Abstract
Thiamin is important for normal function of pancreatic acinar cells, but little is known about its mechanism of uptake and about the effect of chronic alcohol use on the process. We addressed these issues using freshly isolated rat primary and rat-derived cultured AR42J pancreatic acinar cells as models. Results showed thiamin uptake by both primary and cultured AR42J pancreatic acinar cells to be via a specific carrier-mediated mechanism and that both of the thiamin transporters 1 and 2 (THTR-1 and THTR-2) are expressed in these cells. Chronic alcohol feeding of rats was found to lead to a significant inhibition of carrier-mediated thiamin uptake by pancreatic acinar cells and was associated with a significant reduction in level of expression of THTR-1 and THTR-2 at the protein and mRNA levels. Chronic exposure (96 h) of AR42J cells to alcohol also led to a significant decreased carrier-mediated thiamin uptake, an effect that was associated with a significant decrease in the activity of the human SLC19A2 and SLC19A3 promoters expressed in these cells. We also examined the effect of chronic alcohol feeding of rats on level of expression of key thiamin metabolizing enzymes (thiamin phosphokinase and thiamin pyrophosphatase) as well as on level of expression of the mitochondrial thiamin pyrophosphate transporter of pancreatic acinar cells and observed a significant inhibition in all these parameters. These results demonstrate for the first time that thiamin uptake by pancreatic acinar cells is via a carrier-mediated process and that both the THTR-1 as well as THTR-2 are expressed in these cells. Also, chronic alcohol feeding/exposure inhibits thiamin uptake process and the inhibition is, at least in part, being exerted at the transcriptional level. Furthermore, chronic alcohol feeding also negatively impacts intracellular parameters of thiamin metabolism in pancreatic acinar cells.
Keywords: pancreas, THTR-1, THTR-2, TPKase, SLC19A25
thiamin (vitamin b1), a member of the water-soluble family of vitamins, plays an essential role in normal cellular functions, growth, and development. In the cell, imported thiamin is converted to its active forms, mainly thiamin pyrophosphate (TPP), via the action of thiamin pyrophosphokinase (TPKase), a rate-limiting enzyme that plays an important role in regulating cellular thiamin homeostasis. TPP is then either used in the cytoplasm or imported into the mitochondria via a carrier-mediated process that involves the mitochondrial TPP transporter encoded by the SLC25A19 gene (1, 2, 9, 15, 20, 26). Thiamin coenzymes play important role in reactions related to energy metabolism that include the decarboxylation of pyruvic acid and α-ketoglutamic acid and the utilization of pentose in the hexose monophosphate shunt (3, 26). Furthermore, because thiamin bridges the glycolytic and the pentose phosphate metabolic pathways, which are critical for creating chemical reducing power in cells, the vitamin is also considered to have a role in reducing cellular oxidative stress (5, 10). Thus cellular deficiency of thiamin subjects cells to oxidative stress. At the clinical level, thiamin deficiency in humans leads to a variety of abnormalities including neurological and cardiovascular disorders among others (3, 33, 34).
The pancreas contains high levels of thiamin (23), and thiamin deficiency leads to a severe reduction in its content of digestive enzymes (29). Pancreatic acinar cells (which represent an ∼82 percent of total pancreatic cells) cannot synthesize thiamin and therefore must obtain the vitamin from their surroundings via transport across cell membrane. Despite the importance of thiamin for normal metabolism and function of pancreatic acinar cells, there is little known about the mechanisms involved in the vitamin uptake by these cells and about the factors or conditions that negatively affect the process. One such factor could be chronic alcohol use, which is known to be associated with systemic thiamin deficiency and with inhibition in intestinal and renal thiamin absorption and reabsorption (30, 32). Chronic alcohol use has also been shown to negatively impact intracellular thiamin metabolism in a tissue-specific manner (27).
Our aims in these investigations were to identify the mechanism of thiamin uptake by pancreatic acinar cells and to examine the effect of chronic alcohol use on the uptake process. Addressing these issues is of clear physiological and nutritional importance; it may also be of pathophysiological relevance since chronic alcohol use in human is associated with increased risk of pancreatitis. Alcohol is believed to alter, among other things, the resting state of the pancreas and to cause a reduction in its defense mechanisms against stress conditions, injurious agents, or other cellular events (11, 13, 18, 21, 22). One such contributing stress condition could be a cellular deficiency of thiamin, a micronutrient involved in reducing cellular oxidative stress (3, 5, 10). We achieved our stated aims using as models primary pancreatic acinar cells isolated from control rats and rats fed alcohol liquid diet as well as cultured rat-derived pancreatic acinar AR42J cells. Our results showed the thiamin uptake by pancreatic acinar cells to be via a specific carrier-mediated mechanism and showed that both THTR-1 and THTR-2 are expressed in these cells, with expression of the former being markedly higher than that of the latter. The results also showed that chronic alcohol feeding leads to a significant decrease in level of thiamin uptake and in the level of expression of THTR-1 and THTR-2; there was also a decrease in the level of expression of the thiamin-metabolizing enzymes TPKase and thiamin pyrophosphatase (TPPase) as well as in the level of expression of the mitochondrial TPP transporter. Moreover, chronic alcohol exposure of cultured pancreatic acinar AR42J cells was found to lead to a significant decrease in the activity of SLC19A2 and SLC19A3 promoters.
