Abstract
Intermediate-conductance K+ (Kcnn4) channels in the apical and basolateral membranes of epithelial cells play important roles in agonist-induced fluid secretion in intestine and colon. Basolateral Kcnn4 channels have been well characterized in situ using patch-clamp methods, but the investigation of Kcnn4 channels in apical membranes in situ has been hampered by a layer of mucus that prevents seal formation. In the present study, we used patch-clamp methods to characterize Kcnn4 channels in the apical membrane of IEC-18 cells, a cell line derived from rat small intestine. A monolayer of IEC-18 cells grown on a permeable support is devoid of mucus, and tight junctions enable selective access to the apical membrane. In inside-out patches, Ca2+-dependent K+ channels observed with iberiotoxin (a Kcnma1/large-conductance, Ca2+-activated K+ channel blocker) and apamin (a Kcnn1–3/small-conductance, Ca2+-activated K+ channel blocker) present in the pipette solution exhibited a single-channel conductance of 31 pS with inward rectification. The currents were reversibly blocked by TRAM-34 (a Kcnn4 blocker) with an IC50 of 8.7 ± 2.0 μM. The channels were not observed when charybdotoxin, a peptide inhibitor of Kcnn4 channels, was added to the pipette solution. TRAM-34 was less potent in inhibiting Kcnn4 channels in patches from apical membranes than in patches from basolateral membranes, which was consistent with a preferential expression of Kcnn4c and Kcnn4b isoforms in apical and basolateral membranes, respectively. The expression of both isoforms in IEC-18 cells was confirmed by RT-PCR and Western blot analyses. This is the first characterization of Kcnn4 channels in the apical membrane of intestinal epithelial cells.
Keywords: basolateral membrane, intermediate-conductance potassium channel isoforms, potassium secretion, patch clamp
intermediate-conductance k+ (Kcnn4) channels (also known as IK1, mIK1, and KCa3.1) are Ca2+-dependent K+ channels of intermediate conductance that are broadly distributed in both nonepithelial and epithelial cells where they play important roles in regulating membrane potential, influencing Ca2+ signaling, maintaining cell volume, and providing the driving force for anion (Cl− and HCO3−) secretion (12, 18, 24, 26). Kcnn4 channels are present on both apical and basolateral membranes of epithelial cells, including human bronchial (16HBE140 cell line), pancreatic duct adenocarcinoma (HPAF cell line), small intestine, and colon (1, 3, 6, 9, 25). In a recent study, we isolated from rat colon three distinct Kcnn4 splice variants: Kcnn4a, Kcnn4b, and Kcnn4c, which encoded proteins of 425, 424, and 395 amino acids, respectively (1). Kcnn4a transcripts were selectively expressed in smooth muscle, whereas Kcnn4b and Kcnn4c transcripts encoded K+ channels that were targeted in epithelial cells of the rat distal colon to basolateral and apical membranes, respectively (1). In the Xenopus oocyte expression system, Kcnn4c, which lacks the predicted second transmembrane-spanning domain, required an accessory protein for its delivery to the plasma membrane, and it exhibited an unusually low sensitivity to inhibition by TRAM-34 (a Kcnn4 channel blocker), with an IC50 of 7.8 μM (1).
Hyperpolarization of the membrane potential by the Ca2+-dependent opening of Kcnn4 channels plays a critical role in providing the driving force for agonist (cAMP, Ca2+)-induced anion secretion in human and rat colon (8, 13, 22). Although Kcnn4 channels are localized in both the apical and basolateral membranes, only basolateral Kcnn4 channels have been suggested to be responsible for providing the driving force for agonist-induced anion secretion (8, 22). In a recent study, we showed that, in the absence of serosal Kcnn4 channel activity, the activation of mucosal Kcnn4 channels with 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazole-2-one (a Kcnn4 channel opener) enhanced the anion secretion in rat distal colon that is mediated by both the cystic fibrosis transmembrane regulator and the Ca2+-activated Cl− channel (17). In this study, we also suggested that mucosal Kcnn4 channels might contribute to stool K+ losses that accompany diarrheal illnesses (17).
Although basolateral (serosal) Kcnn4 channels have been well characterized, the biophysical and pharmacological properties of apical (mucosal) Kcnn4 channels have not been characterized (5, 8, 14, 19). A few studies have identified large-conductance, Ca2+-activated K+ channels (BK) channels in the apical membranes of colonic surface cells (16, 23), but the characterization of apical ion channels has been generally unsuccessful because the layer of mucus that protects the apical surface of intestinal and colonic epithelial cells also makes it difficult for a patch pipette to access and form a seal on the apical membranes.
In the present study, we used cultured IEC-18 cells as a surrogate for intestinal and colonic epithelial cells to enable us to characterize for the first time the Kcnn4 channels in the apical membrane of intestinal epithelial cells. The IEC-18 cell line is a nontransformed, epithelial cell line from intestinal crypts that forms a confluent monolayer when grown on a permeable support (15). The monolayer exhibits a net electrical resistance that is similar to the intact epithelial layer of rat small intestine (15), and it lacks the layer of mucus that interferes with patch seal formation. Our choice of this cell line was also justified by immunofluoresence studies that have localized Kcnn4-like proteins on both the apical and basolateral membranes of surface and crypt cells in rat ileum (6). We report here that we successfully characterized the physiological properties of Kcnn4 channels in the apical membrane of IEC-18 cells.
