Abstract
A variety of secretory cargoes move through the Golgi, but the pathways and mechanisms of this traffic are still being debated. Here, we evaluate the strengths and weaknesses of five current models for Golgi traffic: (1) anterograde vesicular transport between stable compartments, (2) cisternal progression/maturation, (3) cisternal progression/maturation with heterotypic tubular transport, (4) rapid partitioning in a mixed Golgi, and (5) stable compartments as cisternal progenitors. Each model is assessed for its ability to explain a set of key observations encompassing multiple cell types. No single model can easily explain all of the observations from diverse organisms. However, we propose that cisternal progression/maturation is the best candidate for a conserved core mechanism of Golgi traffic, and that some cells elaborate this core mechanism by means of heterotypic tubular transport between cisternae.
In diverse eukaryotes, a cisternal progression/maturation mechanism coupled with tubular transport between cisternae may best describe the pathways of Golgi traffic.
Golgi traffic is an ancient process (Klute et al. 2011). Certain core mechanisms are likely to be conserved in most or all eukaryotes, but these core mechanisms have undoubtedly been enhanced or modified during evolution. Among the options available in the endomembrane system are vesicular transport, tubular extensions and connections, homotypic fusion of compartments, and compartment maturation (Schnepf 1993; Rothman 1994; Becker et al. 1995; Schekman and Orci 1996; Bannykh and Balch 1997; Glick et al. 1997; Mironov et al. 1997; Marsh et al. 2004; Trucco et al. 2004; Rink et al. 2005). A unifying model should attempt to describe the core mechanisms of Golgi traffic while accounting for species-specific variations.
It is hardly an exaggeration to state that there are as many different models for Golgi traffic as there are Golgi researchers. This lack of consensus reflects the complexity and subtlety of the topic. We believe that a complete model for Golgi traffic should explain the following observations:
-
1.
In most eukaryotes, distinct Golgi compartments can be identified by morphological and biochemical criteria. The number of Golgi compartments is subject to debate (Mellman and Simons 1992), but a typical description includes cis, medial, and trans cisternae plus the trans-Golgi network (TGN) (Dunphy and Rothman 1985; Farquhar 1985). These compartments differ in their structure, cytochemical-staining properties, composition of resident Golgi enzymes, and ability to bud COPI- or clathrin-coated vesicles (Farquhar and Palade 1981; Kleene and Berger 1993; Rabouille et al. 1995; Staehelin and Kang 2008; Nilsson et al. 2009).
-
2.
Glycosylation enzymes in the Golgi show a polarized distribution that reflects the sequence of oligosaccharide processing reactions. In species ranging from mammals to fungi to plants, early-acting glycosylation enzymes are concentrated in cis-cisternae, whereas late-acting glycosylation enzymes are concentrated in trans-cisternae (Kleene and Berger 1993; Rabouille et al. 1995; Nilsson et al. 2009; Schoberer et al. 2010).
-
3.
Secretory cargoes can be observed to move across mammalian Golgi stacks in the cis-to-trans direction. Such “cargo waves” have been seen for both small secretory cargo proteins and large macromolecular aggregates (Bergmann and Singer 1983; Bonfanti et al. 1998; Mironov et al. 2001; Trucco et al. 2004).
-
4.
Golgi structure varies between organisms. Golgi cisternae are organized as individual cisternae in Saccharomyces cerevisiae (Preuss et al. 1992), as single stacks in many plants and unicellular organisms (Melkonian et al. 1991; Yelinek et al. 2009), as pairs of stacks in Drosophila (Kondylis and Rabouille 2009), or as a ribbon of laterally linked stacks in mammalian cells (Rambourg and Clermont 1990). In microsporidia, the Golgi appears to be a membrane network rather than a collection of discrete cisternae (Beznoussenko et al. 2007).
-
5.
COPI vesicles appear to be associated with Golgi structures in most eukaryotes. Electron microscopy and cell-free reconstitution identified COPI vesicles as intra-Golgi carriers (Rothman 1994). As judged by electron tomography, a mammalian Golgi stack may be surrounded by more than a thousand COPI vesicles (Ladinsky et al. 1999). Presumptive COPI vesicles are also abundantly visible around algal Golgi stacks (Farquhar and Palade 1981; Staehelin and Kang 2008). Although peri-Golgi vesicles are less evident in other cell types, COPI is broadly conserved (Dacks et al. 2009).