MATERIALS AND METHODS
Materials
[3H]Thiamin (specific activity 20 Ci/mmol; radiochemical purity >99%) was obtained from American Radiolabeled Chemicals (St. Louis, MO). Nitrocellulose filters (0.45-μm pore size) were from Millipore (Fisher Scientific). Unlabeled thiamin and other chemicals including molecular biology reagents were from commercial vendors and were of analytical grade. Oligonucleotide primers used in this study were synthesized by Sigma Genosys (Sigma, Woodland, TX).
Isolation of Rat Primary Pancreatic Acinar Cells
Rat pancreatic primary acinar cells were isolated from Albino Wistar rats (100–300 g) by a collagenase type-V (Sigma, St. Louis, MO) digestion method as described previously (6, 12, 28). A minor modification is made in isolation of pancreatic primary acinar cells from alcohol-fed rats by reducing the collagenase exposure time for digestion. Isolated acinar cells were suspended in Krebs-Ringer buffer, and uptake was performed within 1 h of isolation. The viability of the isolated pancreatic acinar cells were assessed by Trypan blue exclusion test from control and alcohol-fed rats with viability estimated at >85% in both animal groups.
Cell Culture
AR42J (rat-derived pancreatic acinar) cells were from American Type Tissue Collection (ATCC, Rockville, MD) and cultured in Kaighn's F12-K growth medium containing 20% FBS and antibiotic cocktail. Cells were used between passages 2 and 10. To obtain maximum differentiation prior to use for uptake studies, AR42J cells were cultured in a growth medium containing 100 nM dexamethasone for 48 h (17).
Rat Pancreatic Primary Acinar and AR42J Cell Uptake Studies
All the uptake was carried out at 37°C for both rat primary acinar and cultured AR42J acinar cells suspended in Krebs-Ringer buffer as described before (28). [3H]labeled and unlabeled thiamin was added to the incubation medium during incubation. The uptake reaction was terminated by adding 1 ml ice-cold Krebs-Ringer buffer, and the cell suspension was placed on nitrocellulose filters under negative pressure by use of a vacuum manifold (Hoefer Scientific Instruments, Holliston, MA), washed with 5 ml of ice-cold buffer, and dissolved in scintillation fluid. The radioactivity was counted in a liquid scintillation counter (Beckman Coulter LS6500 multipurpose liquid scintillation counter, Brea, CA). Cell digest samples (100 μl each) were taken for protein quantification using a Bio-Rad Dc protein assay kit (Bio-Rad, Carlsbad, CA).
Chronic Alcohol Feeding of Rats
Male Wistar rats (Charles River, Wilmington, MA) weighing ∼120 g (∼14 wk old) were housed at the Animal Core of the National Institute on Alcohol Abuse and Alcoholism-funded Southern California Research Center for Alcoholic Liver and Pancreatic Disease and Cirrhosis at University of Southern California. The animal experimental protocol was approved by Animal Use Committee of the Veterans Affairs at Long Beach and the University of Southern California. Rats were fed Lieber-Decarli (14) ethanol liquid diet (ethanol provides 36% of total calories) (Bio-Serv); control rats were pair fed the same liquid diet but without ethanol (maltose-dextrin replaced ethanol isocalorically). After 4 wk of chronic alcohol feeding, rats were euthanized and the pancreas was removed and used for isolation of pancreatic primary acinar cells. After isolation of pancreatic acinar cells, some portion was collected and stored at −80°C for protein isolation and portion was stored at −80°C in Trizol (Invitrogen, Carlsbad, CA) for subsequent determination of mRNA and protein expression studies.
Real-Time PCR Analysis
Total RNA (5 μg), isolated from the primary acinar cells of alcohol-fed rats and their pair-fed controls and also from AR42J cells, was treated with DNase I (Invitrogen) enzyme to prevent genomic DNA contamination. The DNase I-treated samples were subjected for cDNA synthesis by using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The coding region of rat THTR-1, THTR-2, TPKase, TPPase, mitochondrial thiamin pyrophosphate (SLC19A25 gene), and β-actin, were PCR amplified by use of gene-specific primers (Table 1) for real-time PCR study. Real-time PCR conditions were same as described previously (30). The data were normalized to β-actin and then quantified by a relative relationship method supplied by the vendor (Bio-Rad) (16).
Table 1.