MATERIALS AND METHODS
IEC-18 cell culture.
IEC-18 cells were grown on transparent, 1 μm PET, 6-Well Millicell TransWell Hanging Cell Culture inserts (Millipore, Billerica, MA) in high-glucose Dulbecco's modified Eagle's medium (Invitrogen-GIBCO) supplemented with insulin (2 U/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), butyrate (500 μM), and 10% FCS and incubated at 37°C with 5% CO2. The resistance of monolayers was measured using the Millicell-ERS electrical resistance system (Millipore). A 10-day postconfluent monolayer exhibited a specific resistance of ∼280 Ω·cm2, which indicates the formation of tight junctions. Therefore, 10-day postconfluent IEC-18 cells were used for patch-clamp studies and for the extraction of RNA and proteins that were used in RT-PCR and Western blot analyses, respectively.
RT-PCR.
RT-PCR analyses were performed as described earlier (1). In brief, RT-PCR analysis of total RNA isolated from rat colonic epithelial cells and IEC-18 cells was performed using Kcnn4-specific sense (5′-GAGCTGGTGACTGGCCT-3′) and antisense (5′-CGTTGTCAGTCATGAACAGCT-3′) primers that amplify 263- and 176-bp cDNA fragments from Kcnn4b and Kcnn4c transcripts, respectively (1). RT-PCR analysis of Kcnma1 (BK) channel α-subunit mRNA was performed using sense (5′-CTCCTCCTCTTCCTCCTCGT) and antisense (5′-GCAAACGGTCCACAGGTACT) primers that amplify a 199-bp cDNA fragment from Kcnma1 transcripts.
Total-RNA was isolated using the RNeasy micro kit (Qiagen), and RT-PCR reactions were performed using the One-Step RT-PCR kit (Qiagen) as described previously (1). RT-PCR products separated on a 2% agarose gel were stained with ethidium bromide and documented using the Gel-Doc System (Bio-Rad Laboratories, Hercules, CA).
Western blot analyses.
Western blot analyses were performed using colonic epithelial cells and 10-day postconfluent IEC-18 cell homogenates. Kcnn4 proteins were detected using an anti-Kcnn4-abc antibody that recognizes all three isoforms of Kcnn4 (1). Kcnma1 was detected using an antibody (Alamone Laboratories) targeted against the α-subunit. In brief, isolated colonocytes and 10-day postconfluent IEC-18 cells were suspended (1:20) in ice-cold lysis buffer [50 mM Tris, pH 8.0, 0.5% SDS, 1 mM phenylmethylsulfonyl fluoride, 4 μg/ml pepstatin A, and one tablet of Complete protease inhibitor/50 ml solution (Roche Applied Science, Indianapolis, IN)]. Following homogenization with a tight-fit Teflon homogenizer, the homogenate was centrifuged for 15 min at 2,000 g, and 16-μl supernatants were mixed with an equal volume of Laemmli buffer and denatured. The proteins were separated on 4–20% Tris·HCl gels (Ready Gel; Bio-Rad) and transferred onto nitrocellulose membrane. The blots were probed with the anti-Kcnn4-abc primary antibody (1:3,000 dilution) and an anti-rabbit secondary antibody labeled with Alexafluor 680 (Invitrogen, Carlsbad, CA). Labeling was detected using an Odyssey Infrared Imaging System (Li-Cor Biosciences).
Electrophysiology.
Patch-clamp recordings were made from the exposed surface of a monolayer of 10-day postconfluent IEC-18 cells attached to a TransWell membrane. The cells were briefly washed with Hanks' buffered salt solution (Sigma-Aldrich, St. Louis, MO) and then with a Cl−-free buffer. The bath (cytosolic) solution contained (in mM): 140 potassium gluconate, 2.5 magnesium acetate, 10 glucose, and 10 HEPES (pH 7.2). For most experiments, calcium acetate and EGTA were added to give a final free Ca2+ concentration of ∼285 μM; in some experiments, the ratio of calcium acetate to EGTA was varied to give lower free Ca2+ concentrations. The concentration of free Ca2+ was calculated using the CaBuf software (G. Droogmans, KU Leuven, Leuven, Belgium). The pipette (extracellular) solution contained (in mM): 140 potassium gluconate, 1 calcium acetate, 2.5 magnesium acetate, 10 glucose, and 10 HEPES (pH 7.4). Apamin (APA; 200 nM) and iberiotoxin (IbTX; 100 nM) were added to the pipette solution to block small-conductance, Ca2+-activated K+ (SK) channels and BK channels, respectively. A Ca2+-free bath solution was prepared by leaving out Ca2+ but including 3 mM EGTA.
