Abstract
Mutations in the MEN1 gene correlate with multiple endocrine neoplasia I (MEN1). Gastrinomas are the most malignant of the neuroendocrine tumors associated with MEN1. Because menin and JunD proteins interact, we examined whether JunD binds to and regulates the gastrin gene promoter. Both menin and JunD are ubiquitous nuclear proteins that we showed colocalize in the gastrin-expressing G cells of the mouse antrum. Transfection with a JunD expression vector alone induced endogenous gastrin mRNA in AGS human gastric cells, and the induction was blocked by menin overexpression. We mapped repression by menin to both a nonconsensus AP-1 site and proximal GC-rich elements within the human gastrin promoter. Chromatin immunoprecipitation assays, EMSAs, and DNA affinity precipitation assays documented that JunD and Sp1 proteins bind these two elements and are both targets for menin regulation. Consistent with menin forming a complex with histone deacetylases, we found that repression of gastrin gene expression by menin was reversed by trichostatin A. In conclusion, proximal DNA elements within the human gastrin gene promoter mediate interactions between JunD, which induces gastrin gene expression and menin, which suppresses JunD-mediated activation.
Keywords: Sp1, AP-1, octreotide, trichostatin A, histone deacetylase inhibitor
menin is the 67-kda protein product of the MEN1 gene with germline mutations responsible for an autosomal-dominant cancer syndrome in which gastrinomas develop and result in hypergastrinemia (5, 11). The exact function of menin has yet to be fully elucidated although a large number of somatic and germline mutations within the gene have been identified that presumably inactivate the protein (3, 27). Menin interacts directly with a number of transcription factors such as JunD, Smad3, and NF-κB (12). Menin has also been shown to exist in a histone methyltransferase complex with the mixed-lineage leukemia protein (43, 46) or with histone deacetylases (HDACs) on the cyclin B2 promoter (42). In the endocrine pancreas, menin promotes methylation of histone H3 at lysine 4, which in turn stimulates the transcription of cyclin-dependent kinase inhibitors p27 and p18 (22).
JunD is a member of the activator protein (AP)-1 transcription factor complex whose components are comprised of Jun and Fos gene family members that mediate the nuclear response to several extracellular stimuli, including growth factors (35). JunD is expressed in all cell types (18) and is a unique member of the Jun protein family because it mediates both positive and negative effects on signaling events depending on the cellular and genetic context (41). Menin physically interacts and represses JunD-dependent transcription (1, 15). Furthermore, it was suggested that histone deacetylase activity might be implicated in the repression of JunD-activated transcription by menin (15, 24).
Gastrin is the only hormone that stimulates gastric acid secretion, and thus hypergastrinemia contributes to peptic ulcer diseases (31). In addition, gastrin has trophic effects on the gastric corpus, implicating the peptide in cancer pathogenesis (31, 39). The regulatory pathways inhibiting gastrin gene transcription and secretion remain to be fully elucidated. Our prior study showed that somatostatin, the major paracrine inhibitor of gastrin gene expression (9), stimulates an increase in menin mRNA and protein (29). In addition menin, which colocalizes with gastrin in antral G cells, inhibits gastrin expression, whereas reduced menin levels increase gastrin gene expression and peptide (29). These results show that menin modulates the gastrin gene promoter. However, because it typically regulates transcription of target genes via cooperation with transcriptional factors, we hypothesized that menin regulates gastrin through a bona fide DNA-binding protein, e.g., JunD. Therefore, we tested whether JunD directly binds the gastrin promoter and provides a possible mechanism by which menin modulates gastrin gene expression.
MATERIALS AND METHODS
Cell culture, plasmid construction, and transfections.
AGS cells (ATCC, Manassas, VA) were routinely cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (GIBCO/Invitrogen, Carlsbad, CA). At 60–80% confluence, the AGS cells were transiently transfected with three gastrin-luciferase (GasLuc) reporter constructs containing, 0.24, 3.3, or 9.8 kb of the human gastrin promoter (29, 36). Mutation in the 0.240 GasLuc within Sp1 (ΔSp1) or gastrin epidermal growth factor response element (gERE) (ΔgERE) were described previously (36). Site-directed mutations within the TGAC site (ΔTGAC TGACTGACTGAC=>TGATTAATTAAC) were introduced into the 0.240-kb construct using the QuickChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). All constructs were confirmed by sequence analysis. Several rounds of single-mutation reactions were performed to generate a construct with a combination of TGAC, Sp1, and gERE mutations (Δcombo). The GasLuc constructs were transfected with either pCMVJunD (ATCC) or pCMVmenin (gift from S. Chandrasekharappa) expression vectors using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The total amount of DNA was normalized to pcDNA3.1(+) vector (Invitrogen). The cells were assayed for firefly luciferase activity using the Dual-Luciferase Assay System (Promega, Madison, WI) and then normalized to Renilla luciferase (Promega). Where indicated, the cells were treated after plasmid transfection with 10 nM of trichostatin A (Sigma-Aldrich, St. Louis, MO) for 28 h.
AGS cells were plated (200,000 cells/ml) onto six-well plates in complete DMEM for 24 h, and then the media was replaced with serum-free Opti-MEM (Invitrogen) for 1 h. Duplex small-interfering RNAs (10 nM siRNA; Santa Cruz Biotechnology, Santa Cruz, CA) against menin (sc-35922) or JunD (sc-35728) were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were harvested for RNA and protein analysis 72 h after transfection. In separate experiments, AGS cells were plated onto six-well plates and transfected for 48 h with indicated plasmids. The total amount of DNA per well (2 μg) was normalized with pcDNA3.1(+). Where indicated, the cells were treated with octreotide (Sigma) for 48 h.