Primers used for amplifying coding region of the respective genes by real-time PCR
| Gene Name | Primers |
|---|---|
| rTHTR-1 | Forward: 5′-GCTGTCATCTACAATGCCGG-3′ |
| Reverse: 5′-GATGTACACTGCAGCAGCAATC-3′ | |
| rTHTR-2 | Forward: 5′-CGTGATACTCTGCTTGTTGTTCGG-3′ |
| Reverse: 5′-GGTAAGAGTACGTCAAACAGG-3′ | |
| rTPKase | Forward: 5′-GACCAAGACCACACTGAC-3′ |
| Reverse: 5′-GTTGGAGGAGGTAGATGAG-3′ | |
| rslc25a19 | Forward: 5′-GGCCATACGCACCATG-3′ |
| Reverse: 5′-GGGTCTTGCTGATGACTC-3′ | |
| rTPPase | Forward: 5′-AGGCCTCAGGACTATCAG-3′ |
| Reverse: 5′-TCAGTGTGGGTGAAAGAC-3′ | |
| rβ-actin | Forward: 5′-CTGCTCTTTCCCAGATGAG-3′ |
| Reverse: 5′-CTCATAGATGGGCACAGTG-3′ |
Western Blot Analysis
Western blot analysis was performed on whole cell lysate from rat primary pancreatic acinar cells from both alcohol-fed and control pair-fed rats. The primary acinar cells were suspended in 200 μl of RIPA buffer (Sigma) according to manufacturer's protocol supplemented with protease inhibitor cocktail (Roche). Equal amounts (60 μg) of pancreatic acinar cell proteins were resolved onto premade 4–12% Bis-Tris minigel (Invitrogen) as described before (28). After electrophoresis, proteins were electroblotted onto immobilon polyvinylidene difluoride membrane (Fisher Scientific) and then blocked overnight with Odyssey blocking solution (Li-COR Bioscience, Lincoln, NE) at 4°C. Along with blocking buffer the membranes were also incubated overnight either with rat THTR-1 or THTR-2-specific (1:500 dilution) polyclonal antibody (raised in rabbit) along with β-actin (1:3,000 dilution) monoclonal antibody (raised in mouse) and with rat THTR-2-specific (1:500 dilution) polyclonal antibodies (raised in rabbit) and β-actin polyclonal antibody (raised in mouse) separately. The THTR-1, THTR-2, and β-actin immunoreactive bands were detected by using anti-rabbit IRDye-800 and anti-mouse IRDye-680 (Li-COR Bioscience) secondary antibodies (1:20,000 dilution) according to manufacturer's protocol. Signals were detected and quantitated with the Odyssey infrared imaging system (Li-COR Bioscience) with accompanied LI-COR software for quantification and normalized to β-actin as an internal control.
Chronic Alcohol Exposure of AR42J Cells and Uptake
Cultured AR42J cells exposed with growth medium containing 50 mM of alcohol [a concentration that mimics the level of alcohol in the blood of chronic alcohol drinkers (8)] was introduced and the plates were kept in an ethanol-saturated incubator for 96 h as described by us recently (31, 32, 35). This step in addition to changing the alcohol-containing growth medium every 12 h were designed to minimize ethanol evaporation (35). Uptake studies were then performed at 37°C in Krebs-Ringer buffer as described above.
Transfection and Reporter Gene Assay
SLC19A2 and SLC19A3 full-length promoter-luciferase reporter constructs utilized in this study were generated previously (19, 25). AR42J cells were cotransfected in 12-well plates at less than 80% confluency with 4 μg of each test construct and 100 ng of the Renilla transfection control plasmid Renilla luciferase-thymidine kinase (pRL-TK) (Promega, Madison, WI). Transfection was performed with lipofectamine reagent (Invitrogen) according to manufacturer's instructions. On the second posttransfection day the cells transfected with SLC19A2 and SLC19A3 were treated with Kaighn's F12-K growth medium containing 50 mM alcohol for 96 h. To ensure proper alcohol effect, the media containing 50 mM alcohol was changed every 12 h. At the end of 96 h of alcohol incubation, Renilla-normalized firefly luciferase activity was determined by using the Dual Luciferase Assay system (Promega).
Statistical Analysis
Uptake data with rat pancreatic primary acinar preparation presented in this paper are means ± SE of at least three separate experiments from different rats and are expressed as femtomoles per milligram protein per unit time. Uptakes with the AR42J cells were also means ± SE from at least three separate experiments. Student's t-test was used for statistical analysis, and P < 0.05 was considered statistically significant. Carrier-mediated thiamin uptake was determined by subtracting uptake by simple diffusion (determined from the slope of the line between uptake at high pharmacological concentration of 1 mM and point of origin) from total uptake. We observed some quantitative variations in the absolute amount of thiamin uptake in certain experiments; for this reason we performed simultaneous controls with each set of experiments. Protein and RNA determinations were performed from at least three sets of samples, and the promoter assay was run on at least three different occasions using three different preparations.