Pipettes were pulled from borosilicate glass (Kwik-Fil, 1.5 mm OD/0.85 mm ID; WPI, Sarasota, FL). The pipette resistance was 15–25 MΩ. The bath was grounded by a AgCl2 pellet, and the junction potential was nulled before each recording. The recordings were performed with an Axopatch-200 amplifier (Axon Instruments, Foster City, CA), and the output was digitized with a Digidata 1321 data acquisition system controlled by PClamp 8 software. Excised, inside-out patches were held at 0 mV, and data were collected by applying 400-ms steps to voltages ranging from −80 to +80 mV in 10-mV increments. The average current during the last 300 ms of the voltage step was calculated following subtraction of the baseline current for analyzing the sensitivity to drugs or the removal of Ca2+. The recording chamber was perfused at a rate of ∼1 ml/min using a gravity-fed perfusion system. Switching between solutions was completed in <1 min. All recordings were performed at room temperature. Curve fitting and statistical analysis were performed using PRISM software (GraphPad, San Diego, CA). All averages are presented ± SE.
Biotinylation of apical proteins.
Apical proteins were isolated from normal distal colon in rat using the EZ-link Sulfo-NHS-SS biotinylation reagent (Pierce Biotechnology, Rockford, IL). A section of distal colon was removed, cleaned by washing in saline, and cooled on ice, and the reagent was added to the intestinal lumen on ice for 30 min. Following quenching with dithiothreitol (DTT) and extensive washing, the epithelial layer was removed and lysed, and the biotinylated proteins were recovered using NeutrAvidin agarose. The bound proteins were released by incubating with SDS-PAGE sample buffer containing DTT.
RESULTS
Expression of Kcnn4b and Kcnn4c in IEC18 cells.
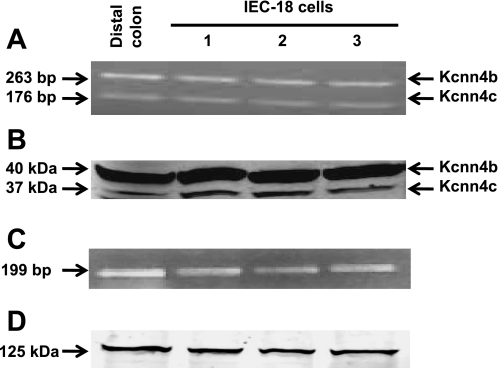
We recently reported that Kcnn4b and Kcnn4c transcripts are specifically expressed in epithelial cells, and the proteins encoded by the Kcnn4b and Kcnn4c transcripts are selectively targeted to the basolateral and apical membranes, respectively, of epithelial cells in the rat distal colon (1). In the present study, we performed RT-PCR and Western blot analyses to determine if Kcnn4b and Kcnn4c isoforms are both expressed in IEC-18 cells. RT-PCR analysis was performed using total RNA isolated from IEC-18 cells and Kcnn4-specific primers that would amplify 263 and 176 bp from Kcnn4b and Kcnn4c transcripts, respectively (1). Both 263- and 176-bp fragments were amplified from IEC-18 cells and distal colon (Fig. 1A). Using the anti-Kcnn4-abc antibody, we detected 37 (Kcnn4c)- and 40 (Kcnn4b)-kDa proteins in IEC-18 cell homogenates and distal colon (Fig. 1B). Thus, Kcnn4b- and Kcnn4c-specific transcripts and proteins are expressed in IEC-18 cells, and this led us to predict that we could characterize Kcnn4 channels in the apical membrane of IEC-18 cells.
Fig. 1.
A: RT-PCR analyses using intermediate-conductance K+ (Kcnn4) channel-specific primer detected transcripts for Kcnn4b (263 bp) and Kcnn4c (176 bp) in IEC-18 cells and epithelial cells from distal colon. B: Western blot analysis using an anti-Kcnn4-abc antibody detected 37 (Kcnn4c)- and 40 (Kcnn4b)-kDa proteins in IEC-18 cells and epithelial cells from rat distal colon. Lanes were loaded as in A. C: RT-PCR analyses using Kcnma1-specific channel primers detected a 199-bp transcript in IEC-18 cells and in epithelial cells from distal colon. D: Western blot analysis using an anti-Kcnma1 antibody detected a 125-kDa protein in IEC-18 cells and in epithelial cells from rat distal colon.
We also expected that Kcnma1/BK channels would be expressed in IEC-18 cells, and we detected a 199-bp transcript (Fig. 1C) and a 125-kDa protein (Fig. 1D) corresponding to the α-subunit (Kcnma1α). However, we did not observe BK channel currents in apical membrane patches when IbTX was not added to the pipette solution.
Patch formation on apical membranes.