EMSA.
Nuclear extracts from AGS cells were prepared using detergent extraction (34). Oligo probes containing the nonconsensus AP-1 (TGAC) binding site at −163 (base pairs upstream of the gastrin gene transcriptional start site) 5′CTGGATGACTGACTGACACTAAATG3′, the Sp1 site at −140 5′GGATCCGGGCGGGGCAGGGAGATCT3′, and gERE at −68 5′GGATCCGGGGCGGGGTGGGGGGAGATCT3′ were flanked by 5′ BamHI and 3′Bgl II isochizomer restriction sites. The sense and antisense strands were hybridized then labeled with [32P]γ-ATP using polynucleotide kinase. One microliter of labeled probe (30,000 cpm/μl) was added to each reaction. The EMSAs were performed using ∼8 μg of AGS nuclear extracts in a final volume of 20 μl containing 10 mM Tris·HCl, 1 mM EDTA, 1 mM DTT, 5 mM MgCl2, 1 mM ZnCl2, 150 mM KCl, 10% glycerol, and 300 ng poly (dI-dC). Antibodies for Sp1 (sc-14027), Sp3 (sc-644), and JunD (sc-74) were purchased from Santa Cruz Biotechnology and for menin (A300–105A) from Bethyl Laboratories (Montgomery, TX). DNA-protein complexes were resolved on a 4% nondenaturing polyacrylamide gel containing 45 mM Tris base, 45 mM boric acid, and 1 mM EDTA after prerunning the gel for at least 2 h.
DNA affinity precipitation assay (DAPA).
The same elements used in the EMSAs were biotinylated and then incubated with nuclear extracts from AGS cells for 30 min as previously described (6, 7). The complex was subsequently incubated with streptavidin-agarose (Invitrogen) beads for 2 h, washed, then resolved on a 4–20% gradient SDS-PAGE gel for immunoblot analysis.
Western blots.
AGS cells were washed with PBS and then collected in RIPA lysis buffer (Sigma), containing the complete protease inhibitor cocktail (Roche, Indianapolis, IN). After homogenization, the samples were centrifuged at 12,000 g for 12 min, and the supernatants were collected. Total protein concentration was measured using the Bradford colorimetric assay Protein Assay Kit (Thermo Scientific, Waltham, MA). One hundred micrograms of protein were boiled for 10 min in Laemmli sample buffer containing β-mercaptoethanol and then resolved on 4–20% gradient-SDS-PAGE gels (Invitrogen). For immunoblotting, the protein cell extracts were electrotransferred onto a nitrocellulose membrane, rinsed in TBS, and then blocked in 5% nonfat milk and 1× TBS with 0.1% Triton-X for 1 h at room temperature, followed by an overnight incubation with the following primary antibodies: a 1:1,000 dilution of the rabbit polyclonal anti-menin antibody (Bethyl Laboratories), a 1:200 dilution of the rabbit polyclonal anti-JunD antibody, a 1:200 dilution of the rabbit polyclonal anti-Sp1 antibody, a 1:200 dilution of the goat polyclonal anti-LaminB antibody (all from Santa Cruz Biotechnology), or a 1-h incubation with a 1:2,000 dilution of the mouse monoclonal anti-GAPDH (Millipore, Temecula, CA) antibody. The horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution, Santa Cruz Biotechnology) and the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) were used to identify the protein bands.
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation assay (ChIP) kit from Millipore was used according to the manufacturer's instructions. Briefly, 1 × 106 AGS cells were treated with 1% (vol/vol) formaldehyde for 10 min at room temperature to cross-link DNA with the associated proteins. Cross-linking was stopped with glycine, and cells were harvested, lysed, and then sonicated. Lysates were precleared with the provided Protein A Agarose/Salmon Sperm DNA (50% slurry), and 1% aliquots were collected to use as “input” DNA. The precleared chromatin samples were then incubated overnight with the following antibodies: 4 μg of rabbit polyclonal anti-menin antibody (Bethyl Laboratories), rabbit polyclonal anti-JunD antibody (Santa Cruz Biotechnology), rabbit polyclonal anti-Sp1 antibody (Santa Cruz Biotechnology), or normal rabbit IgG (Santa Cruz Biotechnology) at 4°C. The antibody-chromatin complex was mixed with Protein A Agarose/Salmon Sperm DNA (50% slurry) for 1 h and then centrifuged. The precipitated immune complexes were then washed, and the cross-linked protein-genomic DNA was eluted with freshly prepared elution buffer (1% SDS, 0.1 M NaHCO3). The cross-links were reversed with an overnight incubation at 65°C followed by treatment with proteinase K for 1 h at 45°C. The DNA released from the precipitated complex was purified by QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and then submitted for PCR analysis using primers: forward 5′GCTCCAGCCCCTCACCATGAAG3′; reverse 5′TTGATGCTCCAGGCCTGCCTTA3′ to amplify the human gastrin gene sequence between −218 and −2 bp upstream of the transcriptional start site.
Immunoprecipitation.
AGS cells were transfected with the menin expression vector for 48 h. Nuclear extracts (500 μg) were precleared with 20 μl of Protein A-Agarose (Santa Cruz Biotechnology) for 30 min, then incubated with 2 μg of rabbit IgG or rabbit polyclonal anti-JunD antibody (Santa Cruz Biotechnology) for 1 h, and then incubated on a rocker platform overnight at 4°C after the addition of 20 μl of Protein A-Agarose. The immunoprecipitates were pelleted and washed twice with RIPA buffer and twice with PBS. The proteins were released from beads by being boiled in Laemmli Sample Buffer for 3 min. The agarose beads were pelleted, and the supernatant was analyzed by SDS-PAGE.
