Abstract
Calcium (Ca2+) is a critical regulator of cardiac myocyte function. Principally, Ca2+ is the link between the electrical signals that pervade the heart and contraction of the myocytes to propel blood. In addition, Ca2+ controls numerous other myocyte activities, including gene transcription. Cardiac Ca2+ signaling essentially relies on a few critical molecular players—ryanodine receptors, voltage-operated Ca2+ channels, and Ca2+ pumps/transporters. These moieties are responsible for generating Ca2+ signals upon cellular depolarization, recovery of Ca2+ signals following cellular contraction, and setting basal conditions. Whereas these are the central players underlying cardiac Ca2+ fluxes, networks of signaling mechanisms and accessory proteins impart complex regulation on cardiac Ca2+ signals. Subtle changes in components of the cardiac Ca2+ signaling machinery, albeit through mutation, disease, or chronic alteration of hemodynamic demand, can have profound consequences for the function and phenotype of myocytes. Here, we discuss mechanisms underlying Ca2+ signaling in ventricular and atrial myocytes. In particular, we describe the roles and regulation of key participants involved in Ca2+ signal generation and reversal.
The primary regulators of cardiac Ca2+ fluxes are ryanodine receptors, voltage-operated Ca2+ channels, and Ca2+ pumps/transporters. Complex signaling networks and accessory proteins impart additional levels of regulation.
OVERVIEW OF THE CARDIAC CYCLE
The mammalian heart is a complex organ consisting of four chambers—the left and right atria and the left and right ventricles. Through a highly coordinated series of events, the muscular heart pumps blood through the pulmonary and systemic vasculature (Fukuta and Little 2008). During diastole, all four chambers are relaxed. Systole is initiated by propagation of a depolarizing action potential from the sino-atrial node located in the apex of the right atrium, through the right and then the left atrium. This depolarization induces contraction of these chambers, forcing blood into the ventricles. On reaching the atrioventricular (AV) node, the depolarization pauses for a short time period (0.1 s in humans) to ensure completion of atrial systole. Importantly, the AV node acts as an electrical insulator between the atria and ventricles. The AV node prevents the transfer of aberrant contraction patterns to the ventricles, such as the spontaneous electrical activity occurring during atrial fibrillation. The lower portion of the AV node is designated the bundle of His, which then splits into the left and right branches, allowing activation of the left and right ventricles, respectively. These branches give rise to thin filaments called Purkinje fibers, composed of noncontractile cells that distribute the action potential to ventricular myocytes and enable the heart to contract in a coordinated fashion. Transduction of the depolarization signal through the His-Purkinje system causes ventricular systole. The contraction wave, traveling up from the ventricular base, expels blood into the pulmonary artery then on to the lungs, or through the aorta into the arterial system. Retrograde flow of blood is prevented by valves between the atria and ventricles.
As indicated above, the SA node situated at the apex of the right atrium is responsible for initiation of the cardiac action potential. At rest, the membrane potential starts around –70 mV (Vm) and slowly depolarizes until an action potential is triggered. Ca2+ signals may play a key role in action potential generation, although there is considerable debate regarding the major mechanisms controlling the rate of SA node depolarization (see Lakatta and DiFrancesco 2009). One primary component of SA node depolarization is known as If (f stands for funny) (Brown et al. 1979), an ion current mediated by hyperpolarizing-activated cyclic nucleotide-gated (HCN) channels (DiFrancesco 1993). Because this current is triggered by hyperpolarization, it is activated at the start of diastole and slowly declines throughout the pacemaker period. HCN channels are relatively nonselective, and they therefore generate an inward current depolarizing Vm toward the threshold for firing an action potential. Intracellular Ca2+ cycling has also been proposed to act as a primary regulator of SA node depolarization. Imaging SA node cells reveals spontaneous elementary Ca2+ signals known as Ca2+ sparks arising from the SR and preceding action potential generation (Huser et al. 2000). Ca2+ sparks reflect the concerted opening of a cluster of RyRs. The Ca2+ sparks activate sodium/calcium exchange (NCX), which promotes membrane depolarization because three Na+ ions enter for each Ca2+ ion that leaves. T-type Ca2+ channels (“transient current;” Cav3) may also provide a source of Ca2+ for triggering Ca2+ sparks (Bogdanov et al. 2001; Berridge 2003). When Vm reaches a critical threshold (−40 to −50 mV), plasma membrane L-type Ca2+ channels are opened (ICa,L), allowing a large influx of Ca2+ into the cytosol and increasing the membrane potential to ∼+10 mV. It is this depolarization signal that is transmitted from the SA node through the cardiac conduction system, culminating in cardiac myocyte contraction. Within the SA node cells, ICa,L activates an outward potassium current (IK) which hyperpolarizes the membrane and curtails the action potential. The hyperpolarization leads to activation of If, T-type Ca2+ channels and Ca2+ sparks, to begin the next conduction cycle.
EXCITATION-CONTRACTION COUPLING (EC-COUPLING)
EC-coupling is the process pairing myocyte depolarization with mechanical contraction. Ca2+ is the critical intermediary (Bers 2008). Indeed, since Ringer’s experiments more than a century ago, Ca2+ has been known to be an essential mediator of this process (Ringer 1883). As the action potential sweeps over the heart, the plasma membrane (sarcolemma) of each myocyte becomes depolarized (∼−90 mV to ∼+20 mV) thereby causing concerted opening of L-type VOCCs (“long-lasting current;” Cav1.2). Ca2+ flows via the VOCCs into a restricted space between the sarcolemma and the underlying sarcoplasmic reticulum (SR) known as the “junctional zone” or “dyadic cleft.” The accumulation of Ca2+ ions during an action potential increases the Ca2+ concentration within this microdomain from ∼100 nM to ∼10 µM. This elementary Ca2+ influx signal, derived from the activation of VOCCs is known as a “Ca2+ sparklet” (Fig. 1) (Wang et al. 2001). The distribution of Ca2+ sparklet magnitudes suggests that one or several VOCCs can give rise to such signals within myocytes (Cheng and Wang 2002).
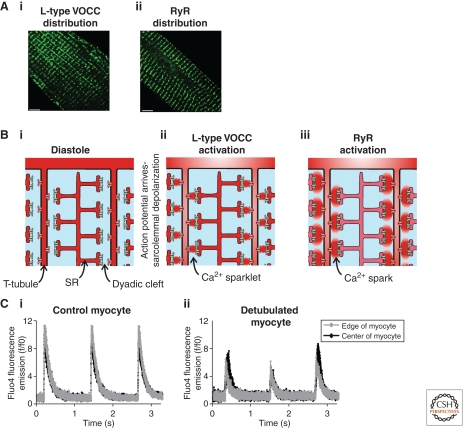
Figure 1.
Excitation contraction coupling in ventricular myocytes. Panel A illustrates the distribution of L-type VOCCs (Ai) and type 2 RyRs (Aii) in a section of a ventricular myocyte. The distributions of these proteins are essentially overlapping at the level of the light microscope. Panel B is a cartoon sequence of events leading to the generation of a Ca2+ signal within a ventricular myocyte. A small section of a ventricular myocyte is depicted with two T-tubule projections (T-tubule spacing ∼1.8 µm). During the diastolic phase (Bi), the L-type VOCCs (red channels on the T-tubule membranes) and RyRs (blue channels on SR membrane) are silent. Arrival of the action potential causes depolarization of the sarcolemma and activation of the L-type VOCCs thereby generating “Ca2+ sparklets” (Bii). The Ca2+ sparklets trigger activation of the RyRs thereby producing “Ca2+ sparks” (Biii). Panel Ci depicts the consistent, global Ca2+ responses observed in an electrically paced ventricular myocyte. The black and gray traces indicate the Ca2+ concentration (measured with fluo4) at the center and edge of the myocyte. The profile of the Ca2+ signal was essentially the same in both locations. Panel Cii illustrates what happens in a ventricular myocyte following detubulation (using formamide treatment). Detubulation decreases the amplitude of systolic Ca2+ transients, and provokes spatial heterogeneity of the resultant Ca2+ signals. The black and gray traces indicate the Ca2+ concentration at the center and edge of the myocyte. Whereas the Ca2+ responses in the edge of the myocyte were reasonably consistent, the signals in the center of the cell showed beat-to-beat variation in amplitude. Such Ca2+ signal alternans are a potential cause of cardiac arrhythmia.
Ca2+ sparklets themselves are not adequate to cause substantial contraction. However, they are sufficient to induce opening of RyRs (type 2 RyRs) on the closely apposed SR, via a process known as “Ca2+-induced Ca2+ release” (CICR). Seminal studies in the 1970s showed that RyR activity is dependent on cytosolic Ca2+ levels, with low concentrations (1–10 µM) being activatory and high concentrations (>10 µM) inhibiting the channel (Fabiato and Fabiato 1972; Fabiato 1983). The concentration of Ca2+ at the dyadic cleft following activation of L-type Ca2+ channels falls within the range of that required for channel activation, thus facilitating CICR.