Our strategy for selectively studying K+ channels in the apical membrane took advantage of the fact that IEC-18 cells grown on a filter membrane produce a confluent monolayer of polarized cells with the basal surface of the cells attached to the permeable support (filter) and the apical surface exposed and accessible to a patch pipette. In a monolayer of polarized, postconfluent cells attached to a filter, the formation of tight junctions and segregation of apical vs. basolateral domains should restrict the region of membrane that is accessible to a patch pipette to the apical membrane only. Giga-ohm seals formed readily (∼50% success rate) when a patch pipette was pressed against the exposed apical surface of a monolayer of 10-day postconfluent cells and gentle suction was applied, and both inside-out and outside-out patches (data not shown) could be excised from the apical surface. There was a tendency for channel activity in inside-out patches to disappear after excision of the patch, but it was usually restored by a transient exposure of the pipette tip to air, perhaps as a result of breaking a vesiculated patch of membrane in the pipette tip.
In addition to Kcnn4 channels, the apical membrane of intestinal epithelial cells also contains Kcnma1/BK channels, Kcnn1–3/SK channels, and Cl− channels. We prevented any interference from Cl− currents by recording in Cl−-free solutions, and K+ currents through Kcnma1 and Kcnn1–3 channels were prevented by adding 100 nM IbTX (a Kcnma1 channel blocker) and 200 nM APA (a Kcnn1–3 channel blocker), respectively, to the pipette solution.
Apical Kcnn4 channel activity.
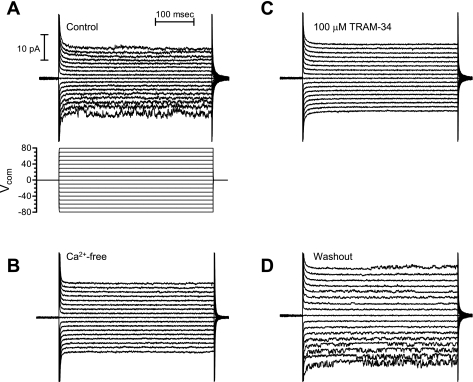
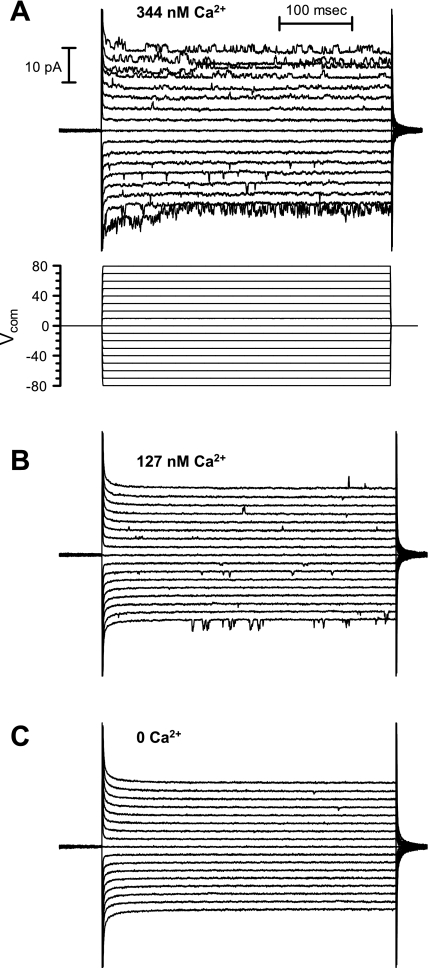
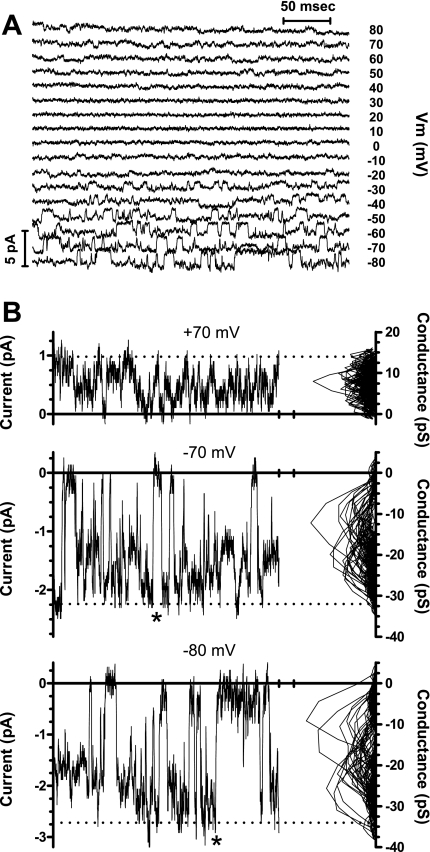
Under the conditions described above, the most common single-channel current exhibited the properties of the intermediate-conductance, Kcnn4 channel. The activity of the K+ channel was completely and reversibly eliminated by the removal of Ca2+ from the cytosolic surface of the patch (Fig. 2B), which confirmed that the channel was a Ca2+-dependent K+ channel. The K+ channel also exhibited a concentration-dependent increase in activity at intermediate free Ca2+ concentrations (Fig. 3). In a symmetric K+ solution, the current was inwardly rectifying (Fig. 2A and see Fig. 5A). The single-channel chord conductance of the fully open channel was 31.3 ± 1.4 pS at −80 mV and 22.3 ± 3.8 pS at +80 mV (n = 15 patches, Fig. 4). Openings of the channel to the fully open state were infrequent, and the channels exhibited considerable subconductance activity (Fig. 4). Collectively, these characteristics indicate that the K+ channels we had patched in the exposed surface of a monolayer of IEC-18 cells were IbTX- and APA-insensitive, Ca2+-activated K+ channels.