Immunofluorescent staining.
Longitudinal sections of the stomach were fixed, embedded in paraffin, and prepared in 5-μm sections. After being blocked with 20% normal donkey serum, slides were incubated with goat anti-gastrin (1:200, Santa Cruz Biotechnology) antibody with sequential incubation with rabbit anti-menin (1:2,000, Bethyl Laboratories) or rabbit anti-JunD (1:200, Santa Cruz Biotechnology) antibody. FITC-conjugated anti-goat secondary antibody or TSA Plus Cy3 System (Perkin Elmer, Waltham, MA) was used to visualize the signal. The sections were then counterstained with DAPI.
qRT-PCR analysis.
Cells were harvested using TRIzol reagent (Invitrogen). After DNase digestion, RNA cleanup was performed with the RNeasy Mini Kit (Qiagen). RNA (0.5 μg) was reverse transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. PCR amplifications were performed using C1000 Thermal Cycler (Bio-Rad) with SYBR Green dye (Molecular Probes, Carlsbad, CA) and Platinum Taq DNA polymerase (Invitrogen). Each reaction was performed three times in triplicate with the following conditions: 3 min at 95°C, 40 cycles of 9 s at 95°C, and 1 min at 60°C, followed by 1 min at 55°C. Melt curve analysis was used to estimate the purity of the product. Gastrin mRNA expression levels were normalized to the expression of β-actin mRNA. The primer sequences for the human gastrin were as follows: 5′GCCCAGCCTCTCATCATC3′ (forward) and 5′GCCGAAGTCCATCCATCC3′ (reverse).
Statistical analysis.
Data are expressed as the means ± SE for at least three independent experiments. A P value <0.05 using t-test (GraphPad Prism5; GraphPad Software, San Diego, CA) was considered significant.
RESULTS
Menin colocalizes with JunD.
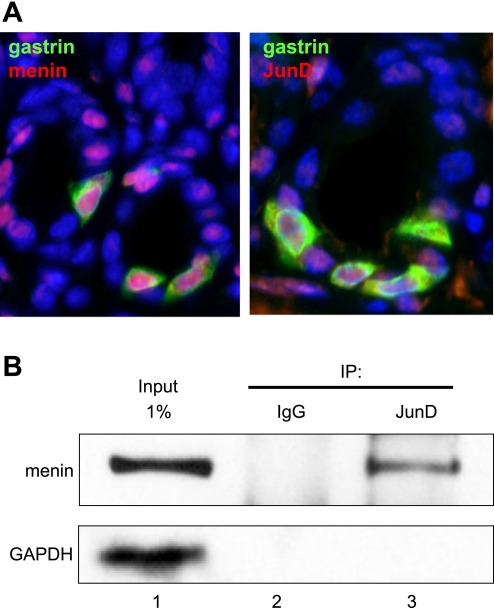
In the antrum, menin is a nuclear protein that is generally expressed ubiquitously and colocalizes with gastrin-expressing endocrine cells called G cells (29). To modulate transcription, menin is known to interact directly with the Jun protein family member JunD (4). JunD acts as either an activator or repressor depending on its cellular context, protein partner, and gene target (41). Therefore, to demonstrate the presence of menin and JunD in G cells, we performed double immunofluorescent staining to colocalize the proteins to G cells in the mouse antrum (Fig. 1A). In addition, we used coimmunoprecipitation of menin with JunD antibody to demonstrate that the two proteins interact in the gastrin-expressing human AGS cell line (Fig. 1B).
Fig. 1.
Menin and JunD colocalize with gastrin in the stomach antrum. A: representative images of immunofluorescent staining in the wild-type mouse gastric antrum. Gastrin-producing G cells (green) expressed menin (red, left) and JunD (red, right). Nuclei were stained with DAPI (blue). Original magnification ×600. B: AGS cells were transfected with menin expression vector for 48 h; 500 μg of nuclear extracts were immunoprecipitated (IP) with either rabbit IgG or rabbit anti-JunD antibody. The immunoprecipitates were analyzed by Western blotting using anti-menin and anti-GAPDH antibodies.
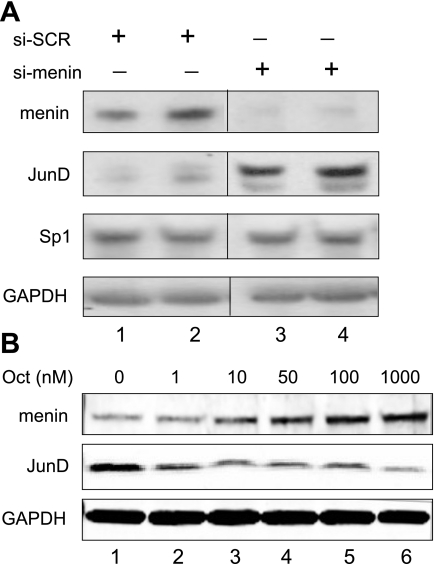
Reduced menin levels increase JunD.