The activation of a cluster of RyRs, and consequent mobilization of Ca2+ from the SR, produces an elementary Ca2+ release signal know as a “Ca2+ spark” (Fig. 1) (Cheng and Lederer 2008). As with Ca2+ sparklets, Ca2+ spark magnitudes can vary, indicating that different numbers of RyRs participate in their generation. Release of Ca2+ through the RyRs increases the concentration of Ca2+ to >100 µM in the dyadic cleft. It is estimated that ∼25 L-type Ca2+ channels and 100 RyRs are closely associated within the dyadic cleft to form a “couplon” (Bers and Guo 2005). Ca2+ ions diffuse out of the cleft to engage the contractile machinery, thereby promoting cell shortening to provide the force for pumping blood. During a single action potential, thousands of Ca2+ spark sites are simultaneously activated by their corresponding Ca2+ sparklet triggers (Cheng and Lederer 2008). Diffusion of Ca2+ ions, and their subsequent spatial and temporal summation, produces an average global Ca2+ increase of 500 nM to ∼1 µM. Troponin C (TnC), the Ca2+-binding component of the contractile filaments, is sensitive to Ca2+ concentrations over that range thereby allowing coupling of the AP-mediated Ca2+ transient and contraction. At the end of an action potential Ca2+ transients are rapidly terminated, and the cells return to resting diastolic levels in preparation for the next depolarization.
In addition to L-type VOCCs, contractile cardiac myocytes express a T-type current (Cav3 family), which is so named because of its transient nature. As described in the section on pacemaking above, this current is activated at a more negative membrane potential than the L-type (Nowycky et al. 1985). Although ICa,T plays a role in depolarizing SA node cells, under normal physiological situations its role is negligible within ventricular and atrial cardiac myocytes as most Ca2+ enters through the L-type channels. However, T-type VOCC expression is up-regulated during cardiac hypertrophy, and they may provide a critical Ca2+ signal to drive hypertrophic remodeling. For example, Cav3.2 knockout mice did not display cardiac hypertrophy in response to pressure overload or angiotensin II (Chiang et al. 2009).
Skeletal muscle EC-coupling is also mediated by elevations in intracellular Ca2+, and has some similarities to the cardiac scheme described above. The key difference in this tissue is Ca2+ sparklets do not trigger Ca2+ release from the SR. Rather, L-type VOCCs (Cav1.1) on the sarcolemma have a direct physical interaction with RyRs (type 1 RyRs) on underlying SR forming the triadic junction (Rios and Brum 1987; Block et al. 1988). Depolarization of the sarcolemma induces a conformational change in the VOCCs, which allosterically activates the RyRs.
REGULATION OF L-TYPE VOCCs
The channels and homeostatic processes underlying Ca2+ release and Ca2+ clearance are subject to regulation by multiple signaling pathways. These signaling pathways can rapidly alter the amplitude and/or spatial properties of myocyte Ca2+ signaling to acutely modulate cardiac output. For example, L-type VOCCs are subject to regulation by cAMP-dependent kinase (protein kinase A; PKA) downstream from β-adrenergic receptor stimulation, e.g., during the fight or flight response. PKA phosphorylation increases channel activity, thereby contributing to the increased cardiac contraction (positive inotropic response) evoked by β-adrenergic stimuli. PKA-mediated phosphorylation increases opening of the VOCC by a twofold mechanism—it increases both the number of channels in an activatable state, and their activation probability (Catterall 2000). PKA phosphorylates Ser-1928 in the amino terminal of the α1 subunit (Perets et al. 1996), in addition to residues on the β2 subunit (Curtis and Catterall 1985; Gerhardstein et al. 1999). Rapid dephosphorylation of the channel is provided by the Ser/Thr phosphatases 1 and 2A (PP1 and PP2A, respectively) (Kamp and Hell 2000).
L-type VOCCs are also phosphorylated by protein kinase C (PKC) in response to activation of Gq-coupled receptors, e.g., α1-adrenergic, endothelin, and angiotensin II receptors. The targets for PKC phosphorylation are proposed to be two Thr residues in the amino terminal of the α1 subunit (Shistik et al. 1998; McHugh et al. 2000). The effects of PKC phosphorylation are less clear than for PKA phosphorylation. It is proposed that the effect may be reliant on the particular PKC isoform activated, the expression of which varies in a complex developmental, species-dependent, and disease-regulated manner in the heart. Additional regulation of the L-type Ca2+ channel is provided by cGMP-dependent protein kinase G (PKG), which has been shown to have an inhibitory effect, thus opposing the effects of PKA (Hartzell and Fischmeister 1986; Abi-Gerges et al. 2001).
Inactivation of the L-type Ca2+ channel is mediated by both membrane repolarization and by Ca2+ itself (Ca2+-dependent inactivation, CDI). The latter acts as a negative feedback loop, and is thought to be the more important of the two mechanisms (Lee et al. 1985). CDI is controlled by calmodulin (CaM), which is constitutively bound to the channel (Peterson et al. 1999; Qin et al. 1999). The CaM binding site is a canonical “IQ” CaM-binding motif within the carboxyl terminus of the α1 subunit that binds the Ca2+-free form of CaM (apo-CaM) (Rhoads and Friedberg 1997; Zuhlke and Reuter 1998). Ca2+ entering through L-type channels during EC-coupling binds to apo-CaM to form Ca-CaM, which inactivates the channel (Tang et al. 2003). Interestingly, Ca-CaM can also enhance Ca2+ entry through L-type Ca2+ channels by Ca2+-dependent facilitation (CDF). CDF is also dependent on the IQ motif in the cytoplasmic tail of the α1C subunit (Zuhlke et al. 1999). This capacity for dual regulation is caused by the presence of both high and low affinity Ca2+ binding sites (EF-hands) within CaM, in the carboxyl and amino terminal lobes, respectively. It is believed that CDI depends on Ca2+ bound to the amino terminal EF-hands, whereas CDF depends on the carboxyl terminal EF hands (DeMaria et al. 2001). Subsequently, it was revealed that the two lobes of CaM can detect Ca2+ arising from distinct sources—the high affinity carboxyl terminal site sensing local Ca2+ arising within the nanodomain of the channel mouth, whereas the low affinity amino terminal lobe detects global Ca2+ signals (Tadross et al. 2008). It therefore appears that Ca2+ binding to apo-CaM at the carboxyl terminus of L-type VOCCs potentiates Ca2+ entry once a Ca2+ sparklet is forming. However, once global Ca2+ is elevated, Ca2+ also binds to the amino terminal lobe of CaM leading to CDI and termination of Ca2+ entry.
Additional modification of ICa,L is provided by Ca2+/CaM-dependent kinase II (CaMKII), which potentiates the influx of Ca2+ (Anderson et al. 1994; Xiao et al. 1994; Yuan and Bers 1994; Wu et al. 2001b). CaMKII interacts with and phosphorylates the carboxyl terminal of the α1 subunit (Hudmon et al. 2005). CaMKII remains tightly bound to the channel even in the absence of Ca2+, although it is only active when it has Ca-CaM bound. Importantly, Ca-CaM can remain bound to CaMKII, and the kinase active, even after global Ca2+ has declined, thereby allowing CaMKII to act as a detector of Ca2+ spike frequency (Hudmon et al. 2005).
REGULATION OF RyRs AND CICR
There are three mammalian RyR isoforms (RyR1–3). RyR2 is predominant in cardiac myocytes, with significantly lesser amounts of RyR3. The RyR channel is a large homotetrameric assembly of ∼2 megadaltons (each subunit has a molecular mass of ∼560 kDa) (Lanner et al. 2010). Structural studies have revealed four-fold symmetry, with a four-leaf clover or mushroom morphology formed by the transmembrane domain and bulky cytoplasmic domain (Anderson et al. 1989; Serysheva 2004). Binding of Ca2+ is proposed to cause conformational changes that evoke channel gating in a mechanism similar to that of a camera iris, with twisting of the transmembrane regions opening the ion pore (Serysheva et al. 1999).
RyRs are bound by a multitude of accessory proteins, comprising a macromolecular signaling complex. These interactions determine the efficiency and specificity of signaling to and from RyRs, and between other signal transduction cascades. Moreover, scaffolding of proteins to RyRs recruits and concentrates important regulatory proteins in the junctional zone where they are ideally located to modulate and/or be regulated by EC-coupling. These interactions occur on both the lumenal and cytosolic face of the RyR (Lanner et al. 2010). In the lumen of the SR, RyRs interact with calsequestrin (CSQ), the major Ca2+ binding/storage protein of muscle. CSQ is a low-affinity Ca2+ storage protein that maintains the SR lumenal free Ca2+ concentration between 100–500 µM (Yano and Zarain-Herzberg 1994; Berridge 2002). The critical role of CSQ in Ca2+ storage was shown by the increase or decrease in SR Ca2+ load observed in experiments in which CSQ expression was enhanced or suppressed, respectively (Terentyev et al. 2003). In addition to acting as the major Ca2+ storage protein in cardiac muscle, CSQ also regulates RyR channel activity (Prins and Michalak 2011). CSQ reversibly changes between monomeric to oligomeric forms in response to changes in luminal Ca2+ concentration. It has been suggested that CSQ oligomers are present when the SR is replete, and that these mainly serve to buffer Ca2+. However, when RyRs open and SR luminal Ca2+ declines, Ca2+ unbinds from calsequestrin and the oligomeric protein dissociates. The calsequestrin monomers bind to RyRs (via a protein intermediate called triadin) and inhibit channel activity. This is an important component of the mechanisms that terminate Ca2+ release during each heartbeat (Gyorke et al. 2009).