Fig. 2.
A: control recordings from an inside-out patch in a symmetric K+ solution. The membrane potential was stepped for 400 ms to potentials ranging from −80 to +80 mV in 10-mV increments. The K+ currents were inwardly rectifying. B: loss of channel activity in a Ca2+-free bath solution. C: block of channel activity with 100 μm TRAM-34 added to the bath solution. D: washout of TRAM-34 and recovery of channel activity. Vcom, command voltage.
Fig. 3.
Activation of Kcnn4 channels in IEC-18 cells at Ca2+ concentrations of 344 nM free Ca2+ (A), 127 nM free Ca2+ (B), and 0 free Ca2+ (C). The recordings were performed as described in the legend for Fig. 2.
Fig. 5.
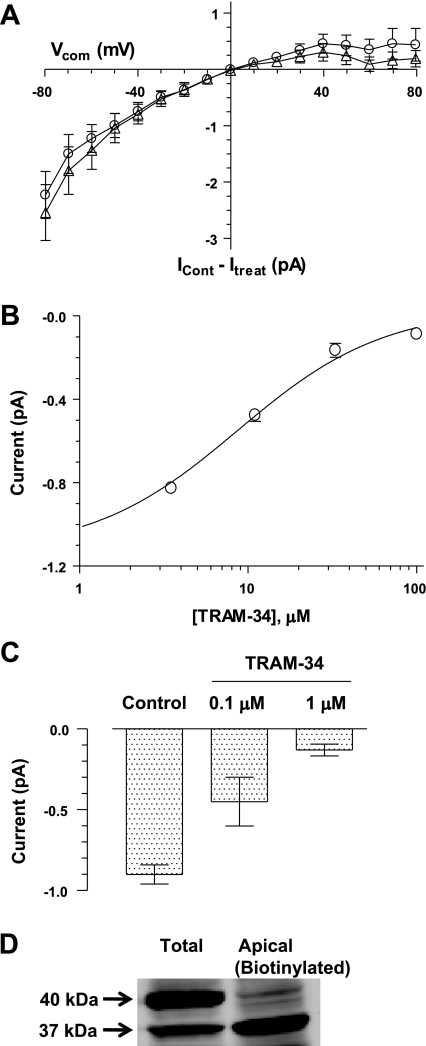
A: overlay of Ca2+-dependent current (circles) and TRAM-34-sensitive current (triangles). For each patch, the current-voltage (I/V) curve after the removal of Ca2+ from the bath or after the addition of 100 μM TRAM-34 to the bath was subtracted from the control I/V curve to calculate the Ca2+-dependent and TRAM-34-sensitive currents, respectively (average ± SE for 10 patches). The Ca2+-dependent and TRAM-34-sensitive currents were very similar in magnitude and inward rectification. B: dose-response relationships for block of the current at −80 mV by TRAM-34. An IC50 of 8.7 ± 2.0 μM was calculated by a fit of a one-site blocking model to the data (circles, average ± SE for 5 patches). C: inhibition of K+ channels in basolateral membranes by TRAM-34 (average ± SE for 3 patches). Basolateral K+ channels were much more sensitive to TRAM-34 than the apical channels shown in B. D: Western blot analysis of epithelial cells in rat distal colon using the anti-Kcnn4-abc antibody detected 37 (Kcnn4c)- and 40 (Kcnn4b)-kDa isoforms in a sample of total proteins (lane on left) but only the 37-kDa isoform in a sample of apical proteins that were biotinylated on the mucosal surface and isolated on NeutrAvidin resin (lane on right). ICont, control current; Itreat, treatment current.
Fig. 4.
Single-channel analysis. A: a family of currents activated by sequentially stepping the membrane potential (Vm) to voltages ranging from −80 to +80 mV for 400 ms in 10-mV increments (as in Fig. 2). The recordings were performed with symmetric K+ solutions. B: expanded views of the currents activated by steps to +70, −70, and −80 mV with a mean-variance plot (20) displayed to the right of the trace. The mean and variance were calculated for a window of 5 consecutive current samples that was moved sequentially through each current trace. For each consecutive pair of mean and variance values, the variance was plotted on the abscissa, and the mean current was rescaled to the corresponding chord conductance by dividing by the membrane potential and plotted on the ordinate. The mean-variance plots reveal that the conductance levels with the lowest variance (corresponding to the best-defined current levels) at negative potentials are primarily intermediate conductance levels between the closed and fully open conductance levels. The current amplitude scaled on the left axis corresponds to the chord conductance scaled on the right axis. The dotted lines indicate the current levels corresponding to a fully open chord conductance of 14 pS (record on top), 31 pS (record in middle), and 34 pS (record on bottom). The direct transitions between the fully open and closed conductance levels (*) indicate that a single channel is active in this recording.