-
6.
COPI vesicles function in retrograde traffic. Strong biochemical and genetic data implicate COPI vesicles in recycling of proteins from the Golgi to the ER (Letourneur et al. 1994; Pelham 1994; Gaynor et al. 1998).
-
7.
Large secretory cargoes can traverse the Golgi. In diverse cell types, secretory cargoes much larger than COPI vesicles are transported through Golgi stacks. The best-characterized examples are cell surface scales in algae (Brown 1977; Melkonian et al. 1991) and procollagen aggregates in mammalian fibroblasts (Leblond 1989; Bonfanti et al. 1998; Mironov et al. 2001; Trucco et al. 2004).
-
8.
Morphological data suggest that Golgi cisternae form at the cis-face of the stack, and peel off and fragment at the trans-face. Electron micrographs of cell types ranging from plants to fungi to mammals suggest that cisternae assemble at the cis-face of a Golgi stack, and separate from the stack at the trans-face while undergoing fission (Morre and Ovtracht 1977; Ladinsky et al. 1999; Mogelsvang et al. 2003; Staehelin and Kang 2008).
-
9.
Resident Golgi proteins can move rapidly within and between compartments. Golgi proteins show overlapping distributions across multiple cisternae, and their concentrations in particular compartments evidently reflect the dynamics of membrane traffic rather than static localization mechanisms (Hoe et al. 1995; Rabouille et al. 1995; Harris and Waters 1996).
-
10.
Golgi compartments in Saccharomyces cerevisiae are transient. The individual Golgi cisternae in S. cerevisiae can be resolved by fluorescence microscopy (Wooding and Pelham 1998). Video imaging of S. cerevisiae cells revealed that over a time-course of several minutes, Golgi compartments exchange their early resident proteins for late resident proteins (Losev et al. 2006; Matsuura-Tokita et al. 2006).
-
11.
In mammalian cells, Golgi cisternae are sometimes linked by heterotypic tubular membrane connections within a single stack. These tubular connections are visible by electron tomography in actively secreting cells (Marsh et al. 2004; Trucco et al. 2004; San Pietro et al. 2009). Treatments that interfere with tubulation inhibit membrane traffic (San Pietro et al. 2009; Bechler et al. 2010).
-
12.
Small soluble secretory cargoes can traverse the mammalian Golgi stack very rapidly. Whereas cargoes such as VSV-G and procollagen require ∼15 minutes to traverse a Golgi stack, some cargoes such as albumin cross the entire stack in under two minutes (A Luini et al. in prep.).
-
13.
In mammalian cells, secretory cargoes exit the Golgi region with exponential kinetics. When fluorescent secretory cargo molecules were tracked over time, exit from the Golgi region followed first-order kinetics, suggesting that the fluorescent molecules were present in a long-lived and well-mixed compartment (Patterson et al. 2008).
In the following sections, we evaluate five current models for their ability to explain these observations.