To determine whether modulating menin affected JunD protein levels, we examined the baseline expression of both proteins in the AGS cells, which express gastrin endogenously. We used siRNA oligonucleotides to reduce menin protein levels and then determined JunD levels by immunoblot analysis. Interestingly, JunD protein levels increased with the decrease in menin protein levels (Fig. 2A). In contrast, there were no changes in the protein levels of the transcription factor Sp1. We previously reported that the somatostatin analog octreotide stimulates menin protein expression in AGS cells (29). Therefore, we used increasing concentrations of this extracellular regulator to induce menin protein levels in AGS cells and found that JunD levels decreased (Fig. 2B). Thus, despite their known ability to interact with each other (15), menin and JunD protein levels varied inversely in these cells. Collectively, these results suggested that somatostatin signaling results in an increase in menin that correlates with a decrease in JunD protein levels. Because both octreotide and menin inhibit gastrin gene expression, we queried whether JunD might induce gastrin gene expression.
Fig. 2.
Reduced menin levels increase JunD protein levels. A: AGS cells were treated with 10 nM of scrambled (si-SCR, lanes 1 and 2) or menin (si-menin, lanes 3 and 4) siRNA. Whole cell extracts were analyzed by immunoblot. B: AGS cells were treated with increasing amounts of Octreotide (Oct) for 48 h, and whole cell extracts were analyzed by immunoblot analysis.
JunD induces gastrin gene expression.
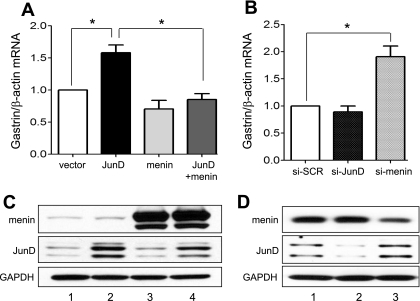
To test the effect of menin and JunD on endogenous gastrin gene expression, AGS cells were transfected with expression vectors for these proteins, and gastrin mRNA was measured by qRT-PCR (Fig. 3A). JunD induced gastrin gene expression. Overexpression of menin had no significant effect on basal levels of gastrin mRNA. However, menin prevented JunD induction. By contrast, reducing JunD with siRNA had little effect on basal levels of gastrin gene expression, suggesting that JunD alone was not required to maintain basal levels of gastrin mRNA (Fig. 3B). However, reduced menin levels increased basal levels of gastrin about twofold, indicating that menin protein contributed to the low basal levels of gastrin gene expression. We used Western blots to confirm the expected increase or decrease in expressed protein levels (Fig. 3, C and D).
Fig. 3.
JunD and menin regulate gastrin gene expression in vitro. Shown are changes in gastrin mRNA expression determined by qRT-PCR. A: AGS cells were transfected for 48 h with the empty vector [pcDNA 3.1(+)], JunD, and menin expression vectors as indicated. *P < 0.05. B: transient transfection of AGS cells for 72 h using scrambled (si-SCR) vs. menin (si-menin) or JunD (si-JunD) siRNA. *P < 0.05. C: Western blot analysis of menin and JunD levels in the AGS cells after transfection for 48 h with either vector (lane 1), JunD (lane 2), menin (lane 3), or JunD and menin together (lane 4). D: AGS cells were transfected for 72 h with either scrambled (lane 1), JunD (lane 2), or menin (lane 3) siRNA, and corresponding cell lysates were used for Western blot analysis. GAPDH is shown as a loading control.
JunD induction maps to the proximal gastrin gene promoter.
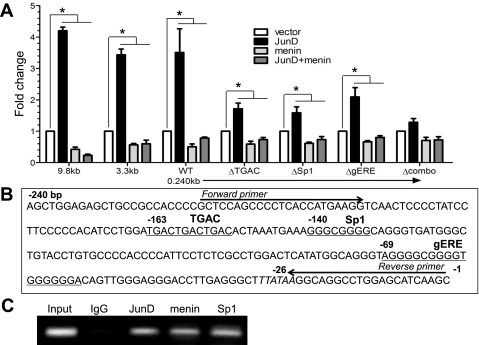
We next used gastrin reporter constructs to map the region required for JunD and menin regulation. Transfecting the 9.8-kb, 3.3-kb, and 0.24-kb gastrin luciferase constructs into AGS cells revealed that JunD alone induces gastrin reporter gene expression, whereas menin alone or when cotransfected with JunD significantly suppressed gastrin reporter gene activity. This regulation was observed in all three constructs, suggesting that the regulation mapped to the proximal 240 bp of the gastrin gene promoter (Fig. 4A). The results are consistent with the fact that menin is more effective at blocking JunD-mediated induction than suppressing basal gastrin levels (Fig. 3A). Jun proteins comprise the AP-1 transcription factor regulatory complex, which typically recognizes the consensus sequence TGACTCA (17, 30, 32). In silico analysis of the proximal human gastrin gene promoter revealed a number of nonconsensus AP-1 binding sites, one of which resided within the first 240 bp of the promoter at −163 (TGACTGACTGAC) (Fig. 4B). In addition, because Jun family members can mediate promoter regulation by forming complexes with Sp1 (13, 21, 25, 40, 44), we considered that Sp1 DNA elements also present in the proximal gastrin gene promoter might cooperate with JunD to activate the promoter. Thus, on the basis of the regulatory studies, we focused on the first 240 bp of the gastrin gene promoter and DNA binding assays.
Fig. 4.