Inhibition by TRAM-34.
In a previous study using the Xenopus oocyte expression system, we observed that transcripts from Kcnn4b (basolateral) and Kcnn4c (apical) splice variants exhibited a high sensitivity (IC50 of 0.6 μM) and a low sensitivity (IC50 of 7.8 μM), respectively, to inhibition by TRAM-34 (a Kcnn4 channel blocker) (1). Therefore, we predicted that preferential expression of Kcnn4c channels in the apical membranes of IEC-18 cells should be evident as TRAM-resistant K+ channels, i.e., K+ channels that are inhibited by TRAM-34 with a relatively low potency. The K+ channels in inside-out patches of apical membrane from IEC-18 cells were completely and reversibly inhibited by 100 μM TRAM-34 (Fig. 2, C and D). Subtraction of the residual currents that remained after the addition of TRAM-34 or after the removal of Ca2+ from the respective control currents revealed that the K+ current blocked by TRAM-34 was identical to the K+ current eliminated by the removal of Ca2+ from the bath (Fig. 5A). This provided evidence that TRAM-34 was blocking a Ca2+-activated K+ channel. Preliminary experiments revealed that TRAM-34 had a low potency in inhibiting the K+ channels in apical membranes (data not shown). We examined in greater detail the dose-dependent inhibition of the inward K+ current measured at −80 mV in inside-out patches of apical membrane (Fig. 5B). We calculated an IC50 of 8.7 ± 2.0 μM (Fig. 5B), based on the fitting of a single-site blocking model to these data. The low sensitivity of K+ channels in the apical membrane to TRAM-34 was consistent with our prediction for the preferential expression of Kcnn4c channels in the apical membranes. The K+ channels in apical membranes were also inhibited by 100 μM clotrimazole (data not shown), an inhibitor of Kcnn4 channels that is structurally related to TRAM-34 (27).
Our biochemical data clearly demonstrated that Kcnn4b channels are also present in IEC-18 cells (Fig. 1). However, we did not observe Kcnn4 channels with a high sensitivity to TRAM-34 in apical membrane patches. Therefore, we predicted that TRAM-sensitive Kcnn4b channels might be targeted to basolateral membranes, as we observed previously in rat colon epithelial cells (1). To test this prediction, we made inside-out patch recordings from monolayers of IEC-18 cells that were partially peeled away from the filter and folded over to provide selective access to the basolateral surface of the monolayer. At 10 days postconfluence, these monolayers are very tough and easy to handle, and the basolateral surface of a large, folded monolayer can be determined unambiguously. We observed K+ channels in recordings from basolateral membranes that were very similar in their conductance and rectification to the Kcnn4 channels in apical membranes (data not shown). However, in basolateral membranes, the K+ channels were nearly completely blocked by 1 μM TRAM-34 (Fig. 5C) (n = 3 patches). We surmise from the higher potency of TRAM-34 in blocking the K+ channels in basolateral membranes that these are predominantly Kcnn4b channels.
The preferential expression of the Kcnn4c isoforms in apical membranes was also examined biochemically using a biotinylating reagent to selectively label apical proteins that could be separated from total protein by recovery on a NeutrAvidin resin. In normal distal colon from rat, application of the biotinylating reagent to the mucosal surface resulted in recovery of only the Kcnn4c isoforms, in contrast to the presence of both Kcnn4b and Kcnn4c isoforms in a sample of total cellular proteins (Fig. 5D). Analogous experiments performed on IEC-18 cells did not yield conclusive results because of the small amount of labeled protein. Thus, our physiological evidence for the preferential expression of Kcnn4c channels in the apical membrane is supported, indirectly, by evidence from rat distal colon.
Inhibition by charybdotoxin.
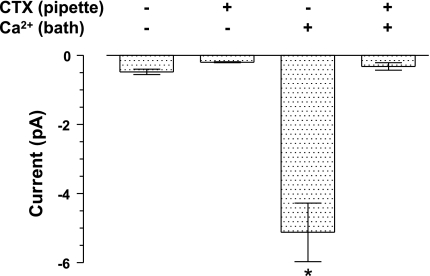
Charybdotoxin (CTX) is a cationic peptide toxin that is a potent inhibitor of Kcnn4 channels (10, 11). It binds to a site on the extracellular face of the channels, which should be exposed to the pipette solution in inside-out patches. To determine if the K+ channels we observed in apical membranes were inhibited by CTX, we compared the average current at −80 mV in five inside-out patches with 100 nM CTX added to the pipette solution with five patches without CTX added to the pipette solution (the pipette solution always contained 100 nM IbTX and 200 nM APA to block Kcnma1 and Kcnn1–3 channels, respectively). K+ channel currents were never observed when Ca2+ was not present in the bath, regardless of the presence of CTX in the pipette solution (Fig. 6). In contrast, when Ca2+ was present in the bath, large inward K+ channel currents were observed in the absence of CTX, but not when CTX was also present in the pipette solution (Fig. 6). Therefore, inside-out patches from the apical membrane of IEC-18 cells contained a Ca2+-activated K+ channel that was inhibited by CTX, but not IbTX or APA. The selective inhibition by CTX, but not IbTX or APA, provided additional evidence that the K+ channel we observed in apical membranes was Kcnn4.