MODEL 1: ANTEROGRADE VESICULAR TRANSPORT BETWEEN STABLE COMPARTMENTS
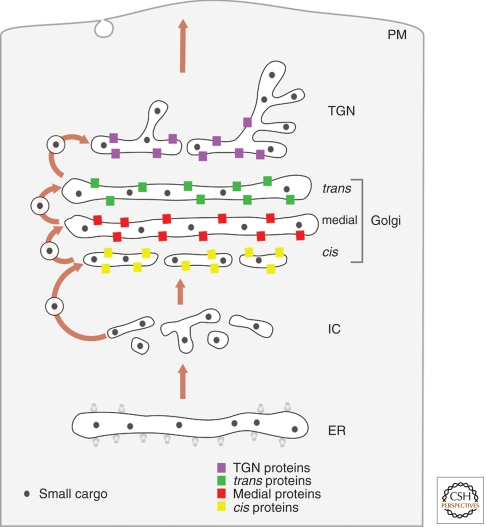
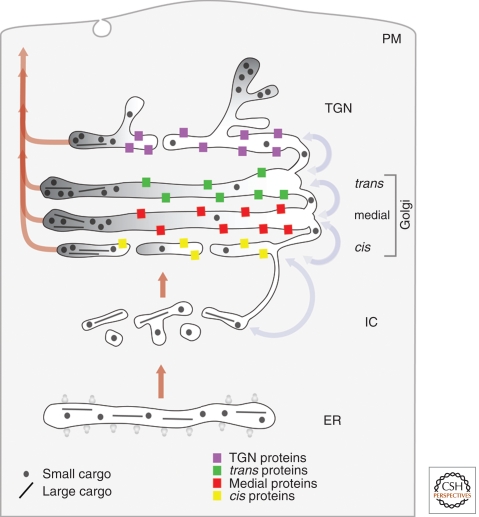
The vesicular transport model (Fig. 1) was widely accepted from the early 1980s until the late 1990s. The Golgi is viewed as a set of stable compartments operating in tandem (Farquhar and Palade 1981; Rothman 1981; Dunphy and Rothman 1985; Farquhar 1985). Each compartment would contain a unique set of resident Golgi proteins, including glycosylation enzymes that operate in assembly line fashion to process secretory cargoes (Kleene and Berger 1993; Rabouille et al. 1995; Nilsson et al. 2009). A newly synthesized secretory cargo would be delivered to the cis-Golgi in COPII-coated vesicles, and would then move from one Golgi compartment to the next in COPI-coated vesicles (Rothman and Wieland 1996). Resident Golgi proteins are assumed to be localized to specific compartments by exclusion from anterograde COPI vesicles. Updated versions of the stable compartments model propose that COPI vesicles move bidirectionally, with anterograde COPI vesicles carrying secretory cargoes forward whereas retrograde COPI vesicles recycle trafficking components (Orci et al. 2000b; Pelham and Rothman 2000).
Figure 1.
Anterograde vesicular transport between stable compartments. Secretory cargoes travel from the ER to the intermediate compartment (IC) and cis-Golgi in dissociative carriers. Golgi compartments are stable and biochemically distinct. Secretory cargoes move across the stack by means of COPI vesicles that bud from one compartment and fuse with the next, whereas resident Golgi proteins stay in place by being excluded from budding vesicles. This model does not provide a mechanism for transporting secretory cargoes that are too large to fit within COPI vesicles.
Strengths
This model can easily explain the existence of distinct Golgi compartments, the polarized distribution of Golgi glycosylation enzymes, and the existence of secretory cargo waves. Moreover, this model can accommodate much of the variability in Golgi structure, and it provides functions for COPI vesicles as bidirectional carriers. Because of these factors, the stable compartments concept remains appealing.
Some studies have identified secretory cargoes in COPI vesicles. Immunoelectron microscopy and biochemical analysis provided evidence that COPI vesicles contain several secretory cargoes, including VSV-G protein, proinsulin, and the polymeric immunoglobulin A receptor (Ostermann et al. 1993; Orci et al. 1997; Malsam et al. 2005).
COPI vesicles seem to come in two types, which could represent anterograde versus retrograde vesicles. Studies of both mammalian and plant cells have revealed the existence of two classes of COPI vesicles that differ in morphological appearance and protein composition (Orci et al. 1997; Malsam et al. 2005; Moelleken et al. 2007; Staehelin and Kang 2008).
Rapidly “percolating” vesicles could explain the fast intra-Golgi traffic of small secretory cargoes, as well as the exponential kinetics of secretory cargo exit from the Golgi region. It is possible that secretory cargoes move bidirectionally across the stack by rapid COPI vesicle-mediated transport (Orci et al. 2000b; Pelham and Rothman 2000). If bidirectional transport between cisternae is fast relative to exit from the Golgi, the exit kinetics should be exponential.
Weaknesses
The abundance of COPI vesicles varies dramatically between organisms and even within a given cell type. COPI vesicles around the mammalian Golgi actually seem to be less abundant during active traffic (Clermont et al. 1993; Rambourg et al. 1993).