RESULTS
Physiological and Molecular Aspects of the Thiamin Uptake Process of Rat Pancreatic Acinar Cells: Studies Utilizing Freshly Isolated Rat Primary Acinar Cells and Cultured Pancreatic Acinar AR42J Cells
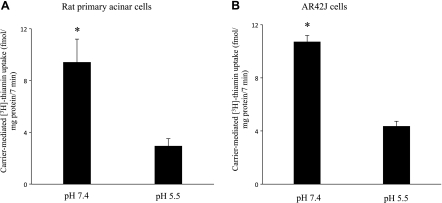
As mentioned earlier, there is nothing known about the mechanism of thiamin uptake by pancreatic acinar cells, and thus we performed a number of studies to shed light onto the process. First we examined the effect of extracellular (buffer) pH on the initial-rate of uptake (7 min; data not shown) of a physiological concentration of (15 nM) of thiamin by rat primary pancreatic acinar and AR42J cells were examined in this study. The results showed a significantly (P < 0.01 for both) higher thiamin uptake at buffer pH 7.4 than pH 5.5 by both freshly isolated primary acinar cells and AR42J cells (Fig. 1, A and B). A buffer of pH 7.4 was used in all our subsequent studies.
Fig. 1.
Effect of buffer pH on initial rate of [3H]thiamin uptake by rat primary and cultured AR42J pancreatic acinar cells. Rat primary pancreatic acinar cells (A) and cultured pancreatic acinar AR42J cells (B) were incubated for 7 min in Krebs-Ringer buffer, pH 7.4 and 5.5, at 37°C in presence of [3H]thiamin (15 nM). Data are means ± SE of at least 3 independent experiments. *P < 0.01.
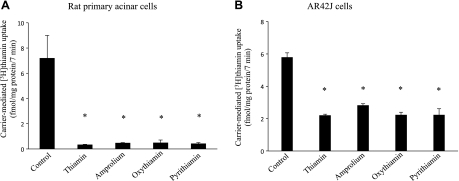
The effect of unlabeled thiamin and its structural analogs amprolium, oxythiamin, and pyrithiamin (all 100 μM) on the initial rate of [3H]thiamin (15 nM) uptake by freshly isolated primary pancreatic acinar and AR42J cells was then examined. The result showed that unlabeled thiamin and its structural analogs cause a significant (P < 0.01 for all) inhibition in [3H]thiamin uptake by both primary pancreatic acinar and AR42J cells (Fig. 2, A and B). We also examined at the effect of the unrelated organic cations tetraethylammonium (TEA) and choline on initial rate of [3H]thiamin (15 nM) uptake by AR42J cells and observed no significant effect (in percentage, uptake was 100 ± 8.58, 99.49 ± 9.12, and 95.94 ± 13.22% for control and in the presence of TEA and choline, respectively). These findings suggest the involvement of a specific, carrier-mediated mechanism for thiamin uptake by rat pancreatic acinar cells.
Fig. 2.
Effect of thiamin structural analogs on the initial rate of [3H]thiamin uptake by rat primary and cultured AR42J pancreatic acinar cells. Rat primary pancreatic acinar cells (A) and cultured AR42J cells (B) were incubated for 7 min in Krebs-Ringer buffer, pH 7.4 containing 100 μM unlabeled thiamin, amprolium, oxythiamin, or pyrithiamin in the presence of [3H]thiamin (15 nM). Data are means ± SE of at least 3 independent experiments. *P < 0.01.
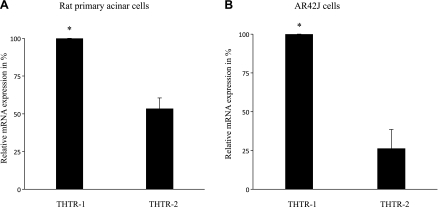
Two specific and high-affinity transport systems for thiamin have been identified in mammals: the thiamin transporters 1 and 2 (THTR-1 and THTR-2, respectively). Thus we examined the mRNA expression of these two thiamin transport systems in both primary and AR42J pancreatic acinar cells using real-time PCR (see methods). The results showed that both transporters are expressed in primary and AR42J pancreatic acinar cells, with expression of THTR-1 being significantly (P < 0.01) higher than that of THTR-2 in both cell types (Fig. 3, A and B).
Fig. 3.
Relative expression of THTR-1 and THTR-2 mRNA in both rat primary and cultured AR42J pancreatic acinar cells. mRNA was isolated from rat primary pancreatic acinar cells (A) and cultured AR42J cells (B) and subjected to real-time PCR using rat THTR-1 and THTR-2 gene-specific primers (Table 1). Data are means ± SE of at least 3 separate experiments from 3 different samples and were normalized relative to β-actin. *P < 0.01.