JunD activates the 9.8-, 3.2-, and 0.240-kb gastrin gene reporter constructs. A: 9.8-, 3.2-, and 0.240-kb GasLuc constructs or 0.240-kb GasLuc construct with mutations in the TGAC (ΔTGAC), Sp1 (ΔSp1), gastrin epidermal growth factor response element (gERE) (ΔgERE), or all three sites (Δcombo) were cotransfected into the AGS cell line with pcDNA (set to 1), JunD alone, menin alone, or with JunD and menin expression vectors. *P < 0.05. B: proximal promoter sequence of the human gastrin gene is shown. TGAC, Sp1, and gERE binding sites are underlined. C: chromatin immunoprecipitation assay analysis of the immunoprecipitated chromatin with either rabbit IgG, anti-JunD, anti-menin, or anti-Sp1 antibodies.
To determine whether the nonconsensus AP-1 site (TGAC) and/or the putative Sp1 sites (Sp1 and gERE) mediate JunD and menin regulation, mutations were introduced into each site alone or in combination. The single mutations were sufficient to reduce JunD induction of the gastrin gene promoter. However, complete abrogation of JunD induction required mutations in all three sites (Fig. 4A). The mutations did not dramatically affect the ability of menin to inhibit the promoter and block residual JunD induction (Fig. 4A). The latter result suggests that menin works through protein-protein and chromatin interactions as opposed to a specific DNA sequence. We used ChIP assays to demonstrate that JunD, menin, and Sp1 proteins are present in the protein complexes binding to the proximal 220 bp of the gastrin gene promoter (Fig. 4C).
Menin and JunD bind proximal gastrin gene DNA elements.
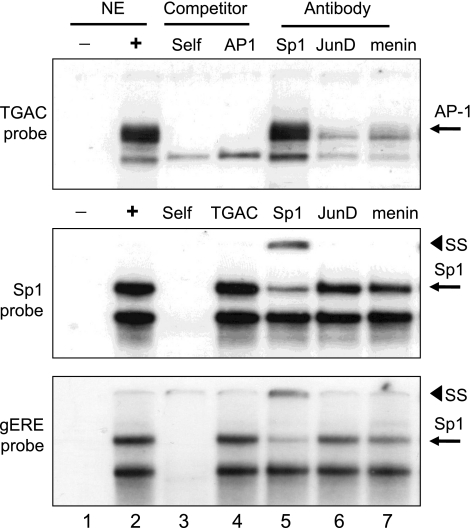
We used EMSAs to identify the protein complexes that recognized the proximal gastrin gene promoter elements (Fig. 5). A protein complex bound to the TGAC probe that was competed by both the unlabeled TGAC probe and a canonical AP-1 element. Both JunD and menin but not Sp1 antibody disrupted the complex binding to the TGAC sequence. Nuclear proteins recognized both GC-rich Sp1 and gERE elements and were competed with unlabeled probe sequence but not by the TGAC element. Sp1 antibodies supershifted a portion of the upper complex. JunD and menin antibodies did not disrupt the complexes. However, there was a slight reduction in the complex binding to these two GC-rich elements with both JunD and menin antibodies, suggesting the presence of these proteins at the GC-rich sites. Therefore, JunD recognized the TGAC element and to a lesser extent the two Sp1 elements. Menin antibody disrupted the complex at the TGAC site to a greater extent than the one at the two Sp1 sites, suggesting that menin interacts directly with JunD.
Fig. 5.
EMSA analysis of proteins binding to proximal gastrin gene promoter elements. Shown are EMSAs using AGS nuclear extracts with either TGAC, Sp1, or gERE probe from the human gastrin gene promoter. NE, nuclear extract; SS, supershift; AP, activator protein.
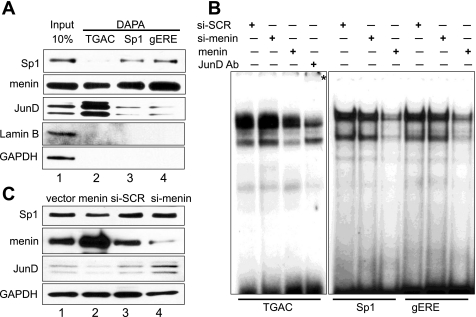
To detect the proteins at these three sites directly, we used DAPA, which uses Western blots to visualize the proteins pulled down by a biotinylated probe (Fig. 6A). As determined by the EMSAs, both JunD and menin bind the TGAC probe compared with Sp1. However, a small amount of JunD was detected at the Sp1 sites with about equal affinity, whereas menin was detected at all sites examined. To study the effect of menin overexpression or underexpression, we transfected the cells with either a menin expression vector or menin siRNA oligos. Indeed, the effect of menin levels on the TGAC probe was more dramatic than on the Sp1 probes. Reduced menin levels resulted in an increase in JunD binding as confirmed by the supershifted JunD, whereas an increase in menin protein reduced JunD binding (Fig. 6B). By contrast, an increase in menin protein reduced both JunD and Sp1 binding but had no effect when menin levels were reduced (Fig. 6B). Because reduced menin increased JunD but not Sp1 protein levels (Fig. 2A), we concluded that the changes at the TGAC site might represent changes in JunD protein levels rather than binding. We confirmed that the menin expression vector and siRNA oligos used in Fig. 6B increased or decreased menin levels, respectively (Fig. 6C). Collectively, the results are consistent with reduced menin levels, creating a permissive environment for gastrin gene promoter induction through AP-1 and Sp1 sites, in which more JunD is available to bind, whereas Sp1 binding to its respective sites remains stable.
Fig. 6.