A number of studies have not detected small secretory cargoes in COPI vesicles. Various investigators have analyzed the composition of COPI vesicles using immunoelectron microscopy or proteomics, and have concluded that secretory cargoes such as VSV-G protein and albumin are depleted rather than enriched in these vesicles (Dahan et al. 1994; Martinez-Menarguez et al. 2001; Gilchrist et al. 2006).
The evidence for anterograde COPI vesicle traffic is inconclusive, and the two types of COPI vesicles could both be retrograde carriers. Morphological analysis of plant and algal cells suggested that one type of COPI vesicle functions in Golgi-to-ER retrograde transport, whereas the second type is an intra-Golgi carrier that may recycle resident Golgi proteins (Staehelin and Kang 2008) (see Fig. 6B). A parsimonious interpretation of the data is that COPI vesicles act exclusively as retrograde carriers (Pelham 1994).
Vesicular transport would need to be unrealistically rapid and extensive to explain the fast intra-Golgi traffic of small secretory cargoes. Although bidirectional vesicular transport could theoretically explain how small secretory cargoes can sample the whole Golgi in a short time, calculations indicate that each cisterna would need to produce hundreds of vesicles per second (A Luini et al. in prep.).
This model cannot easily explain the traffic of large secretory cargoes, the fused Golgi network in microsporidia, the apparent formation and peeling off of Golgi cisternae, the mobility of resident Golgi enzymes between compartments, the transient nature of yeast Golgi cisternae, or the existence of heterotypic tubular connections between cisternae. In particular, the finding that large secretory cargoes can transit through the Golgi has long served as an argument against the generality of the stable compartments model (Becker et al. 1995; Bonfanti et al. 1998).
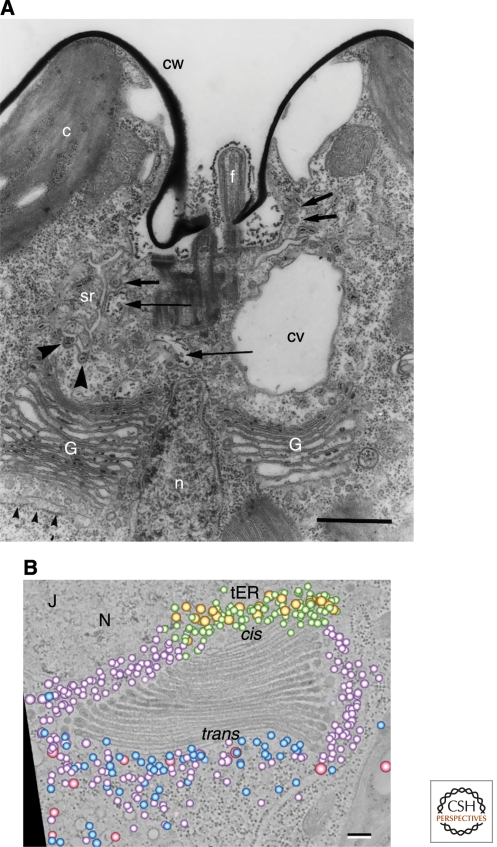
Figure 6.
Two views of Golgi stacks in the alga Scherffelia dubia. (A) Thin-section electron micrograph of the apical region of a S. dubia cell. The two Golgi stacks (G) are separated by the nucleus (n). Small arrowheads indicate the transitional ER, which is the site of ER exit. Electron-dense scales can be seen in Golgi cisternae but not in peri-Golgi vesicles. Scale bar, 1.0 µm. (Panel A adapted from Perasso et al. [2000] and reprinted with permission from Springer ©2000.) (B) An electron tomographic slice of a single S. dubia Golgi stack next to a transitional ER site (tER). Superimposed on this slice is a 3D model of the Golgi-associated vesicles, which were interpreted as falling into five classes: two morphologically distinct classes of COPI vesicles (purple and green), COPII vesicles (gold), secretory vesicles (blue), and clathrin-coated vesicles (pink). Scale bar, 100 nm. (Panel B reproduced, with permission, from Staehelin and Kang [2008].)