Effect of Chronic Alcohol Feeding/Exposure on Cellular and Molecular Parameters of Thiamin Uptake by Pancreatic Acinar Cells
Studies with primary pancreatic acinar cells isolated from alcohol-fed rats and their pair-fed controls.
EFFECT ON THIAMIN UPTAKE.
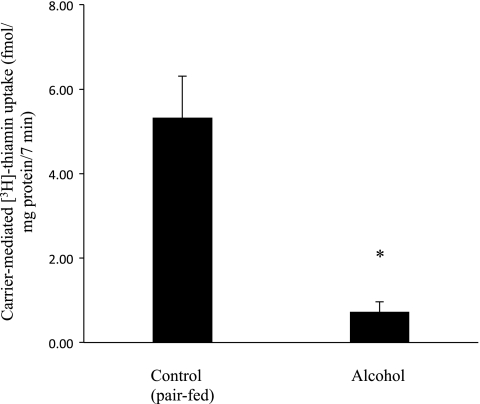
The effect of chronic alcohol feeding of rats (for 4 wk; see methods) on initial rate of carrier-mediated thiamin (15 nM) uptake by freshly isolated pancreatic acinar cells was examined in this study and the results were compared with thiamin uptake by primary pancreatic acinar cells isolated from control rats that were pair fed the same (isocalorically balanced) liquid diet but without ethanol. The results showed a significant (P < 0.01) reduction in carrier-mediated thiamin uptake by pancreatic acinar cells isolated from the alcohol-fed rats compared with uptake by cells isolated from pair-fed control animals (Fig. 4).
Fig. 4.
Effect of chronic alcohol feeding (4 wk) on carrier-mediated [3H]thiamin uptake by rat primary pancreatic acinar cells. Primary pancreatic acinar cells were isolated from rats fed alcohol-liquid diet (4 wk) and from their pair-fed controls. Incubation was done for 7 min in Krebs-Ringer buffer, pH 7.4 at 37°C in the presence of [3H]thiamin (15 nM). Carrier-mediated thiamin uptake was determined as described in methods. Data are means ± SE of at least 3 separate uptake determinations from multiple sets of rats. *P < 0.01.
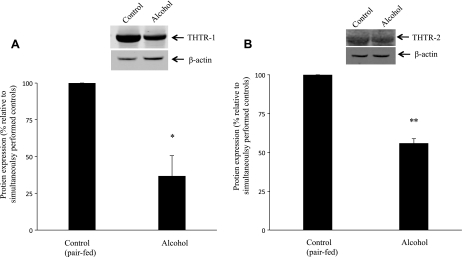
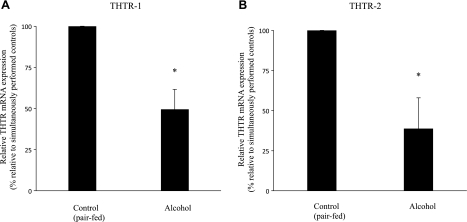
EFFECT ON LEVEL OF EXPRESSION OF THTR-1 AND THTR-2 PROTEIN AND MRNA.
The effect of chronic alcohol feeding on the level of expression of THTR-1 and THTR-2 in primary pancreatic acinar cells at the protein and mRNA levels was examined in this study by means of Western blot analysis and real-time PCR, respectively. Western blot analysis was performed by using specific polyclonal antibodies against THTR-1 and THTR-2 and whole lysate of primary acinar cells isolated from rats chronically fed alcohol liquid-diet and their pair-fed controls (see methods). The results showed that chronic alcohol feeding led to a significant (for THTR-1 and for THTR-2) reduction in the level of expression of the THTR-1 (P < 0.02) and THTR-2 proteins (P < 0.01) (Fig. 5, A and B). Real-time PCR was performed using total RNA from pancreatic acinar cells isolated from alcohol-fed rats and their pair-fed controls. The results again showed a significant (P < 0.05) reduction in the level of expression of the THTR-1 and THTR-2 message upon chronic alcohol feeding (Fig. 6, A and B). These data suggest that chronic alcohol feeding may exert part of its effect at the transcriptional level, and thus we tested this possibility in the next set of experiments.
Fig. 5.
Effect of chronic alcohol feeding on level of expression of THTR-1 and THTR-2 proteins in rat primary pancreatic acinar cells. Western blot analysis was done using pancreatic acinar whole cell proteins (60 μg) isolated from chronic alcohol-fed rats (4 wk) and their pair-fed controls and were resolved on 4–12% SDS-PAGE and electroblotted onto polyvinylidene difluoride membrane as described in methods. A: blots were incubated with rabbit polyclonal THTR-1 antibody. B: blots were incubated with rabbit polyclonal THTR-2 antibody. Data are means ± SE from 3 different sets of samples from multiple rats and were normalized relative to β-actin protein expression. *P < 0.02, **P < 0.01.