Menin is present and decreases complex formation at the TGAC, Sp1, and gERE site from proximal gastrin gene promoter. A: TGAC, Sp1, and gERE probes were biotinylated and then incubated with nuclear extracts from AGS cells for DNA affinity precipitation assay (DAPA). A nuclear protein, LaminB, and GAPDH were used as the negative controls. The input shows proteins, which are present in the AGS nuclear extracts. DAPA detected proteins, which bind to TGAC, Sp1, and gERE sites. B: EMSA using AGS cells transfected with either scrambled siRNA (si-SCR) as a control, siRNA against menin (si-menin), or menin expression vector (menin). After 48 h, the corresponding nuclear extracts were isolated and incubated with TGAC, Sp1, or gERE probes. The asterisk (*) indicates a shifted complex. C: representative immunoblot for menin, showing that overexpression of menin resulted in increased menin protein level and transfection with menin siRNA-reduced menin protein level.
Trichostatin A reverses menin repressor activity of gastrin gene promoter.
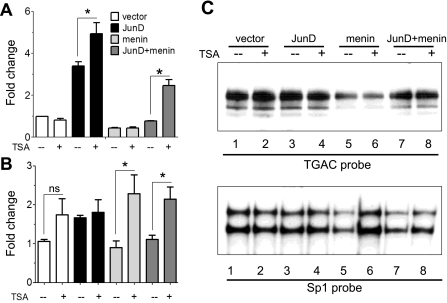
It has been reported that HDAC inhibitors, e.g., trichostatin A (TSA), reverse the inhibitory effect of menin on JunD-mediated transcriptional activity (15). We therefore examined whether TSA reversed the inhibitory effect of menin on gastrin gene promoter activity and endogenous gastrin mRNA (Fig. 7, A and B). Although TSA alone did not affect gastrin reporter activity (Fig. 7A), we observed an increase in endogenous gastrin mRNA (Fig. 7B), reflecting the known effect of histone deacetylase inhibition by TSA on gene expression through chromatin modification (14, 37). JunD induced both gastrin gene promoter activity and endogenous gene expression, with some potentiation of the activation with the transfected reporter (Fig. 7, A and B). However, TSA reversed the inhibitory effect of menin on endogenous gastrin gene expression (Fig. 7B) but not on the transfected gastrin reporter plasmid (Fig. 7A), reinforcing the notion that TSA is most effective in the presence of chromatin as opposed to plasmid DNA (38). Nevertheless, TSA blocked the inhibitory effect of menin on JunD because induction of gastrin was observed with both menin and JunD only with TSA (Fig. 7, A and B). Because the effect was observed with both the transfected reporter and endogenous gastrin gene, the results suggest either a direct or indirect effect of TSA on the JunD transcription factor.
Fig. 7.
Trichostatin A (TSA) reversed the inhibitory effect of menin and JunD on the gastrin gene promoter. A: 0.240 GasLuc construct was cotransfected with either an empty vector, JunD alone, menin alone, or with JunD and menin expression vector and then treated with TSA. *P < 0.05. B: shown are changes in gastrin mRNA expression determined by qRT-PCR. AGS cells were transfected with empty vector, with menin without and with JunD expression vector for 48 h and then treated with TSA. *P < 0.05. ns, not statistically significant. C: EMSAs with TGAC and Sp1 probe and nuclear extracts from AGS cells. The cells were transfected with empty vector, with menin without and with JunD expression vector for 48 h and then treated with TSA.
Because an increase in JunD protein levels induced gastrin gene expression, we examined the effect of these complexes on protein binding to DNA (Fig. 7C). At the TGAC site, protein binding was minimally affected, and JunD binding to this element overall was reduced, most likely attributable to reduced JunD protein levels, as shown in Fig. 6C. By contrast, TSA treatment restored complex binding at the Sp1 element that was normally reduced by menin overexpression (Fig. 7C, bottom, lanes 5, 6). Overall, TSA altered complex binding on the Sp1 probe more dramatically than on the TGAC probe, which likely reflects the known interaction of Sp1 with chromatin-modifying proteins such as HDAC1 (20) and the formation of repressive chromatin structures at this GC-rich site (19).
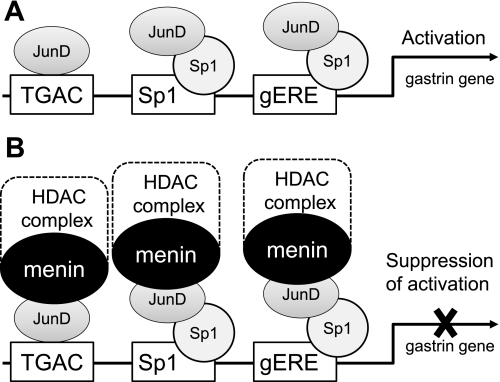
Finally, we propose the following mechanism for menin and JunD regulation of gastrin gene expression through proximal DNA elements (Fig. 8). We found that overexpression of JunD induces gastrin gene expression (Fig. 8A); overexpression of menin blocks this induction (Fig. 8B). We found that the proximal 240 bp of the gastrin gene promoter is responsible for the regulation. The TGAC, Sp1, and gERE elements appeared to mediate this regulation. The ability of menin to repress JunD-mediated activation was reversed by TSA, an HDAC inhibitor most effectively on the endogenous gene, which is likely attributable to an effect on chromatin (Fig. 8B). In conclusion, proximal DNA elements within the human gastrin gene promoter mediate interactions between JunD, which induces gastrin gene expression and menin, which suppresses JunD-mediated activation.
Fig. 8.