Fig. 6.
Inhibition of Kcnn4 channels in inside-out patches from apical membrane by charybdotoxin (CTX). K+ channel currents were not observed when Ca2+ was not present in the bath solution (bars 1 and 2) (average ± SE for 5 patches with CTX and 5 patches without CTX). However, a large inward current (bar 3, indicated by *) was observed when Ca2+ was added to the bath solution and CTX was not present in the pipette solution. This current was significantly greater than the current observed with all other conditions (P < 0.001; ANOVA followed by Tukey's multiple-comparison test). The Ca2+-activated current was not observed when 100 nM CTX was present in the pipette solution (bar 4).
We did not observe any K+ channel activity when IbTX, APA, and CTX were added simultaneously to the pipette or when Ca2+ was removed from the bath (cytosolic face of the membrane). Thus, Ca2+-activated K+ channels were the only class of K+ channels evident in the apical membranes of IEC-18 cells.
DISCUSSION
K+ channels play important roles in maintaining the membrane potential of epithelial cells sufficiently hyperpolarized to provide the driving force for agonist (cAMP and Ca2+)-induced anion (Cl−/HCO3−)/fluid secretion (2, 4). Immunofluoresence and functional studies have shown that Kcnn4 channels are localized on both the apical and basolateral membranes of intestinal and colonic epithelial cells in human, guinea pig, and rat (6, 9, 12, 21). Molecular studies have identified the Kcnn4 channel as the basolateral K+ channel that provides the driving force for anion secretion (12, 26). We recently reported that apical and basolateral Kcnn4 channels are encoded by the splice variants Kcnn4c and Kcnn4b, respectively, in rat distal colon (1). Physiological studies have demonstrated that, in the absence of basolateral Kcnn4 channel activity, the activation of apical Kcnn4 channels can also provide the driving force for anion secretion (17). Although previous studies have established the presence of Kcnn4 channels in both apical and basolateral membranes, only the biophysical and pharmacological properties of basolateral Kcnn4 channels have been characterized (8, 26). With the exception of Kcnma1 channels in the apical membranes of rat and human colonic surface cells (16, 23), the characterization of K+ channels in apical membranes has been hampered by the layer of protective mucus that overlies the apical membrane and interferes with patch seal formation.
We have demonstrated in the present study that a 10-day postconfluent monolayer of IEC-18 cells grown on a permeable support exhibits a high specific membrane resistance (280 Ω·cm2), which is strong evidence for the formation of tight junctions and the segregation of apical domains that are exposed on the surface of the monolayer from basolateral domains that are attached to the filter and inaccessible. The apical membrane is devoid of mucus, and ion channels in the apical membrane can be easily studied using the patch-clamp method. By recording in a Cl−-free medium, including IbTX and APA in the pipette solution, and focusing on Ca2+-dependent K+ currents, we were able to eliminate channels other than Kcnn4 from our recordings.
We were successful in using this strategy to excise inside-out patches from the apical membranes of IEC-18 cells and identify Kcnn4 channels in these patches. This conclusion is supported by the observations that: 1) the K+ channels were blocked by CTX, but not by IbTX and APA; 2) the K+ channels were reversibly inhibited by the removal of cytosolic Ca2+; 3) the K+ channels exhibited inward rectification and a single-channel chord conductance of 31 pS at −80 mV; and 4) the K+ channels were inhibited by the Kcnn4 channel inhibitors TRAM-34 and clotrimazole. The physiological evidence for the presence of Kcnn4 channels in IEC-18 cells was also supported by our detection by RT-PCR of transcripts for the Kcnn4b and Kcnn4c isoforms, as well as our detection by Western blot analysis of Kcnn4b (40 kDa) and Kcnn4c (37 kDa) proteins in the IEC-18 cells. The gating of Kcnn4 channels in the apical membrane of IEC-18 cells was predominantly flickering openings to intermediate conductance levels, more than is typically apparent in published recordings from Kcnn4 channels in other cells (e.g., see Ref. 10). Further studies will be required to determine if this behavior might be related to deletion of the predicted second transmembrane domain in the Kcnn4c isoform.