Fig. 6.
Effect of chronic alcohol feeding on level of expression of THTR-1 and THTR-2 mRNA in rat primary pancreatic acinar cells. Real-time PCR was performed using rat THTR-1 (A) and THTR-2 (B) gene-specific primers (see Table 1). Data are means ± SE from separate sets of samples from multiple rats and were normalized relative to β-actin and calculated by the relative relationship method supplied by manufacturer (Bio-Rad). *P < 0.05.
Studies with cultured pancreatic acinar AR42J cells chronically exposed to alcohol.
EFFECT ON THIAMIN UPTAKE.
The effect of chronic exposure (96 h) of the cultured AR42J cells to ethanol [50 mM; a concentration that mimics blood alcohol level in chronic alcoholics (8)] on initial rate of carrier-mediated thiamin uptake. Chronic alcohol exposure of cells was performed as described recently by us and others (31, 32, 35). The results showed that chronic alcohol exposure of AR42J cells to lead to a significant (P < 0.01) inhibition in thiamin uptake compared with uptake by control (unexposed) cells (100 ± 3.13 and 61.78 ± 14.86 fmol·mg protein−1·7 min−1 for control cells and those exposed to alcohol, respectively).
EFFECT ON ACTIVITY OF THE SLC19A2 AND SLC19A3 PROMOTERS.
The effect of chronic alcohol exposure of AR42J cells on the activity of full-length SLC19A2 (the gene that encodes the human THTR-1; −2250 to −36 bp) and SLC19A3 (the gene that encodes the human THTR-2; −1957 to +59 bp) promoters was examined. In these investigations, SLC19A2 and SLC19A3 promoters fused to the firefly luciferase reporter gene were transiently transfected into AR42J cells chronically exposed to ethanol followed by determination of luciferase activity as described by us previously (19, 24, 25). The results showed a significant reduction in the activity of the both SLC19A2 (P < 0.01) and SLC19A3 (P < 0.05) in cells chronically exposed to alcohol compared with their activity in control cells (Fig. 7, A and B). These data suggest that effects of chronic alcohol exposure on physiological and molecular parameters of thiamin uptake by pancreatic acinar cells are exerted, at least in part, at the level of transcription.
Fig. 7.
Effect of chronic alcohol exposure of cultured AR42J cells on the activity of SLC19A2 and SLC19A3 promoters. Full-length SLC19A2 and SLC19A3 promoters in pGL3-Basic were transfected into AR42J cells. Cells were then exposed to 50 mM ethanol treatment for 96 h followed by determination of luciferase activity. Data were normalized relative to Renilla luciferase activity and presented as means ± SE of at least 4 independent experiments. *P < 0.01, **P < 0.05.
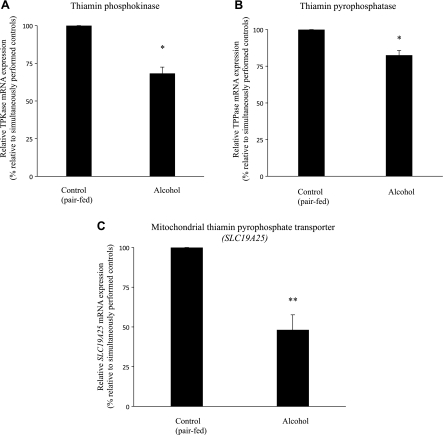
Effect of Chronic Alcohol Feeding of Rats On Intracellular Parameters of Thiamin Metabolism and Compartmentalization in Rat Pancreatic Acinar Cells
In this study, we examined the effect of chronic alcohol feeding of rats (4 wk) on the level of mRNA expression of key thiamin-metabolizing enzymes, TPKase and TPPase, as well as on the level of expression of the mitochondrial TPP transporter. We used real-time PCR and total RNA isolated from fresh primary pancreatic cells isolated from rats fed alcohol chronically and their pair-fed controls. The results showed a significant reduction in the level of expression of TPKase, TPPase (P < 0.05), and the thiamin mitochondrial TPP transporter (P < 0.01) in pancreatic acinar cells of alcohol-fed rats compared with their pair-fed controls (Fig. 8, A, B, and C).
Fig. 8.
Effect of chronic alcohol feeding on level of expression of thiamin pyrophosphokinase (TPKase), thiamin pyrophosphatase (TPPase), and mitochondrial thiamin pyrophosphate (TPP) transporter (SLC19A25) mRNA in rat primary pancreatic acinar cells. Real-time PCR was performed using rat TPKase (A), TPPase (B), and mitochondrial TPP transporter (C) gene-specific primers (see Table 1). Data are means ± SE from 3 separate sets of samples from multiple rats (normalized relative to β-actin) and are calculated by the relative relationship method supplied by the manufacturer (Bio-Rad). *P < 0.05, **P < 0.01.