Proposed model for menin and JunD regulation of the gastrin gene expression through proximal DNA elements. A: JunD binds the TGAC (noncanonical AP-1) element as well as Sp1 and gERE binding sites (via cooperation with Sp1) in the proximal gastrin gene promoter. JunD activates the gastrin gene promoter and induces basal levels of gastrin mRNA expression. B: Menin, possibly as part of the histone deacetylase (HDAC) complex, suppresses JunD-mediated gastrin gene activation. Rectangles (TGAC, Sp1 and gERE) show DNA-binding sites within the proximal gastrin promoter. Ovals indicate transcriptional factors.
DISCUSSION
Menin is known to partner with JunD to regulate the transcription of target genes (1, 15). We observed that both of these nuclear proteins are expressed in antral G cells and form a protein-protein complex. We have previously reported that menin is an inhibitor of gastrin (29). Here we showed that there is an inverse relationship between menin and JunD protein levels in the AGS cells, which occurred despite the fact that the two proteins coprecipitate together. Moreover, JunD overexpression induced gastrin mRNA levels, and menin overexpression blocked JunD-mediated activation. Collectively our study demonstrates that modulating menin levels is sufficient to regulate gastrin gene expression. Using gastrin gene promoter deletion constructs, we mapped regions responsible for JunD activation to the proximal promoter. In silico analysis of the proximal 240 bp of the promoter revealed a nonconsensus AP-1 element that we confirmed binds JunD. In vitro DNA-binding assays revealed that menin recognizes both the AP-1 and Sp1 sites, whereas JunD prefers to bind to the nonconsensus AP-1 site (TGACTGAC). In addition, we found that JunD also sits on the Sp1 sites, albeit with significantly lower affinity. This result is likely due to Sp1 and JunD protein:protein interactions. Elevated menin levels decreased complex formation at all three of the DNA elements tested, consistent with the ability of menin to modulate transcription through multiple sites presumably through both sequence-specific DNA-binding protein as well as chromatin remodeling (16, 45).
Despite the presumed importance of menin in the generation of gastrinomas, there is essentially no information on how menin might regulate gastrin gene expression. Thus the studies here demonstrate that menin exerts its regulatory effect on the human gastrin gene through several elements in the proximal promoter. Although several DNA-binding proteins interact with menin, e.g., JunD, SMAD, and NF-κB, none have been shown to bind the human gastrin promoter. However, AP-1 and SMAD3/4 factors have been shown to recognize sequences at the mouse gastrin gene promoter (10, 23, 28). Kidd et al. (23) identified changes in menin and JunD expression in carcinoid type gastrin-mediated tumors of the rodent Mastomys (23), underscoring the potential relevance of analyzing JunD regulation of the human gastrin gene.
Menin has the ability to recruit transcription factors (12), and its interactions with HDAC proteins might be a mechanism by which these complexes regulate transcription of target genes. Consistent with this hypothesis, we demonstrated that the HDAC inhibitor TSA alleviates the inhibitory effect of menin on the gastrin gene promoter. Moreover, JunD induction of the gastrin gene promoter in the presence of menin correlated with restoration of Sp1 factor binding. We speculate that TSA inhibition of menin function might be related to inhibition of HDACs and perhaps other corepressors such as Sin3A. A prior study has indicated that menin is a component of the HDAC-Sin3A corepressor complex (24).
In summary, we show that JunD and menin regulate gastrin gene expression via proximal DNA elements that include two Sp1 binding sites and a nonconsensus AP-1 site. It is known that gastrinomas can be detected using octreotide scans because they overexpress somatostatin receptors (8, 26). Thus understanding how octreotide regulates menin protein expression and subsequently the gastrin gene promoter will facilitate the refinement of this molecule in diagnosis and therapies.
GRANTS
This work was supported by NIH grant R37-DK45729 to J. Merchant, and the use of core facilities was supported by the University of Michigan Digestive Disease Research Center (Peptide Center) P30 DK34933.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, Burns AL. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 96: 143–152, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal SK, Impey S, McWeeney S, Scacheri PC, Collins FS, Goodman RH, Spiegel AM, Marx SJ. Distribution of menin-occupied regions in chromatin specifies a broad role of menin in transcriptional regulation. Neoplasia 9: 101–107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal SK, Kennedy PA, Scacheri PC, Novotny EA, Hickman AB, Cerrato A, Rice TS, Moore JB, Rao S, Ji Y, Mateo C, Libutti SK, Oliver B, Chandrasekharappa SC, Burns AL, Collins FS, Spiegel AM, Marx SJ. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res 37: 369–374, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci USA 100: 10770–10775, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anlauf M, Perren A, Meyer CL, Schmid S, Saremaslani P, Kruse ML, Weihe E, Komminoth P, Heitz PU, Kloppel G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 128: 1187–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bai L, Merchant JL. Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J Biol Chem 275: 30725–30733, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Billon N, Carlisi D, Datto MB, van Grunsven LA, Watt A, Wang XF, Rudkin BB. Cooperation of Sp1 and p300 in the induction of the CDK inhibitor p21WAF1/CIP1 during NGF-mediated neuronal differentiation. Oncogene 18: 2872–2882, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA, Paganelli G. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest 32: 360–369, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem 263: 5341–5347, 1988 [PubMed] [Google Scholar]