The key role of CSQ in regulating Ca2+ storage and RyR function is highlighted by a pathological condition known as catecholaminergic polymorphic ventricular tachycardia (CPVT) that is observed in patients with CSQ mutations. CPVT is a life-threatening form of cardiac dysrhythmia typically brought about by emotional or physical stress. Recessive CSQ mutations are found in ∼3% of CPVT patients (Katz et al. 2009). The effects of some CPVT-inducing CSQ mutations on calcium fluxes in cardiac myocytes are known. For example, mutation of the aspartate to histidine at residue 307 in CSQ (CSQD307H) causes decreased SR Ca2+ storage and release, and increases the frequency of delayed afterdepolarizations (DADs; spontaneous electrical depolarization of cardiac myocyte independent of the SA node-evoked AP) (Viatchenko-Karpinski et al. 2004). Another mutation, arginine to glutamic acid at residue 33 (CSQR33Q), decreases the interaction between the RyR and CSQ, resulting in abnormal regulation of the RyR by lumenal Ca2+ and increased Ca2+ release (Terentyev et al. 2006). A substantial proportion of CPVT patients (∼50%) express mutated RyRs that have altered association with accessory proteins.
CaM is an important regulator of the RyR in cardiac myocytes, both in its Ca2+ bound and Ca2+ free form (Ca-CaM and apo-CaM, respectively). The binding site for CaM on the RyR was mapped to the carboxyl terminal of the receptor by site-directed mutagenesis (Porter Moore et al. 1999a; Porter Moore et al. 1999b), in agreement with cryo-EM studies (Wagenknecht et al. 1997). Binding of CaM decreases Ca2+ efflux through these channels (Meissner and Henderson 1987). This is thought to be facilitated by a reduction in the opening probability and in the Ca2+-dependent activation of the channel (Balshaw et al. 2001).
Further Ca2+-dependent modification of RyRs is provided by CaMKII-dependent phosphorylation. Sequence analysis revealed six consensus phosphorylation sites in the RyR (Zucchi and Ronca-Testoni 1997). Both Ser-2809 (Witcher et al. 1991) and Ser-2815 (Wehrens et al. 2004) have been shown to be crucial for CaMKII-dependent phosphorylation. CaMKII phosphorylation increases RyR activity (Witcher et al. 1991; Wehrens et al. 2004), although some studies have contested this idea (Lokuta et al. 1995; Wu et al. 2001a). Phosphorylation by CaMKII is emerging as the dominant mode of regulation of RyR activity during adrenergic stimulation and in the greater contractility associated with increased frequency of myocyte contraction (Wu et al. 2009; Grimm and Brown 2010).
Ca2+ release through RyRs is regulated by interaction with FK binding proteins (FKBPs), named because of their binding of the immunosuppressant drug FK506. Cardiac myocytes express two isoforms of the 12 kDa FKBP, namely FKBP12 and FKBP12.6 (also known as calstabin1 and calstabin2, respectively). The cardiac RyR2 binds FKBP12.6 with a higher affinity (Timerman et al. 1996; Jeyakumar et al. 2001). FKBP12.6 binding stabilizes the coordinated gating of RyR subunits within a tetramer, thereby enabling channels to transition between the fully closed and the fully open state, while also shifting the Ca2+ dependence of channel opening to a higher Ca2+ concentration (Bers 2004). CPVT-inducing mutations within the type 2 RyR channel decrease the association between the receptor and FKBP12.6, although only under conditions of β-adrenergic stimulation. FKBP12.6 dissociation may lead to increased Ca2+ release from the SR (Wehrens et al. 2003).
RyRs can also be phosphorylated by PKA, which is tethered by an A kinase anchoring protein (mAKAP) (Marx et al. 2000). PKA-dependent phosphorylation of the receptor was reported to increase its responsiveness to Ca2+ (Valdivia et al. 1995), although the precise consequences of PKA-dependent phosphorylation have been controversial. A widely discussed model proposed that PKA phosphorylation of RyRs causes dissociation of FKBP12.6, thereby leading to increased probability of channel opening (Marx et al. 2000; Wehrens et al. 2003). This model of RyR regulation would not only be relevant physiologically, coupling β-adrenergic stimulation with enhanced Ca2+ release, but would also be important in disease conditions in which elevated PKA phosphorylation had been reported to decrease FKBP12.6-RyR associations and result in increased spontaneous diastolic RyR activity and DADs. However, the dependence of FKBP12.6 association with the RyR on PKA phosphorylation has been much disputed (Xiao et al. 2007). Indeed, a recent report provides substantial evidence that the association of FKBP12.6 interaction with the RyR is insensitive to the degree of PKA phosphorylation (Guo et al. 2010).
PKA may also regulate RyR activity by phosphorylation of Sorcin, another RyR-interacting protein. Sorcin is a ubiquitously expressed 22 kDa Ca2+ binding protein that inhibits Ca2+ release via the RyR. This inhibitory effect is lost following its phosphorylation by PKA (Lokuta et al. 1997).
A cAMP phosphodiesterase (PDE) has also been identified within the RyR macromolecular complex (specifically, the PDE4D3 isoform). The presence of this enzyme provides a mechanism to tightly regulate cAMP, and thus PKA activity, in the vicinity of the receptor. The levels of this PDE isoform have been reported to be reduced in failing hearts (Lehnart et al. 2005).
Type 2 RyRs are associated with phosphatases to mediate rapid receptor dephosphorylation and return it to basal levels of activity. For example, calcineurin is suggested to be an accessory protein of cardiac RyRs (Bandyopadhyay et al. 2000), and is proposed to decrease Ca2+ channel activity. In addition, the phosphatases PP1 and PP2A are associated with cardiac RyRs (Marx et al. 2001). PP1 associates with RyRs via an interaction with spinophilin, whereas PP2A binds to a targeting protein PR130, which then anchors it to RyRs (Marx et al. 2001).
CARDIAC MYOCYTE CONTRACTION
Contraction of cardiac myocytes is facilitated by myofilaments organized into sarcomeres, situated along the long axis of the cell. The sarcomere consists of myosin-containing thick filaments surrounded by a hexagonal array of thin filaments, which are made up of actin polymers and troponin/α-tropomyosin (Tn/Tm) regulatory units (Parmacek and Solaro 2004). Additionally, each thin filament is separated at the Z-line by an actin binding protein, α-actinin.
Every seventh actin monomer comprising the thin filament is bound to a Tn/Tm complex. Tn is composed of three subunits: TnC, Troponin I (TnI), and Troponin T (TnT) (Greaser and Gergely 1971). TnC has a similar structure to CaM, and contains Ca2+ binding EF-hands (Parmacek and Solaro 2004). TnI is an inhibitory subunit, and TnT constitutively interacts with Tm. Binding of Ca2+ to TnC causes a conformational change in associated TnI. This enables Tn/Tm to slide into the groove between actin monomers, allowing the myosin thick filament to bind actin, thus forming a cross-bridge. By repetitive, transient actin-myosin interactions and utilizing energy from ATP hydrolysis, the two filaments slide relative to each other, thus shortening the cell. Coordinated shortening of the entire myocyte population by the spreading AP leads to cardiac contraction. As cytosolic Ca2+ levels decline, Ca2+ is released from TnC leading to cross-bridge detachment, and the thick and thin filaments slide past each other back to their original positions. Cross-bridges cannot form between the filaments as they travel past in that direction.
Troponin proteins are regulated by phosphorylation, which alters their activity and thus affects cardiac myocyte contraction. PKA phosphorylation downstream from β-adrenergic stimulation is of particular importance, as this would facilitate altered cardiac contraction during, for example, physical exertion. Much work has been completed in elucidating the functional outcome of this modification, and the consensus of opinion is that PKA phosphorylation leads to increased cardiac contraction (for a review see Metzger and Westfall 2004). PKC phosphorylates regions on TnT and TnI. Early studies reported divergent results regarding the effects of PKC phosphorylation, although more recent studies point toward an inhibition of contractile function following PKC phosphorylation (Takeishi et al. 1998; Sumandea et al. 2004).
A large number of mutations have been identified in Ca2+-dependent contractile proteins that are linked with specific cardiomyopathies (reviewed in Morimoto 2008). Clinically, cardiomyopathies can be divided into four main groups: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM), and arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C). HCM is characterized by thickening of the ventricular walls with accompanying decreases in ventricular chamber volume. In this condition, systolic function is preserved at the expense of diastolic function, which is responsible for symptoms of heart failure and death in these patients. Thickened ventricular walls are also observed in DCM, in addition to an increase in chamber volume. The clinical outcomes include systolic dysfunction, which also leads to heart failure and sudden cardiac death. RCM is linked to diastolic dysfunction, although there is little or no effect on systolic function or ventricular wall thickness. Mutations leading to all three of these myopathies have been identified in the contractile proteins of the sarcomere, although interestingly none have yet been found that are linked to ARVD/C (Morimoto 2008). Considering TnT, for example, 27 mutations have been identified that have been linked to HCM, which act by increasing the Ca2+ sensitivity of cardiac muscle contraction. Two TnT mutations have been linked to DCM, the functional consequence being decreased Ca2+ sensitivity and an increased affinity of TnT for tropomyosin. Thirty-three mutations in TnI have been found that are linked with HCM, DCM, and RCM, mainly causing increased Ca2+ sensitivity and impaired interaction of TnT with TnI. Regarding TnC, a HCM-causing mutation has been identified that abolishes its interaction with TnI, thus losing the altered Ca2+ sensitivity imparted by PKA-dependent phosphorylation. Thirteen tropomyosin mutations have been identified, leading to HCM and DCM in the manner described previously.