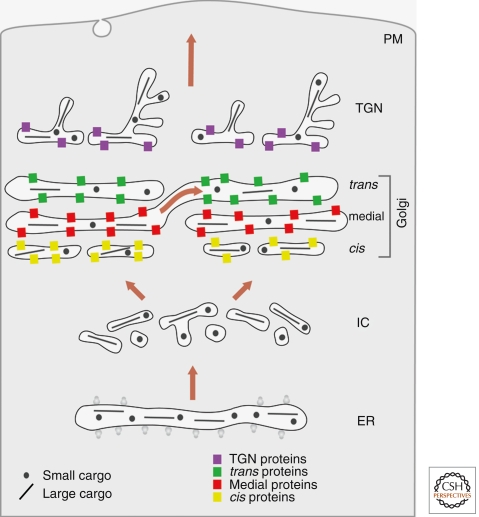
MODEL 2: CISTERNAL PROGRESSION/MATURATION
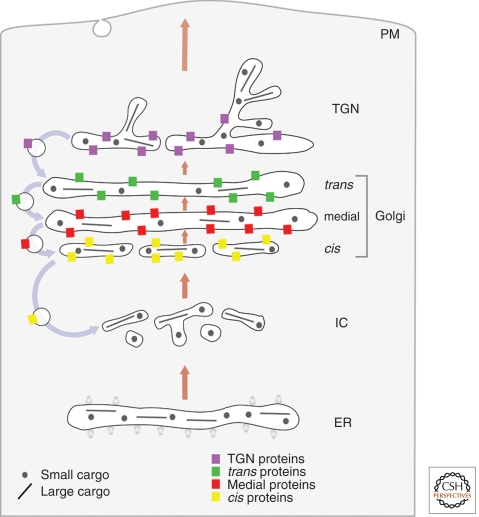
The cisternal progression/maturation model (Fig. 2) builds on the cisternal progression concept that was put forth by morphologists several decades ago (Grasse 1957; Morre and Ovtracht 1977). Cisternae are viewed as transient carriers. Homotypic fusion of COPII vesicles or other ER-derived carriers (Bannykh and Balch 1997; Mironov et al. 2003) is proposed to nucleate the formation of a new cis-cisterna, which gradually matures into a TGN cisterna, which in turn disintegrates into secretory vesicles and other types of carriers. As the cisternae carry the secretory cargoes forward (Bonfanti et al. 1998), COPI vesicles would recycle resident Golgi proteins from older to younger cisternae (Glick and Malhotra 1998; Rabouille and Klumperman 2005). Differential recycling efficiencies for different Golgi proteins could explain the biochemical polarity of the Golgi (Glick et al. 1997; Weiss and Nilsson 2000). It was recently postulated that the various Golgi compartments represent discrete kinetic stages of maturation, with the transition from one stage to the next being regulated by Rab GTPases (Glick and Nakano 2009).
Figure 2.
Cisternal progression/maturation. Secretory cargoes exit the ER in dissociative carriers, which coalesce with one another and with COPI vesicles derived from the cis-Golgi to form the intermediate compartment, which coalesces in turn to form a new cis-cisterna. In subsequent rounds of COPI-mediated recycling, the new cisterna matures by receiving medial and then trans-Golgi proteins from older cisternae while exporting cis and then medial-Golgi proteins to younger cisternae. Meanwhile, the cisterna progresses through the stack, carrying forward both small and large secretory cargoes. In the final stage of maturation, the cisterna is a TGN element that breaks down into anterograde and retrograde transport carriers.
Strengths
This model can readily explain the existence of distinct Golgi compartments, the polarized distribution of Golgi glycosylation enzymes, the existence of secretory cargo waves, the transport of large secretory cargoes, the apparent formation and peeling off of Golgi cisternae, the mobility of resident Golgi enzymes between compartments, and the transient nature of yeast Golgi cisternae. Moreover, this model can accommodate much of the variability in Golgi structure, and it provides a function for COPI vesicles as retrograde carriers. The idea that COPI vesicles recycle resident Golgi proteins resolves many of the issues that led to the downfall of the original cisternal progression model (Glick and Malhotra 1998).