DISCUSSION
As mentioned earlier, thiamin assumes important roles in pancreatic acinar cells via its involvement in a variety of metabolic reactions including those that lead to a reduction in cellular oxidative stress. At the functional level, thiamin deficiency leads to a severe reduction in pancreatic content of digestive enzymes (29). Our aims in this study were to characterize the mechanism of thiamin uptake by pancreatic acinar cells and to examine the effect of chronic alcohol feeding/exposure on the uptake process. Using both freshly isolated rat primary pancreatic acinar cells and cultured rat-derived pancreatic acinar AR42J cells we show that uptake of the positively charged thiamin is via a specific carrier-mediated process. This conclusion is based on the observations that initial rate of uptake of physiological concentrations of [3H]thiamin by these cells is significantly inhibited by unlabeled thiamin and by thiamin structural analogs but not by the unrelated organic cations TEA and choline. Both of these pancreatic acinar cell types were also found to express the two known high-affinity mammalian thiamin transporters, i.e., THTR-1 and -2, at the mRNA level, with expression of the former being significantly higher than that of the latter. These findings suggest possible involvement of these systems in thiamin uptake by pancreatic acinar cells.
One of the important targets of the deleterious effects of chronic alcohol use in humans is the pancreas. In fact, alcohol abuse is one of the most common causes of pancreatitis. For this reason and because chronic alcohol use is known to interfere with normal thiamin physiology and metabolism in other cellular systems, we examined the effect of chronic alcohol feeding/exposure on physiological and molecular parameters of the pancreatic acinar cell thiamin uptake process. Using an established chronic alcohol feeding regiment of rats, we observed a significant inhibition in initial-rate of carrier-mediated thiamin uptake by pancreatic acinar cells of alcohol-fed animals compared with uptake by acinar cells of their pair-fed controls. This inhibition was associated with a significant reduction in the level of expression of the THTR-1 and THTR-2 protein and mRNA. This effect of alcohol on expression of THTR-1 and THTR-2 is similar to what we have recently observed in the kidneys (30); it is, however, in contrast to what was observed in the intestine where expression of THTR-1 but not THTR-2 was affected by chronic alcohol use (32). These findings demonstrate a differential sensitivity of thiamin transporters to chronic alcohol exposure in different tissues.
The decrease in the level of mRNA expression of THTR-1 and THTR-2 in pancreatic acinar cells of rats fed alcohol chronically compared with their pair-fed controls raises the possibility that the inhibition may be (in part) exerted at the level of transcription of the respective genes. To test this possibility, we used cultured pancreatic acinar AR42J cells exposed to alcohol chronically and examined the effect of such exposure on initial-rate of carrier-mediated thiamin uptake and on activity of the human SLC19A2 and SLC19A3 promoters expressed in these cells. The results showed a significant inhibition in thiamin uptake and in the activity of the SLC19A2 and SLC19A3 promoters in AR42J cells chronically exposed to alcohol compared with unexposed control cells. These findings support the above suggestion that the effect of chronic alcohol exposure on thiamin uptake by pancreatic acinar cells is at least in part being exerted at the level of transcription of the involved transporters.
Since chronic alcohol feeding also affect intracellular thiamin metabolism and that the effect appears to be tissue-specific in nature (27), we also sought to examine the effect of chronic alcohol feeding of rats on level of expression of important indexes involved in the handling of thiamin intracellularly. Thus we examined the effect of chronic alcohol feeding on level of expression of the thiamin-metabolizing enzymes TPKase and TPPase, which play important roles in regulating intracellular thiamin homeostasis. We also examined the effect of chronic alcohol feeding on the level of expression of the mitochondria TPP transporter, a protein that is responsible for the compartmentalization of thiamin in cells [up to 30% of cellular TPP are localized in the mitochondria and mitochondria cannot synthesis TPP from free thiamin, i.e., rely on its import from the cytoplasm (9)]. Our results showed that chronic alcohol feeding leads to a significant inhibition in the level of expression of both TPKase and TPPase as well as in the level of expression of the mitochondrial TPP transporter.
The mechanism through which chronic alcohol feeding/exposure brings about such negative effects on thiamin transport (and metabolism) is not clear, but chronic alcohol exposure is known to lead to an increase in intracellular level of reactive oxygen species that can react with and damage complex cellular molecules like DNA and proteins (7). Similarly, acetaldehyde (a product of ethanol oxidative metabolism) is a very reactive intermediate that can affect gene expression (4). In summary, results of these studies show for the first time that thiamin uptake by rat pancreatic acinar cells is via a specific carrier-mediated process and that both the THTR-1 and THTR-2 are expressed at the mRNA and protein levels in these cells. In addition, chronic alcohol feeding/exposure inhibits thiamin uptake by these cells and the effect is (at least in part) being exerted at the transcriptional level. In addition, chronic alcohol feeding also appears to inhibit the level of expression of important intracellular thiamin metabolizing enzymes as well as the level of expression of the mitochondrial TPP transporter.