Ca2+ EFFLUX
EC-coupling events are short-lived—atrial and ventricular myocytes reach peak contraction within a few tens of milliseconds of action potential initiation at the SA node. After cytosolic Ca2+ has activated the contractile units, it is rapidly extruded from the cytosol in preparation for the following action potential (Shannon and Bers 2004). The main efflux mechanisms in cardiac myocytes are the plasma membrane NCX and the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump on the SR. The plasma membrane Ca2+ ATPase (PMCA) and the mitochondrial uniporter may also play a more minor role. The relative contribution of these mechanisms varies in a species-dependent manner, for example in rabbit ventricles ∼70% of cytosolic Ca2+ is removed by SERCA2a, 28% by NCX, and the remaining 2% by the mitochondrial uniporter. A similar pattern is apparent in ventricles from dog, cat, guinea pig, and human. Alternately, in mouse and rat ventricles, SERCA2a removes ∼92% of the Ca2+, leaving only ∼7% for the NCX (Bassani et al. 1994; Bers 2001). The difference in the relative contributions of the Ca2+ clearance pathways is reflected by the observed kinetics of NCX activity between different species (Sham et al. 1995). NCX current density is lowest in myocytes from rat, and highest in those from guinea pig. The adaptability of this system was shown in a recent report showing minimal cardiac dysfunction in a cardiac-specific SERCA2 KO mouse (Andersson et al. 2009). Ca2+ fluxes were maintained by increased activity of the L-type Ca2+ channel and NCX, demonstrating that these proteins can compensate for a major reduction in SERCA levels (<5% SERCA2 protein remained in myocardial tissue 4 weeks after gene excision).
In its forward mode, NCX uses the electrochemical gradient across the sarcolemma to translocate three Na+ ions into the cytosol and expel one Ca2+. In this situation, NCX is a depolarizing current. If Na+ levels are high, the exchanger may change to its reverse mode, and bring Ca2+ into the cell. The exchanger was discovered around 40 years ago in the squid giant axon (Baker et al. 1969) and in the mammalian heart (Reuter and Seitz 1968). Following cloning of the canine NCX (Nicoll et al. 1990), three isoforms were identified (NCX1-3). Of these, NCX1 (110 kDa) is predominant in cardiac myocytes. It is responsible for exporting an amount of Ca2+ approximately equivalent to the flux entering via L-type Ca2+ channels (Bridge et al. 1990). The adaptability of cardiac myocytes is again shown in the NCX KO mouse, in which EC-coupling is maintained by a compensatory reduction in Ca2+ influx (Pott et al. 2007).
Cardiac NCX is regulated by the concentration of cytoplasmic ions, specifically activation by Ca2+ (Hilgemann 1990) and inhibition by Na+ (Hilgemann et al. 1992). Maintenance of the ionic gradient between the extracellular space and cytosol is critical for NCX activity. In particular, intracellular Na+ must remain low for NCX activity. This is facilitated by the action of the sodium potassium ATPase, which extrudes Na+ entering the cell during the action potential. Inhibition of this pump by cardiac glycosides such as digoxin and ouabain results in an accumulation of intracellular Na+, leading to a suppression of NCX activity and thereby attenuating Ca2+ efflux. As a consequence, Ca2+ transient amplitude and myocyte contraction are increased. Because of these positive inotropic effects, digoxin has long been used as therapy for failing heart. However, this manipulation can also lead to arrhythmia and myocyte death.
NCX has been shown to be phosphorylated by PKC and PKA suggesting that its activity may be regulated by these posttranslational modifications (Iwamoto et al. 1996; Ruknudin et al. 2000). NCX interacts with a number of accessory proteins. In particular, it interacts with the transmembrane protein phospholemman, which exerts an inhibitory effect (Zhang et al. 2003; Ahlers et al. 2005; Cheung et al. 2007). Phosphorylation of phospholemman is reported to occur on residues within the cytoplasmic carboxyl terminal of the protein, specifically Ser-68, and acts to inhibit NCX (Song et al. 2005a; Zhang et al. 2006; Cheung et al. 2007). This appears to be mediated by PKC and not PKA (Zhang et al. 2006). NCX has been identified within a macromolecular complex containing PKA and its anchoring protein mAKAP, together with PKC and the phosphatases PP1 and PP2A (Schulze et al. 2003), providing a robust mechanism for the phosphorylation and regulation of NCX and/or phospholemman.
Ca2+ reuptake into the SR is mediated by the SERCA pump, which uses energy from ATP hydrolysis. Molecular cloning has identified three SERCA isoforms (SERCA1–3), all of which undergo alternative splicing. The alternately spliced isoforms of SERCA2 (SERCA2a and SERCA2b) possess distinct carboxyl terminal residues. SERCA isoforms differ in their relative affinity for Ca2+ and their transport rates (Lytton et al. 1992). SERCA2a, the main cardiac isoform, possesses a K1/2 of ∼0.4 µM; however, in vivo, this is ∼0.9 µM because of its association with its regulatory transmembrane phosphoprotein, phospholamban (PLB). Cardiac SERCA pumps are usually situated on a region of the SR separate from the junctional zone where the RyRs are located. During recovery of a Ca2+ transient, Ca2+ is pumped into the SR at the location of the SERCA enzymes. The Ca2+ ions return to the dyadic SR lumen by tunneling through the SR network.
SERCA regulation in the heart is primarily governed by PLB. Inactive, phosphorylated PLB exists as a pentamer. It depolymerizes when not phosphorylated and can then interact with SERCA (Kimura et al. 1997). Unphosphorylated PLB monomers decrease the affinity of SERCA for Ca2+ (Tada et al. 1974). Subsequent phosphorylation of PLB prevents its interaction with SERCA, increasing the apparent activity of the pump and the rate of Ca2+ accumulation within the SR. PLB can be phosphorylated on three sites: Ser-16 by PKA, Thr-17 by CaMKII, and Ser-10 by PKC (Movsesian et al. 1984; Simmerman et al. 1986), facilitating regulation by a variety of signaling pathways. CaMKII is present in a multiprotein complex with SERCA2a, and can directly phosphorylate the Ca2+ pump (Toyofuku et al. 1994; Narayanan and Xu 1997) leading to enhanced activity (Xu and Narayanan 1999; Xu et al. 1999). Modulation of PLB-mediated SERCA inhibition is a major mechanism for acute enhancement of cardiac function following β-adrenergic receptor activation. As a result of reduced PLB interaction with the pump, the rates of clearance of the Ca2+ transient and relaxation is increased (positive lusitropic response), and SR store loading is enhanced. Because of greater Ca2+ within the store, the magnitude of Ca2+ fluxes is elevated thereby producing enhanced contraction.
SUBCELLULAR ORGANIZATION OF CARDIAC MYOCYTES
As described previously, the action potential generated at the SA node sweeps rapidly through the heart, coordinating cardiac contraction by activating atrial and then ventricular myocytes in synchrony. Within individual myocytes, the depolarization signal culminates in activation of the sarcomeric contractile units by elevating intracellular Ca2+. The spatial properties of the Ca2+ increase depending on the structure of the different myocytes within the heart. For example, adult ventricular myocytes possess numerous invaginations of the sarcolemma, which form a regular array of inwardly directed membranous structures known as transverse tubules (T-tubule) (Fig. 1) (Song et al. 2005b). T-tubules are narrow (∼200 nm diameter) and occur at regular intervals of ∼2 µm (Brette and Orchard 2003). Additional branches project from the main T-tubules to give a complex network of sarcolemmal intrusions (Ayettey and Navaratnam 1978). T-tubules are a feature of mammalian ventricular myocytes, and are absent in the ventricles of birds (Bossen et al. 1978), reptiles, and amphibians (Bossen and Sommer 1984).
The presence of T-tubules facilitates homogenous Ca2+ transients during EC-coupling in ventricular myocytes. T-tubules serve to create dyadic junctions deep within the volume of a ventricular myocyte. In this way, Ca2+ sparks can be triggered simultaneously throughout a cell. The alternative to T-tubules is observed in neonatal myocytes and atrial myocytes, where dyadic junctions occur solely at the periphery of the cells (Fig. 2). T-tubules also act as important scaffolding regions for many of the proteins essential for Ca2+ signaling (Chase and Orchard 2011). For example, the sarcolemmal L-type Ca2+ channels and NCX are abundant on T-tubule membranes (Orchard and Brette 2008). It has been estimated that >75% of ICa flows into a myocyte through the T-tubules because of the high concentration of L-type Ca2+ channels in this region. As mentioned in the previous section, Ca2+-dependent inactivation of ICa is one of the main mechanisms to curtail Ca2+ influx. This feedback mechanism appears to be more potent at T-tubules, meaning ICa at T-tubules is large but inactivates rapidly. In contrast, inactivation of ICa at the peripheral sarcolemma is slower overall, resulting in more Ca2+ entering the cell across the outer region of a cell than at T-tubule sites. This prolonged Ca2+ entry occurs during the latter stages of ICa, and it has been proposed to promote SR Ca2+ loading in preparation for the following next cycle of EC-coupling.