Certain resident Golgi proteins have been convincingly identified as components of COPI vesicles, and some studies have identified Golgi glycosylation enzymes in COPI vesicles. There is general agreement that mammalian COPI vesicles contain the KDEL receptor (Orci et al. 1997; Aoe et al. 1998), Golgi-localized SNARE proteins (Kweon et al. 2004; Volchuk et al. 2004), and the tethering protein giantin (Sonnichsen et al. 1998). In some studies, resident Golgi glycosylation enzymes were also found to be concentrated in COPI vesicles (Martinez-Menarguez et al. 2001; Malsam et al. 2005; Gilchrist et al. 2006).
Weaknesses
Several groups have reported that Golgi glycosylation enzymes are actually depleted in COPI vesicles. Some immunoelectron microscopy studies have not detected significant levels of Golgi glycosylation enzymes in COPI vesicles, contradicting a key prediction of the cisternal progression/maturation model (Orci et al. 2000a; Cosson et al. 2002; Kweon et al. 2004).
This model cannot easily explain the fused Golgi network in microsporidia, the existence of heterotypic tubular connections between cisternae, the rapid intra-Golgi traffic of small secretory cargoes, or the exponential kinetics of secretory cargo exit from the Golgi region. In a simple progression/maturation model, all secretory cargoes should progress through the Golgi at the same rate and should exit the Golgi with linear kinetics. Recent studies have presented evidence against both of these predictions (Patterson et al. 2008; A Luini et al., in prep.). To explain the exponential kinetics of secretory cargo exit, the cisternal progression/maturation model would need to be modified to include a long-lived TGN or post-TGN compartment (Patterson et al. 2008; Glick and Nakano 2009).
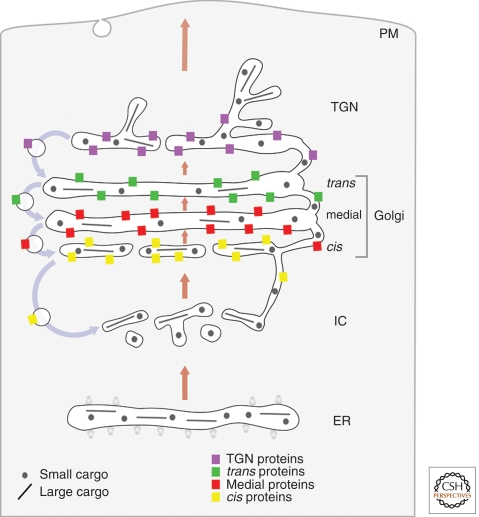
MODEL 3: CISTERNAL PROGRESSION/MATURATION WITH HETEROTYPIC TUBULAR TRANSPORT
The cisternal progression/maturation model can be extended to incorporate tubular connections between cisternae (Fig. 3). It is known that mammalian Golgi stacks are linked “horizontally” by homotypic tubular connections to form the Golgi ribbon (Rambourg and Clermont 1990), and for many years, the idea has been discussed that Golgi cisternae within a stack might also be linked “vertically” by heterotypic tubular connections (Mellman and Simons 1992; Weidman 1995; Mironov et al. 1997). Recent electron tomography studies have shown the presence of such heterotypic tubular connections (Marsh et al. 2004; Trucco et al. 2004). Meanwhile, functional studies have implicated enzymes of phospholipid metabolism in the generation of Golgi-derived membrane tubules, and have indicated that tubules are important for anterograde and retrograde traffic in the ER-Golgi system (Weigert et al. 1999; Brown et al. 2003; San Pietro et al. 2009; Schmidt and Brown 2009). Heterotypic tubular connections are proposed to complement cisternal progression/maturation by allowing either fast anterograde traffic of small secretory cargoes, or retrograde traffic of resident Golgi proteins, or both (San Pietro et al. 2009; A Luini et al. in prep.).
Figure 3.
Cisternal progression/maturation with heterotypic tubular transport. This model is identical to the cisternal progression/maturation model, except that cisternae within a given stack are connected by tubular continuities through which small secretory cargoes and resident Golgi proteins can diffuse. Tubular continuities may also exist between heterotypic cisternae in adjacent stacks (not shown).
