, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res 64: 3014–3021, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Guanti G, Resta N, Simone C, Cariola F, Demma I, Fiorente P, Gentile M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum Mol Genet 9: 283–287, 2000 [DOI] [PubMed] [Google Scholar]
- 10. He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39: 189–198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med 11: 687–694, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring P, Dela Vega T, Kenski DM, Bowman KK, Lorenzo M, Li H, Wu J, Modrusan Z, Stinson J, Eby M, Yue P, Kaminker JS, de Sauvage FJ, Backer JM, Seshagiri S. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16: 463–474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L, Augenlicht LH, Perez-Soler R, Mariadason JM. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res 68: 1953–1961, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langlois MJ, Roy SA, Auclair BA, Jones C, Boudreau F, Carrier JC, Rivard N, Perreault N. Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB J 23: 1835–1844, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32: 148–149, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–33283, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht LH. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res 61: 3465–3471, 2001 [PubMed] [Google Scholar]
- 18. Mariadason JM, Nicholas C, L'Italien KE, Zhuang M, Smartt HJ, Heerdt BG, Yang W, Corner GA, Wilson AJ, Klampfer L, Arango D, Augenlicht LH. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 128: 1081–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Mariadason JM, Velcich A, Wilson AJ, Augenlicht LH, Gibson PR. Resistance to butyrate-induced cell differentiation and apoptosis during spontaneous Caco-2 cell differentiation. Gastroenterology 120: 889–899, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Marsh V, Winton DJ, Williams GT, Dubois N, Trumpp A, Sansom OJ, Clarke AR. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet 40: 1436–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Lengauer C, Velculescu VE. Colorectal cancer: mutations in a signalling pathway (Abstract). Nature 436: 792, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96: 1563–1568, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saito Y, Swanson X, Mhashilkar AM, Oida Y, Schrock R, Branch CD, Chada S, Zumstein L, Ramesh R. Adenovirus-mediated transfer of the PTEN gene inhibits human colorectal cancer growth in vitro and in vivo. Gene Ther 10: 1961–1969, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157–170, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Shin KH, Park YJ, Park JG. PTEN gene mutations in colorectal cancers displaying microsatellite instability. Cancer Lett 174: 189–194, 2001 [DOI] [PubMed] [Google Scholar]
- 26. van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol 7: 381–386, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Wilson AJ, Byun DS, Nasser S, Murray L, Ayyanar K, Arango D, Figueroa M, Melnick A, Kao GD, Augenlicht LH, Mariadason JM. HDAC4 promotes growth of colon cancer cells via repression of p21. Mol Biol Cell 19: 4062–4075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young GP, Rose IS, Cropper S, Seetharam S, Alpers DH. Hepatic clearance of rat plasma intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol 247: G419–G426, 1984 [DOI] [PubMed] [Google Scholar]
- 29. Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clin Cancer Res 16: 4325–4330, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Zhou XP, Loukola A, Salovaara R, Nystrom-Lahti M, Peltomaki P, de la Chapelle A, Aaltonen LA, Eng C. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol 161: 439–447, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]