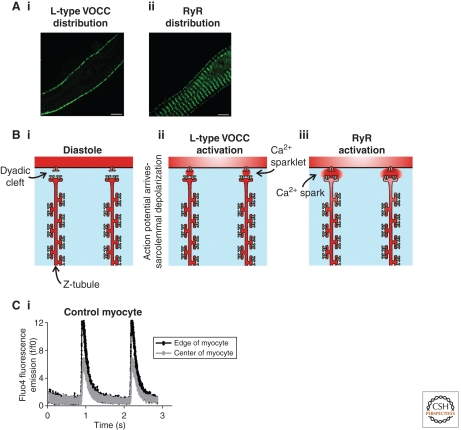
Figure 2.
Excitation contraction coupling in atrial myocytes. Panel A illustrates the distribution of L-type VOCCs (Ai) and type 2 RyRs (Aii) in a section of an atrial myocyte. The pattern of L-type VOCC expression is clearly different from that in ventricular cells. The distribution of RyRs is similar to that in ventricular cells, except that there is an evident population of peripheral RyRs around the edge of the myocyte. Solely these peripheral RyRs align with the L-type VOCCs to produce functional dyads. Panel B is a cartoon sequence of events leading to the generation of a Ca2+ signal within an atrial myocyte. A small section of an atrial myocyte is depicted. There are no T-tubules, but instead two prominent SR tubules with a spacing of ∼1.8 µm. Such SR tubules have previously denoted as “Z-tubules,” as they occupy the Z-line (just like T-tubules). During the diastolic phase (Bi), the L-type VOCCs (red channels on the T-tubule membranes) and RyRs (blue channels on SR membrane) are silent. Arrival of the action potential causes depolarization of the sarcolemma and activation of the L-type VOCCs thereby generating “Ca2+ sparklets” at the periphery of the cell (Bii). The Ca2+ sparklets trigger activation of nearby RyRs thereby producing “Ca2+ sparks” (Biii). Panel Ci depicts the gradient of Ca2+ typically observed during electrical pacing of atrial myocytes. The Ca2+ signal at the edge of the cell (black trace) is larger and more rapidly rising than the central response (gray trace). The extent to which the Ca2+ signal occurs in the center of the cell (and thereby causes contraction) is dependent on the inotropic status of the cell. Application of a β-adrenergic agonist can make atrial Ca2+ signals become homogenous.
Cardiac myocytes display an important homeostatic principle known as “autoregulation” (Eisner et al. 1998). This is essentially a balance between ICa, Ca2+ release from the SR and Ca2+ efflux via NCX. Acute changes in any one of these fluxes will exert compensatory changes in the others that bring systolic Ca2+ transients back to normal levels. For example, under conditions of increased SR, Ca2+ release following RyR phosphorylation Ca2+ influx through ICa is reduced and Ca2+ efflux via NCX is increased, thus maintaining the steady-state systolic Ca2+ transient. Taking into consideration that Ca2+-dependent inactivation following SR Ca2+ release occurs predominantly at the T-tubules, and NCX is also concentrated in this region, it is evident that autoregulation occurs mainly at the T-tubules. Because of the principle of autoregulation, prolonged changes in inotropic status require sustained changes in more than one component of the ICa/Ca2+ release/NCX triumvirate (Eisner et al. 2009).
The T-tubular network of atrial myocytes is generally not as well developed as that observed in ventricular myocytes, especially in the atrial myocytes of small mammals. The sarcolemmal L-type Ca2+ channels of atrial myocytes provide a triggering Ca2+ signal for a small population of “junctional” RyRs situated below the sarcolemma at the periphery of the cells (Bootman et al. 2006). The consequence of this arrangement is that the initial Ca2+ influx (ICa) activates Ca2+ sparks solely from the peripheral SR (Figs. 1 and 2). In the absence of a positive inotropic agonist, the Ca2+ signal remains confined to the cellular periphery and contraction is minimal (Bootman et al. 2011).
In addition to the junctional RyRs, atrial myocytes express a major population of nonjunctional RyRs that form a 3-dimensional lattice of Ca2+ release sites within the cells (the distribution of RyRs is actually very similar between ventricular and atrial myocytes—the location of L-type VOCCs is different) (Figs. 1 and 2) (Chen-Izu et al. 2006; Schulson et al. 2011). Because these nonjunctional RyRs are not located within a dyadic junction, they are not activated by ICa (Mackenzie et al. 2001). However, they can be activated by CICR if the peripheral Ca2+ signal is sufficient to act as a trigger for a Ca2+ wave. The depth that such centripetal Ca2+ waves propagate within an atrial myocyte determines the extent of contraction—the deeper a Ca2+ wave spreads the more contractile filaments will be engaged (Mackenzie et al. 2004). The extent of atrial myocyte contraction is regulated by controlling the spread of the centripetal Ca2+ waves. The atrial myocytes in some mammalian species display a relatively high degree of T-tubule membrane. Why some atrial cells should rely on T-tubules when others do not is unclear. However, it has been shown that the presence of T-tubules within individual atrial myocytes is correlated with cell diameter, suggesting that tubulation acts to coordinate Ca2+ signaling in larger cells (Smyrnias et al. 2010).
Neonatal rat ventricular myocytes are similar to atrial cells in that they lack a fully formed T-tubule network. The T-tubules appear progressively through development (Sedarat et al. 2000). Just as with atrial myocytes, the main region of VOCC-RyR interaction within a neonatal myocyte is at the periphery of the cell. Therefore, Ca2+ signals within a neonatal myocyte closely resemble those in an adult atrial cell, and change to become homogenous Ca2+ signals when T-tubules arise.
Ventricular myocytes from hearts that are progressing toward failure show decreased organization of their T-tubules, and loss of T-tubules (Fig. 3) (He et al. 2001). As a result, there is reduced coupling efficiency between the L-type VOCCs on the sarcolemma and RyRs on the underlying SR. The loss of T-tubule membranes means that dyadic junctions are lost and RyRs become “orphaned.” This leads to abnormal EC-coupling characterized by an increased propensity for arrhythmia and decreased magnitude of the Ca2+ response and contraction (Louch et al. 2004). EC-coupling in detubulated ventricular myocytes resembles that observed in atrial myocytes, whereby Ca2+ signals are initiated at the plasma membrane and propagate through the myocyte by the relatively slow process of CICR (Smyrnias et al. 2010).
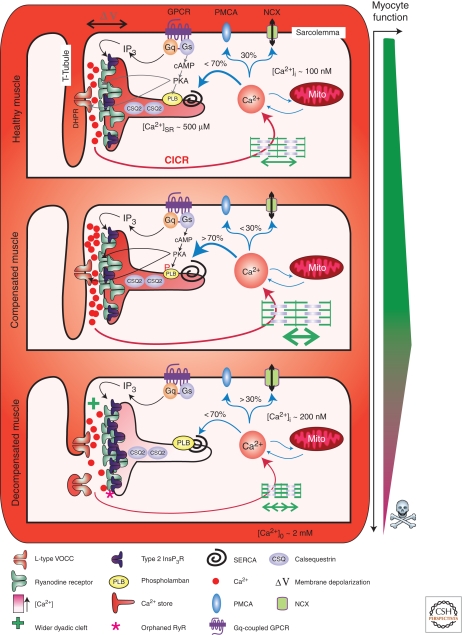
Figure 3.