GRANTS
This study was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (AA 018071, DK 56061).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Barile M, Passarella S, Quagliariello E. Thiamine pyrophosphate uptake into isolated rat liver mitochondria. Arch Biochem Biophys 280: 352–357, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Barile M, Valenti D, Brizio C, Quagliariello E, Passarella S. Rat liver mitochondria can hydrolyse thiamine pyrophosphate to thiamine monophosphate which can cross the mitochondrial membrane in a carrier-mediated process. FEBS Lett 435: 6–10, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Berdanier CD. Advanced Nutrition: Micronutrients. New York: CRC, 1998 [Google Scholar]
- 4. Brenner DA, Chojkier M. Acetaldehyde increases collagen gene transcription in cultured human fibroblasts. J Biol Chem 262: 17690–17695, 1987 [PubMed] [Google Scholar]
- 5. Calingasan NY, Gandy SE, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol 149: 1063–1071, 1996 [PMC free article] [PubMed] [Google Scholar]
- 6. Capdevila A, Decha-Umphai W, Song KH, Borchardt RT, Wagner C. Pancreatic exocrine secretion is blocked by inhibitors of methylation. Arch Biochem Biophys 345: 47–55, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83: 519–548, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Devenyi P, Kapur BM, Roy JH. High-density lipoprotein response to alcohol consumption and abstinence as an indicator of liver function in alcoholic patients. Can Med Assoc J 130: 1445–1447, 1984 [PMC free article] [PubMed] [Google Scholar]
- 9. Dianzani MU, Dianzani Mor MA. Displacement of thiamine pyrophosphate from swollen mitochondria. Biochim Biophys Acta 24: 564–568, 1957 [DOI] [PubMed] [Google Scholar]
- 10. Frederikse PH, Farnsworth P, Zigler JS., Jr Thiamine deficiency in vivo produces fiber cell degeneration in mouse lenses. Biochem Biophys Res Commun 258: 703–707, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Greenberger NJ, Toskes PP. Acute and chronic pancreatitis. In: Harrison's Principles of Internal Medicine (15th ed.), edited by Kasper DL. New York: McGraw-Hill, 2001, p. 1792–1804 [Google Scholar]
- 12. Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest 100: 1853–1862, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek DE, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 15. Lindhurst MJ, Fiermonte G, Song S, Struys E, De Leonardis F, Schwartzberg PL, Chen A, Castegna A, Verhoeven N, Mathews CK, Palmieri F, Biesecker LG. Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc Natl Acad Sci USA 103: 15927–15932, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol 100: 1200–1208, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 282: G501–G507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim Biophys Acta 1777: 564–578, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, Solomon TE, Gukovskaya AS, Tsukamoto H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 117: 706–716, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Ponnappa BC, Marciniak R, Schneider T, Hoek JB, Rubin E. Ethanol consumption and susceptibility of the pancreas to cerulein-induced pancreatitis. Pancreas 14: 150–157, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Prasannan KG, Sundaresan R, Venkatesan D. Thiamine deficiency and protein secretion by pancreatic slices in vitro. Experientia 33: 169–170, 1977 [DOI] [PubMed] [Google Scholar]
- 24. Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim Biophys Acta 1561: 180–187, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Rindi G, Patrini C. Thiamin. In: Encyclopedia of Dietary Supplements, edited by Coates PM. New York: Dekker, 2005, p. 677–686. [Google Scholar]
- 27. Rindi G, Reggiani C, Patrini C, Laforenza U. Effect of ethanol administration on the in vivo kinetics of thiamine phosphorylation and dephosphorylation in different organs. I. Chronic effects. Alcohol Alcohol 26: 285–301, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Said HM, Mee L, Sekar VT, Ashokkumar B, Pandol SJ. Mechanism and regulation of folate uptake by pancreatic acinar cells: effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol 298: G985–G993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh M. Effect of thiamin deficiency on pancreatic acinar cell function. Am J Clin Nutr 36: 500–504, 1982 [DOI] [PubMed] [Google Scholar]
- 30. Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am J Physiol Renal Physiol 299: F28–F34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Subramanya SB, Subramanian VS, Kumar JS, Hoiness R, Said HM. Inhibition of intestinal biotin absorption by chronic alcohol feeding: cellular and molecular mechanisms. Am J Physiol Gastrointest Liver Physiol 300: G494–G501, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299: G23–G31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanphaichirt V. Thiamin. In: Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, Shike M. New York: Lea and Febiger, 1994, p. 359–375. [Google Scholar]
- 34. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: Davis, 1989 [Google Scholar]
- 35. You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008 [DOI] [PubMed] [Google Scholar]