- 10. Chakladar A, Dubeykovskiy A, Wojtukiewicz LJ, Pratap J, Lei S, Wang TC. Synergistic activation of the murine gastrin promoter by oncogenic Ras and beta-catenin involves SMAD recruitment. Biochem Biophys Res Commun 336: 190–196, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276: 404–407, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Chandrasekharappa SC, Teh BT. Functional studies of the MEN1 gene. J Intern Med 253: 606–615, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Chen BK, Chang WC. Functional interaction between c-Jun and promoter factor Sp1 in epidermal growth factor-induced gene expression of human 12(S)-lipoxygenase. Proc Natl Acad Sci USA 97: 10406–10411, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferguson LR, Tatham AL, Lin Z, Denny WA. Epigenetic regulation of gene expression as an anticancer drug target. Curr Cancer Drug Targets 11: 199–212, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Gobl AE, Berg M, Lopez-Egido JR, Oberg K, Skogseid B, Westin G. Menin represses JunD-activated transcription by a histone deacetylase-dependent mechanism. Biochim Biophys Acta 1447: 51–56, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hendy GN, Kaji H, Canaff L. Cellular functions of menin. Adv Exp Med Biol 668: 37–50, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Hirai S, Yaniv M. Jun DNA-binding is modulated by mutations between the leucines or by direct interaction of fos with the TGACTCA sequence. New Biol 1: 181–191, 1989 [PubMed] [Google Scholar]
- 18. Hirai SI, Ryseck RP, Mechta F, Bravo R, Yaniv M. Characterization of JunD: a new member of the jun proto-oncogene family. EMBO J 8: 1433–1439, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hodny Z, Li R, Barath P, Nelson BD. Sp1 and chromatin environment are important contributors to the formation of repressive chromatin structures on the transfected human adenine nucleotide translocase-2 promoter. Biochem J 346: 93–97, 2000 [PMC free article] [PubMed] [Google Scholar]
- 20. Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW. Trichostatin A induces transforming growth factor beta type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1. NF-Y complex. J Biol Chem 280: 10047–10054, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kardassis D, Papakosta P, Pardali K, Moustakas A. c-Jun transactivates the promoter of the human p21(WAF1/Cip1) gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J Biol Chem 274: 29572–29581, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci USA 102: 14659–14664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kidd M, Hinoue T, Eick G, Lye KD, Mane SM, Wen Y, Modlin IM. Global expression analysis of ECL cells in Mastomys natalensis gastric mucosa identifies alterations in the AP-1 pathway induced by gastrin-mediated transformation. Physiol Genomics 20: 131–142, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kim H, Lee JE, Cho EJ, Liu JO, Youn HD. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res 63: 6135–6139, 2003 [PubMed] [Google Scholar]
- 25. Kuhlmann AS, Villaudy J, Gazzolo L, Castellazzi M, Mesnard JM, Duc Dodon M. HTLV-1 HBZ cooperates with JunD to enhance transcription of the human telomerase reverse transcriptase gene (hTERT) (Abstract). Retrovirology 4: 92, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, Krenning EP. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 40: 78–88, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Lairmore TC, Chen H. Role of menin in neuroendocrine tumorigenesis. Adv Exp Med Biol 668: 87–95, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Lei S, Dubeykovskiy A, Chakladar A, Wojtukiewicz L, Wang TC. The murine gastrin promoter is synergistically activated by transforming growth factor-beta/Smad and Wnt signaling pathways. J Biol Chem 279: 42492–42502, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mensah-Osman E, Zavros Y, Merchant JL. Somatostatin stimulates menin gene expression by inhibiting protein kinase A. Am J Physiol Gastrointest Liver Physiol 295: G843–G854, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakabeppu Y, Ryder K, Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell 55: 907–915, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol 63: 49–76, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Ryder K, Lanahan A, Perez-Albuerne E, Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci USA 86: 1500–1503, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scacheri PC, Davis S, Odom DT, Crawford GE, Perkins S, Halawi MJ, Agarwal SK, Marx SJ, Spiegel AM, Meltzer PS, Collins FS. Genome-wide analysis of menin binding provides insights into MEN1 tumorigenesis (Abstract). PLoS Genet 2: e51, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schreiber E, Harshman K, Kemler I, Malipiero U, Schaffner W, Fontana A. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res 18: 5495–5503, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Shiotani A, Merchant JL. cAMP regulates gastrin gene expression. Am J Physiol Gastrointest Liver Physiol 269: G458–G464, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Sowa Y, Orita T, Hiranabe-Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites. Ann NY Acad Sci 886: 195–199, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Spenger A, Ernst W, Condreay JP, Kost TA, Grabherr R. Influence of promoter choice and trichostatin A treatment on expression of baculovirus delivered genes in mammalian cells. Protein Expr Purif 38: 17–23, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Walsh JH. Role of gastrin as a trophic hormone. Digestion 47, Suppl 1: 11–16; discussion 49–52, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Wang CH, Tsao YP, Chen HJ, Chen HL, Wang HW, Chen SL. Transcriptional repression of p21((Waf1/Cip1/Sdi1)) gene by c-jun through Sp1 site. Biochem Biophys Res Commun 270: 303–310, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Weitzman JB, Fiette L, Matsuo K, Yaniv M. JunD protects cells from p53-dependent senescence and apoptosis. Mol Cell 6: 1109–1119, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Wu T, Zhang X, Huang X, Yang Y, Hua X. Regulation of cyclin B2 expression and cell cycle G2/m transition by menin. J Biol Chem 285: 18291–18300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu X, Hua X. Menin, histone h3 methyltransferases, and regulation of cell proliferation: current knowledge and perspective. Curr Mol Med 8: 805–815, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu Y, Zhang X, Zehner ZE. c-Jun and the dominant-negative mutant, TAM67, induce vimentin gene expression by interacting with the activator Sp1. Oncogene 22: 8891–8901, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell 14: 36–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol 24: 5639–5649, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]