Excitation contraction coupling in healthy and decompensated hypertrophic cardiac muscle. The figure depicts the architecture and molecular composition of a cardiac dyad in a normal, healthy myocyte (upper panel), a compensated hypertrophic situation (middle panel), and a decompensated failing myocyte (lower panel). A key depicting the symbols used to represent the major players in EC-coupling is provided at the bottom of the figure. Depolarization of the plasma membrane results in Ca2+ influx through L-type voltage gated channels in the T-Tubule, which stimulates Ca2+ release via RyRs located on the juxtaposed SR. Following diffusion out of the dyadic cleft, Ca2+ encounters the contractile filaments causing myocyte contraction. Myocyte relaxation is then brought about by Ca2+ recycling back into the SR by the SERCA pump or extrusion across the plasma membrane via NCX. Neurohormonal activation of Gαq-coupled receptors leads to the formation of InsP3, which stimulates Ca2+ release via InsP3Rs located in the dyad. This InsP3-stimulated Ca2+ release sensitizes neighboring RyRs, causing enhanced Ca2+ fluxes and increasing the frequency of arrhythmic events. Activation of β-adrenergic receptors increases intracellular cAMP, which activates PKA leading to phosphorylation of PLB. On phosphorylation, PLB dissociates from SERCA thereby enhancing Ca2+ transport activity. Compensated/adaptive hypertrophy is associated with enhanced EC-coupling. Significantly contributing to this phenotype is an increase in SR store loading. This is brought about by an up-regulation of SERCA activity mediated by either an increase in SERCA or decrease in PLB expression. Alternatively, as a result of PKA-dependent phosphorylation, PLB interaction with SERCA and suppression of Ca2+ transport may also be decreased. Increased SERCA activity also serves to increase the rate of relaxation thereby allowing more rapid cycles of myocyte contraction. The width of the dyadic cleft may also marginally increase at this stage of hypertrophic remodeling. However, IP3Rs are up-regulated in the dyad during hypertrophy supplementing the Ca2+ signal arising via RyRs to possibly further support EC-coupling. During decompensated hypertrophy, myocyte architecture and protein expression are remodeled. Specifically, the width of the dyadic cleft is increased making it harder for Ca2+ arising via VOCCs to activate Ca2+ release from RyRs. T-Tubules also atrophy resulting in orphaned RyRs. The SERCA-PLB ratio is also modified to favor decreased SERCA activity. Notably, InsP3R expression in the dyad is increased. As a result, more of the RyRs that are located in this region are close enough to InsP3Rs to be affected by Ca2+ arising from them. Overactivation of these InsP3Rs, for example, by the elevated levels of circulating ET-1 present during heart failure, promotes arrhythmias thereby contributing to the pathology associated with heart failure.
ADAPTATION TO DISEASE
In response to pressure or volume overload, damage, or genetic factors, the heart mounts an adaptive hypertrophic response. Because cardiac myocytes are terminally differentiated, enlargement of the heart results mainly from growth of existing myocytes and not as a result of proliferation. For many hypertrophic stimuli, the remodeling of the heart is initially beneficial by acting to increase cardiac output. However, under conditions in which stress persists, for example, because of hypertension, the remodeled heart undergoes a process known as “decompensation.” Specifically, the thickness of the ventricle wall diminishes, and the ability of the heart to supply the cardiovascular requirements of the organism is lost. Such pathological cardiac hypertrophy has a poor prognosis and leads to cardiac failure and sudden death (Levy et al. 1990).
A widely supported hypothesis is that changes in Ca2+ cycling mediate the hypertrophic response (Molkentin 2006). Although initial modifications of Ca2+ handling are beneficial, as the heart progresses to failure they may contribute to pathology (Fig. 3) (Roderick et al. 2007). A general feature of calcium fluxes during adaptive hypertrophy is an increase in the amplitudes of the Ca2+ transient, and also of the Ca2+ sparks that underlie them. Contributing to this is an increase in SR store loading mediated by increased SERCA pump activity. SERCA activity is enhanced through several mechanisms: increased SERCA expression, decreased PLB expression, and increased PLB phosphorylation. A decrease in NCX current and an increase in RyR activity have also been detected during this stage of hypertrophy.
As the heart progresses to failure, further changes in Ca2+ regulation and flux are observed. Contributing to the decreased contractility of the failing heart is a general decrease in the amplitude of each action potential-evoked Ca2+ transient. Underlying this reduced Ca2+ signal are a number of factors, foremost of which is a decrease in SERCA activity (Fig. 3) (Hoshijima et al. 2006). SERCA activity may be modified through a reduction in its expression, increased PLB expression or decreased PLB phosphorylation. As a result of this decrease in SERCA function, diastolic Ca2+ is elevated (a common feature of the failing heart) while myocyte relaxation is prolonged and SR store content is diminished (Roderick et al. 2007). The key contribution of SERCA dysfunction to the decrease in cardiac function during failure has led to the development of viral-mediated SERCA overexpression for gene therapy (Lyon et al. 2011).
Failing hearts are also characterized by more arrhythmic Ca2+ signals. This is perhaps surprising given that SR store loading—a key determinant of RyR activity—is decreased. RyRs isolated from failing hearts and incorporated into lipid bilayers are more spontaneously active than RyR isolated from control hearts, perhaps explaining this conundrum (Kubalova et al. 2005). Indeed, myocytes from failing hearts showed decreased store loading, increased spontaneous RyR opening (observed as Ca2+ sparks) and decreased SR Ca2+ reuptake. Another mechanism for increased arrhythmic Ca2+ signaling during heart failure is the expression of inositol 1,4,5-trisphosphate (InsP3) gated Ca2+ release channels (InsP3Rs). InsP3Rs are typically >50-fold less abundant than RyR in healthy myocytes (Kockskamper et al. 2008). However, their expression increases significantly during hypertrophy and heart failure. In particular, their expression within dyadic junctions increases (Fig. 3) (Harzheim et al. 2009, 2010). The up-regulation of InsP3Rs provides a positive-feedback loop that can promote further hypertrophic remodeling (Nakayama et al. 2010). Although InsP3Rs are not capable of mounting significant Ca2+ signals by themselves, their location next to RyRs within dyadic junctions means that they can trigger CICR and thereby augment EC-coupling. The expression of InsP3Rs may be an initially beneficial adaptive aspect of myocyte remodeling in that they can help to increase systolic Ca2+ transients and evoke greater contraction. However, concomitant with this potentially beneficial aspect of enhanced InsP3R expression is an undesirable increase in the propensity of cells to show spontaneous Ca2+ release events that may cause arrhythmia. This is because of the fact that InsP3Rs are not solely tuned to the activation of ICa, but can open independently whenever cytosolic InsP3 levels are sufficient (Harzheim et al. 2009).
The importance of cellular architecture to EC-coupling is highlighted by its contribution to Ca2+ dysregulation during cardiac failure. In myocytes from failing heart, the synchronicity of the initiation of the Ca2+ transient is reduced and the amplitude of the transient diminished (Gomez et al. 1997). Work from a number of laboratories using live and fixed cell staining approaches, have now established that T-tubules are lost or atrophied as hypertrophy develops, whereas the distribution of RyRs on the SR is unaffected (Gomez et al. 2001; Song et al. 2005b). As a consequence of this membrane remodeling, RyRs become progressively orphaned. Deficiencies in EC-coupling because of T-tubule remodeling have also been observed during the earlier stages of hypertrophy, albeit with less dramatic consequences (Xu et al. 2007). During this stage, the width of the dyadic cleft is marginally increased, altering the kinetic properties of coupling between membrane depolarization and Ca2+ release from the SR. Inotropic stimuli such as adrenaline, which serve to increase Ca2+ influx and sensitivity of RyRs to release, overcome this deficiency in EC-coupling thus indicating that the Ca2+ signaling machinery is still intact. Similarly, dyadic InsP3Rs, which as indicated earlier are elevated during hypertrophy, may also contribute to overcoming the decreased efficiency of coupling.
CONCLUSION
The raison d’etre of cardiac myocytes is controlled contraction in response to repetitive electrical depolarization signals—a function that is fundamentally controlled by Ca2+. Although there are a limited number of key players—ICa, NCX, SERCA, RyRs, TnC— involved in generating and reversing Ca2+ signals, they are subject to numerous levels of regulation and an array of interactions with other proteins. Furthermore, the cellular location of the Ca2+ signaling systems is critical in determining the spatial properties of the Ca2+ signals during EC-coupling. Cardiac Ca2+ signals can be acutely altered to provide rapid changes in cardiac output. The heart responds to long-term increased hemodynamic demand by remodeling. This remodeling encompasses both structural changes and altered gene expression. Depending on the stimulus, this can be an adaptive, reversible remodeling that promotes Ca2+ signaling and cardiac function. Alternatively, a heart can become committed to an irreversible form of remodeling that causes progressively weaker and more arrhythmic Ca2+ signaling. The remodeling causes changes in Ca2+ signaling and vice versa. Therefore, within the context of the heart Ca2+ is very much a signal for both life and death.