Although the physiological properties of the K+ channels we observed in apical membrane patches from IEC-18 cells were similar to the properties of Kcnn4 channels observed in other membranes (7, 8, 27), the potency of TRAM-34 in inhibiting the apical K+ channels was significantly lower than what has been observed for classical Kcnn4 channels, which are blocked by TRAM-34 with an IC50 of 20 nM (27). The fact that much higher concentrations of TRAM-34 are required to inhibit prostaglandin E2-induced K+ secretion in colonic epithelium led Halm et al. (9) to conclude that K+ secretion involved K+ channels other than Kcnn4 channels. However, since the publication of that study, we have identified a TRAM-34-resistant isoform of Kcnn4 (i.e., Kcnn4c) that exhibited an IC50 of 7.8 μM when expressed in Xenopus oocytes (1), which is close to the IC50 of 8.7 μM we observed in apical membranes of IEC-18 cells. Thus, Kcnn4c is a good candidate for a TRAM-34-resistant mediator of K+ secretion. Given the overall similarity of the K+ channels we observed in apical membranes to Kcnn4 channels, we conclude that the low sensitivity to TRAM-34 can be accounted for by the expression of the Kcnn4c isoform as the predominant species of Kcnn4 in the apical membrane of IEC-18 cells. This conclusion is indirectly supported by our biochemical evidence that the Kcnn4c isoform was selectively targeted to the apical membrane in epithelial cells from rat distal colon. TRAM-34 has also been shown to inhibit voltage-gated K+ channels (i.e., Kv channels) at high concentrations (27). However, the presence of purely voltage-dependent channels in apical membranes can be ruled out by the complete dependence of the TRAM-34-sensitive K+ channel currents in apical membranes on the presence of Ca2+ in the bath (cytosol). In conclusion, IEC-18 cells should provide a useful surrogate for intestinal epithelial cells in future investigations of the expression and physiological function of Kcnn4 channels in epithelial cells.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-018777.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Barmeyer C, Rahner C, Yang Y, Sigworth FJ, Binder HJ, Rajendran VM. Cloning and identification of tissue specific expression of Kcnn4 splice variants in rat colon. Am Physiol Cell Physiol 299: C251–C263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Ann Rev Physiol 62: 535–572, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bernard K, Bogliolo S, Soriani O, Ehrenfeld J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J Membr Biol 196: 15–31, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Binder HJ, Sandle GI. Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York, NY: Raven, 1987 [Google Scholar]
- 5. Bowley KA, Morton MJ, Hunter M, Sandle GI. Non-genomic regulation of intermediate conductance potassium channels by aldosterone in human colonic crypt cells. Gut 52: 854–860, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furness JB, Robbins HL, Selmer IS, Hunne B, Chen MX, Hicks GA, Moore S, Neylon CB. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res 314: 179–189, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem 275: 37137–37149, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Greger R, Bleich M, Riedemann N, van Driessche W, Ecke D, Warth R. The role of K+ channels in colonic Cl- secretion. Comp Biochem Physiol 118: 271–275, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol 291: C636–C648, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651–11656, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen BS, Strobaek D, Christophersen P, Jorgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol Cell Physiol 275: C848–C856, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol 285: G185–G196, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Liao T, Wang L, Halm ST, Lu L, Fyffe RE, Halm DR. K+ channel KVLQT1 located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am J Physiol Cell Physiol 289: C564–C575, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Lomax RB, Warhurst G, Sandle GI. Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut 38: 243–247, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma TY, Hollander D, Bhalla D, Nguyen H, Krugliak P. IEC-18, a nontransformed small intestinal cell line for studying epithelial permeability. J Lab Clin Med 120: 329–341, 1992 [PubMed] [Google Scholar]
- 16. Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 206: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Nanda Kumar N, Singh S, Rajendran V. Mucosal potassium efflux mediated via Kcnn4 channels provides the driving force for electrogenic anion secretion in colon. Am J Physiol Gastrointest Liver Physiol 299: G707–G714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca(2+)-activated K(+) channel in vascular smooth muscle: relationship between K(Ca) channel diversity and smooth muscle cell function. Circ Res 85: e33–e43, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Nielsen MS, Warth R, Bleich M, Weyand B, Greger R. The basolateral Ca2+-dependent K+ channel in rat colonic crypt cells. Pflugers Arch 435: 267–272, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Patlak JB. Measuring kinetics of complex single ion channel data using mean-variance histograms. Biophys J 65: 29–42, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rufo PA, Jiang L, Moe SJ, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotrimazole, inhibits Cl- secretion by polarized monolayers of human colonic epithelial cells. J Clin Invest 98: 2066–2075, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rufo PA, Merlin D, Riegler M, Ferguson-Maltzman MH, Dickinson BL, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J Clin Invest 100: 3111–3120, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandle GI, Butterfield I. Potassium secretion in rat distal colon during dietary potassium loading: role of pH regulated apical potassium channels. Gut 44: 40–46, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandorpe DH, Shmukler BE, Jiang L, Lim B, Maylie J, Adelman JP, de Franceschi L, Cappellini MD, Brugnara C, Alper SL. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J Biol Chem 273: 21542–21553, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Morishima S, Okada Y. IK channels are involved in the regulatory volume decrease in human epithelial cells. Am J Physiol Cell Physiol 284: C77–C84, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Molecular and functional characterization of the small Ca(2+)-regulated K+ channel (rSK4) of colonic crypts. Pflugers Arch 438: 437–444, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]