Footnotes
Editors: Martin Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Abi-Gerges N, Fischmeister R, Mery PF 2001. G protein-mediated inhibitory effect of a nitric oxide donor on the L-type Ca2+ current in rat ventricular myocytes. J Physiol 531: 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, et al. 2005. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem 280: 19875–19882 [DOI] [PubMed] [Google Scholar]
- Anderson K, Lai FA, Liu QY, Rousseau E, Erickson HP, Meissner G 1989. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem 264: 1329–1335 [PubMed] [Google Scholar]
- Andersson KB, Birkeland JA, Finsen AV, Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien KR, Sejersted OM, et al. 2009. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol 47: 180–187 [DOI] [PubMed] [Google Scholar]
- Ayettey AS, Navaratnam V 1978. The T-tubule system in the specialized and general myocardium of the rat. J Anat 127: 125–140 [PMC free article] [PubMed] [Google Scholar]
- Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA 1969. The influence of calcium on sodium efflux in squid axons. J Physiol 200: 431–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G 2001. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem 276: 20144–20153 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Shin DW, Ahn JO, Kim DH 2000. Calcineurin regulates ryanodine receptor/Ca2+-release channels in rat heart. Biochem J 352 (Pt 1): 61–70 [PMC free article] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM 1994. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J Physiol 476: 279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ 2002. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium 32: 235–249 [DOI] [PubMed] [Google Scholar]
- Berridge MJ 2003. Cardiac calcium signalling. Biochem Soc Trans 31: 930–933 [DOI] [PubMed] [Google Scholar]
- Bers DM 2004. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37: 417–429 [DOI] [PubMed] [Google Scholar]
- Bers DM 2008. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49 [DOI] [PubMed] [Google Scholar]
- Bers DM, Guo T 2005. Calcium signaling in cardiac ventricular myocytes. Ann NY Acad Sci 1047: 86–98 [DOI] [PubMed] [Google Scholar]
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C 1988. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107: 2587–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov KY, Vinogradova TM, Lakatta EG 2001. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: Molecular partners in pacemaker regulation. Circ Res 88: 1254–1258 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Higazi DR, Coombes S, Roderick HL 2006. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci 119: 3915–3925 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Smyrnias I, Thul R, Coombes S, Roderick HL 2011. Atrial cardiomyocyte calcium signalling. Biochim Biophys Acta 1813: 922–934 [DOI] [PubMed] [Google Scholar]
- Bossen EH, Sommer JR 1984. Comparative stereology of the lizard and frog myocardium. Tissue Cell 16: 173–178 [DOI] [PubMed] [Google Scholar]
- Bossen EH, Sommer JR, Waugh RA 1978. Comparative stereology of the mouse and finch left ventricle. Tissue Cell 10: 773–784 [DOI] [PubMed] [Google Scholar]
- Brette F, Orchard C 2003. T-tubule function in mammalian cardiac myocytes. Circ Res 92: 1182–1192 [DOI] [PubMed] [Google Scholar]
- Bridge JH, Smolley JR, Spitzer KW 1990. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science 248: 376–378 [DOI] [PubMed] [Google Scholar]
- Brown HF, DiFrancesco D, Noble SJ 1979. How does adrenaline accelerate the heart? Nature 280: 235–236 [DOI] [PubMed] [Google Scholar]
- Catterall WA 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555 [DOI] [PubMed] [Google Scholar]
- Chase A, Orchard CH 2011. Ca efflux via the sarcolemmal Ca ATPase occurs only in the t-tubules of rat ventricular myocytes. J Mol Cell Cardiol 50: 187–193 [DOI] [PubMed] [Google Scholar]
- Chen-Izu Y, McCulle SL, Ward CW, Soeller C, Allen BM, Rabang C, Cannell MB, Balke CW, Izu LT 2006. Three-dimensional distribution of ryanodine receptor clusters in cardiac myocytes. Biophys J 91: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ 2008. Calcium sparks. Physiol Rev 88: 1491–1545 [DOI] [PubMed] [Google Scholar]
- Cheng H, Wang SQ 2002. Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells. Front Biosci 7: d1867–d1878 [DOI] [PubMed] [Google Scholar]
- Cheung JY, Rothblum LI, Moorman JR, Tucker AL, Song J, Ahlers BA, Carl LL, Wang J, Zhang XQ 2007. Regulation of cardiac Na+/Ca2+ exchanger by phospholemman. Ann NY Acad Sci 1099: 119–134 [DOI] [PubMed] [Google Scholar]
- Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, et al. 2009. The Cav3.2 T-type Ca2+ channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res 104: 522–530 [DOI] [PubMed] [Google Scholar]
- Curtis BM, Catterall WA 1985. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci 82: 2528–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT 2001. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411: 484–489 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D 1993. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55: 455–472 [DOI] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW, Diaz ME, Overend CL, O’Neill SC 1998. The control of Ca release from the cardiac sarcoplasmic reticulum: Regulation versus autoregulation. Cardiovasc Res 38: 589–604 [DOI] [PubMed] [Google Scholar]
- Eisner DA, Kashimura T, O’Neill SC, Venetucci LA, Trafford AW 2009. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol 46: 474–481 [DOI] [PubMed] [Google Scholar]
- Fabiato A 1983. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245: C1–C14 [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F 1972. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res 31: 293–307 [DOI] [PubMed] [Google Scholar]
- Fukuta H, Little WC 2008. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin 4: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardstein BL, Puri TS, Chien AJ, Hosey MM 1999. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltage-dependent calcium channels. Biochemistry 38: 10361–10370 [DOI] [PubMed] [Google Scholar]
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ 1997. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276: 800–806 [DOI] [PubMed] [Google Scholar]
- Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ 2001. Heart failure after myocardial infarction: Altered excitation-contraction coupling. Circulation 104: 688–693 [DOI] [PubMed] [Google Scholar]
- Greaser ML, Gergely J 1971. Reconstitution of troponin activity from three protein components. J Biol Chem 246: 4226–4233 [PubMed] [Google Scholar]
- Grimm M, Brown JH 2010. β-adrenergic receptor signaling in the heart: Role of CaMKII. J Mol Cell Cardiol 48: 322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM 2010. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res 106: 1743–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke S, Stevens SC, Terentyev D 2009. Cardiac calsequestrin: Quest inside the SR. J Physiol 587: 3091–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell HC, Fischmeister R 1986. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature 323: 273–275 [DOI] [PubMed] [Google Scholar]
- Harzheim D, Movassagh M, Foo RS, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL 2009. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci 106: 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D, Talasila A, Movassagh M, Foo RS, Figg N, Bootman MD, Roderick HL 2010. Elevated InsP3R expression underlies enhanced calcium fluxes and spontaneous extra-systolic calcium release events in hypertrophic cardiac myocytes. Channels 4: 67–71 [DOI] [PubMed] [Google Scholar]
- He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ 2001. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49: 298–307 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW 1990. Regulation and deregulation of cardiac Na+-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature 344: 242–245 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Matsuoka S, Nagel GA, Collins A 1992. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol 100: 905–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M, Knoll R, Pashmforoush M, Chien KR 2006. Reversal of calcium cycling defects in advanced heart failure toward molecular therapy. J Am Coll Cardiol 48: A15–A23 [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS 2005. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol 171: 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huser J, Blatter LA, Lipsius SL 2000. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524 (Pt 2): 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M 1996. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem 271: 13609–13615 [DOI] [PubMed] [Google Scholar]
- Jeyakumar LH, Ballester L, Cheng DS, McIntyre JO, Chang P, Olivey HE, Rollins-Smith L, Barnett JV, Murray K, Xin HB, et al. 2001. FKBP binding characteristics of cardiac microsomes from diverse vertebrates. Biochem Biophys Res Commun 281: 979–986 [DOI] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW 2000. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Katz G, Arad M, Eldar M 2009. Catecholaminergic polymorphic ventricular tachycardia from bedside to bench and beyond. Curr Prob Cardiol 34: 9–43 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH 1997. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem 272: 15061–15064 [DOI] [PubMed] [Google Scholar]
- Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD 2008. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, et al. 2005. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci 102: 14104–14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, DiFrancesco D 2009. What keeps us ticking: A funny current, a calcium clock, or both? J Mol Cell Cardiol 47: 157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL 2010. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Marban E, Tsien RW 1985. Inactivation of calcium channels in mammalian heart cells: Joint dependence on membrane potential and intracellular calcium. J Physiol 364: 395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR 2005. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP 1990. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566 [DOI] [PubMed] [Google Scholar]
- Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH 1995. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. J Physiol 487 (Pt 3): 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuta AJ, Meyers MB, Sander PR, Fishman GI, Valdivia HH 1997. Modulation of cardiac ryanodine receptors by sorcin. J Biol Chem 272: 25333–25338 [DOI] [PubMed] [Google Scholar]
- Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR 2004. Reduced synchrony of Ca2+ release with loss of T-tubules—a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73 [DOI] [PubMed] [Google Scholar]
- Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, et al. 2011. SERCA2a gene transfer decreases SR calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH 1992. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267: 14483–14489 [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Berridge MJ, Lipp P 2001. Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J Physiol 530: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD 2004. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci 117: 6327–6337 [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell 101: 365–376 [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR 2001. Phosphorylation-dependent regulation of ryanodine receptors: A novel role for leucine/isoleucine zippers. J Cell Biol 153: 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Sharp EM, Scheuer T, Catterall WA 2000. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci 97: 12334–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G, Henderson JS 1987. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem 262: 3065–3073 [PubMed] [Google Scholar]
- Metzger JM, Westfall MV 2004. Covalent and noncovalent modification of thin filament action: The essential role of troponin in cardiac muscle regulation. Circ Res 94: 146–158 [DOI] [PubMed] [Google Scholar]
- Molkentin JD 2006. Dichotomy of Ca2+ in the heart: Contraction versus intracellular signaling. J Clin Invest 116: 623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S 2008. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res 77: 659–666 [DOI] [PubMed] [Google Scholar]
- Movsesian MA, Nishikawa M, Adelstein RS 1984. Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. Stimulation of cardiac sarcoplasmic reticulum calcium uptake. J Biol Chem 259: 8029–8032 [PubMed] [Google Scholar]
- Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD 2010. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res 107: 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N, Xu A 1997. Phosphorylation and regulation of the Ca2+-pumping ATPase in cardiac sarcoplasmic reticulum by calcium/calmodulin-dependent protein kinase. Basic Res Cardiol 92 (Suppl 1): 25–35 [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD 1990. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science 250: 562–565 [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW 1985. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316: 440–443 [DOI] [PubMed] [Google Scholar]
- Orchard C, Brette F 2008. T-tubules and sarcoplasmic reticulum function in cardiac ventricular myocytes. Cardiovasc Res 77: 237–244 [DOI] [PubMed] [Google Scholar]
- Parmacek MS, Solaro RJ 2004. Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis 47: 159–176 [DOI] [PubMed] [Google Scholar]
- Perets T, Blumenstein Y, Shistik E, Lotan I, Dascal N 1996. A potential site of functional modulation by protein kinase A in the cardiac Ca2+ channel α1C subunit. FEBS Lett 384: 189–192 [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron 22: 549–558 [DOI] [PubMed] [Google Scholar]
- Porter Moore C, Rodney G, Zhang JZ, Santacruz-Toloza L, Strasburg G, Hamilton SL 1999a. Apocalmodulin and Ca2+ calmodulin bind to the same region on the skeletal muscle Ca2+ release channel. Biochemistry 38: 8532–8537 [DOI] [PubMed] [Google Scholar]
- Porter Moore C, Zhang JZ, Hamilton SL 1999b. A role for cysteine 3635 of RYR1 in redox modulation and calmodulin binding. J Biol Chem 274: 36831–36834 [DOI] [PubMed] [Google Scholar]
- Pott C, Henderson SA, Goldhaber JI, Philipson KD 2007. Na+/Ca2+ exchanger knockout mice: Plasticity of cardiac excitation-contraction coupling. Ann NY Acad Sci 1099: 270–275 [DOI] [PubMed] [Google Scholar]
- Prins D, Michalak M 2011. Organellar calcium buffers. Cold Spring Harb Perspect Biol 3: a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L 1999. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc Natl Acad Sci 96: 2435–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H, Seitz N 1968. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol 195: 451–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads AR, Friedberg F 1997. Sequence motifs for calmodulin recognition. FASEB J 11: 331–340 [DOI] [PubMed] [Google Scholar]
- Ringer S 1883. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol 4: 29–42.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E, Brum G 1987. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325: 717–720 [DOI] [PubMed] [Google Scholar]
- Roderick HL, Higazi DR, Smyrnias I, Fearnley C, Harzheim D, Bootman MD 2007. Calcium in the heart: When it’s good, it’s very very good, but when it’s bad, it’s horrid. Biochem Soc Trans 35: 957–961 [DOI] [PubMed] [Google Scholar]
- Ruknudin A, He S, Lederer WJ, Schulze DH 2000. Functional differences between cardiac and renal isoforms of the rat Na+-Ca2+ exchanger NCX1 expressed in Xenopus oocytes. J Physiol 529 (Pt 3): 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulson MN, Scriven DR, Fletcher P, Moore ED 2011. Couplons in rat atria form distinct subgroups defined by their molecular partners. J Cell Sci 124: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM 2003. Sodium/calcium exchanger (NCX1) macromolecular complex. J Biol Chem 278: 28849–28855 [DOI] [PubMed] [Google Scholar]
- Sedarat F, Xu L, Moore ED, Tibbits GF 2000. Colocalization of dihydropyridine and ryanodine receptors in neonate rabbit heart using confocal microscopy. Am J Physiol Heart Circ Physiol 279: H202–H209 [DOI] [PubMed] [Google Scholar]
- Serysheva II 2004. Structural insights into excitation-contraction coupling by electron cryomicroscopy. Biochemistry (Moscow) 69: 1226–1232 [DOI] [PubMed] [Google Scholar]
- Serysheva II, Schatz M, van Heel M, Chiu W, Hamilton SL 1999. Structure of the skeletal muscle calcium release channel activated with Ca2+ and AMP-PCP. Biophys J 77: 1936–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham JS, Hatem SN, Morad M 1995. Species differences in the activity of the Na+-Ca2+ exchanger in mammalian cardiac myocytes. J Physiol 488 (Pt 3): 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Bers DM 2004. Integrated Ca2+ management in cardiac myocytes. Ann NY Acad Sci 1015: 28–38 [DOI] [PubMed] [Google Scholar]
- Shistik E, Ivanina T, Blumenstein Y, Dascal N 1998. Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J Biol Chem 273: 17901–17909 [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR 1986. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem 261: 13333–13341 [PubMed] [Google Scholar]
- Smyrnias I, Mair W, Harzheim D, Walker SA, Roderick HL, Bootman MD 2010. Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation-contraction coupling and inotropic stimulation. Cell Calcium 47: 210–223 [DOI] [PubMed] [Google Scholar]
- Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, et al. 2005a. Serine 68 of phospholemman is critical in modulation of contractility, [Ca2+]i transients, and Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 288: H2342–H2354 [DOI] [PubMed] [Google Scholar]
- Song LS, Guatimosim S, Gomez-Viquez L, Sobie EA, Ziman A, Hartmann H, Lederer WJ 2005b. Calcium biology of the transverse tubules in heart. Ann NY Acad Sci 1047: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumandea MP, Burkart EM, Kobayashi T, De Tombe PP, Solaro RJ 2004. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann NY Acad Sci 1015: 39–52 [DOI] [PubMed] [Google Scholar]
- Tada M, Kirchberger MA, Repke DI, Katz AM 1974. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 249: 6174–6180 [PubMed] [Google Scholar]
- Tadross MR, Dick IE, Yue DT 2008. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell 133: 1228–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi Y, Chu G, Kirkpatrick DM, Li Z, Wakasaki H, Kranias EG, King GL, Walsh RA 1998. In vivo phosphorylation of cardiac troponin I by protein kinase Cβ2 decreases cardiomyocyte calcium responsiveness and contractility in transgenic mouse hearts. J Clin Invest 102: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Halling DB, Black DJ, Pate P, Zhang JZ, Pedersen S, Altschuld RA, Hamilton SL 2003. Apocalmodulin and Ca2+ calmodulin-binding sites on the CaV1.2 channel. Biophys J 85: 1538–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S 2003. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci 100: 11759–11764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, et al. 2006. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res 98: 1151–1158 [DOI] [PubMed] [Google Scholar]
- Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S 1996. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem 271: 20385–20391 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Curotto Kurzydlowski K, Narayanan N, MacLennan DH 1994. Identification of Ser38 as the site in cardiac sarcoplasmic reticulum Ca2+-ATPase that is phosphorylated by Ca2+/calmodulin-dependent protein kinase. J Biol Chem 269: 26492–26496 [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ 1995. Rapid adaptation of cardiac ryanodine receptors: Modulation by Mg2+ and phosphorylation. Science 267: 1997–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Terentyev D, Gyorke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Gyorke S 2004. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res 94: 471–477 [DOI] [PubMed] [Google Scholar]
- Wagenknecht T, Radermacher M, Grassucci R, Berkowitz J, Xin HB, Fleischer S 1997. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J Biol Chem 272: 32463–32471 [DOI] [PubMed] [Google Scholar]
- Wang SQ, Song LS, Lakatta EG, Cheng H 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410: 592–596 [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, et al. 2003. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840 [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR 2004. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94: e61–e70 [DOI] [PubMed] [Google Scholar]
- Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR 1991. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem 266: 11144–11152 [PubMed] [Google Scholar]
- Wu Y, Colbran RJ, Anderson ME 2001a. Calmodulin kinase is a molecular switch for cardiac excitation-contraction coupling. Proc Natl Acad Sci 98: 2877–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dzhura I, Colbran RJ, Anderson ME 2001b. Calmodulin kinase and a calmodulin-binding “IQ” domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism. J Physiol 535: 679–687 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, et al. 2009. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci 106: 5972–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG 1994. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci 91: 9659–9663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Tian X, Jones PP, Bolstad J, Kong H, Wang R, Zhang L, Duff HJ, Gillis AM, Fleischer S, et al. 2007. Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J Biol Chem 282: 34828–34838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Narayanan N 1999. Ca2+/calmodulin-dependent phosphorylation of the Ca2+-ATPase, uncoupled from phospholamban, stimulates Ca2+-pumping in native cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 258: 66–72 [DOI] [PubMed] [Google Scholar]
- Xu L, Tripathy A, Pasek DA, Meissner G 1999. Ruthenium red modifies the cardiac and skeletal muscle Ca2+ release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem 274: 32680–32691 [DOI] [PubMed] [Google Scholar]
- Xu M, Zhou P, Xu SM, Liu Y, Feng X, Bai SH, Bai Y, Hao XM, Han Q, Zhang Y, et al. 2007. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol 5: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Zarain-Herzberg A 1994. Sarcoplasmic reticulum calsequestrins: Structural and functional properties. Mol Cell Biochem 135: 61–70 [DOI] [PubMed] [Google Scholar]
- Yuan W, Bers DM 1994. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol 267: H982–H993 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Qureshi A, Song J, Carl LL, Tian Q, Stahl RC, Carey DJ, Rothblum LI, Cheung JY 2003. Phospholemman modulates Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 284: H225–H233 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY 2006. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S 1997. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: Modulation by endogenous effectors, drugs and disease states. Pharmacol Rev 49: 1–51 [PubMed] [Google Scholar]
- Zuhlke RD, Reuter H 1998. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the α1C subunit. Proc Natl Acad Sci 95: 3287–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399: 159–162 [DOI] [PubMed] [Google Scholar]