Strengths
This model has all of the strengths of the cisternal progression/maturation model. In addition, it can explain the rapid intra-Golgi traffic of small secretory cargoes, and it provides functions for heterotypic tubular connections. Narrow tubular connections may provide a “fast track” that allows small secretory cargoes to traverse the Golgi without requiring extensive membrane transport. Moreover, these connections may allow Golgi glycosylation enzymes to recycle independently of COPI vesicles (San Pietro et al. 2009).
For small secretory cargoes, this model can explain the exponential kinetics of exit from the Golgi region. If a protein can diffuse rapidly between cisternae, this protein should behave as if the Golgi were a single well-mixed compartment.
The fused Golgi network in microsporidia can be viewed as a variation of a vertically connected Golgi stack. Heterotypic tubular connections may be so extensive in microsporidia that the Golgi is effectively a single compartment.
Weaknesses
This model cannot easily explain the exponential kinetics of exit of the large secretory cargo procollagen from the Golgi region. Procollagen is too large to diffuse through heterotypic tubular connections. Instead, procollagen seems to traverse Golgi stacks by cisternal progression, which should produce linear kinetics of exit from the Golgi region (Bonfanti et al. 1998; Patterson et al. 2008).
Heterotypic tubular connections between Golgi cisternae have not been convincingly described in fungal and plant cells, and the prevalence of these connections in mammalian cells is still debated. Different groups disagree about whether heterotypic tubular connections are common or rare in mammalian Golgi stacks (Martinez-Menarguez et al. 2001; Marsh et al. 2004; Trucco et al. 2004; Vivero-Salmeron et al. 2008; Mavillard et al. 2010). Moreover, such connections have not yet been detected by tomographic analysis of fungal and plant cells (Mogelsvang et al. 2003; Staehelin and Kang 2008). These caveats raise the possibility that heterotypic tubular connections are a specialization of certain cell types or a response to overloading the secretory pathway.
Questions remain about how Golgi compartmentation can be maintained in the presence of heterotypic membrane continuities. If Golgi cisternae are connected vertically, unknown mechanisms must exist to preserve gradients across the stack of resident Golgi protein distribution, lipid composition, and pH.
MODEL 4: RAPID PARTITIONING IN A MIXED GOLGI
A recent paper proposed a dramatic revision of traditional perspectives on the Golgi (Patterson et al. 2008; Lippincott-Schwartz and Phair 2010). According to the rapid partitioning model (Fig. 4), the Golgi operates as a single compartment that contains processing domains and export domains. Secretory cargoes would arrive from the ER, partition between the two domains of the Golgi, and then stochastically exit from every level of the Golgi to their final destinations. This model was inspired by the finding that multiple secretory cargoes exited the Golgi region with exponential kinetics. The concept of distinct domains within the Golgi is based on fluorescence microscopy data suggesting that VSV-G protein, a trans-membrane secretory cargo, was partially segregated from Golgi glycosylation enzymes (Patterson et al. 2008).
Figure 4.
Rapid partitioning in a mixed Golgi. As soon as secretory cargoes enter the Golgi, they equilibrate across the stack via intercisternal continuities. Secretory cargoes partition dynamically between processing domains, which contain resident Golgi proteins, and export domains. These various domains are established by segregation of different lipid species. Secretory cargoes can exit the Golgi at every level of the stack.
Strengths
This model can readily explain the transport of large secretory cargoes, the mobility of resident Golgi enzymes, the existence of heterotypic tubular connections between cisternae, the rapid intra-Golgi traffic of small secretory cargoes, and the exponential kinetics of exit of both small and large secretory cargoes from the Golgi region. Notably, a number of variations on the cisternal progression/maturation model were unable to explain the observed exponential kinetics of secretory cargo exit (Patterson et al. 2008).
The Golgi of microsporidia seems to be a single mixed compartment. This organism lacks obvious COPI vesicles, and evidently has a single Golgi compartment between the ER and plasma membrane (Beznoussenko et al. 2007).
Weaknesses
This model cannot easily explain the existence of discrete cisternae and distinct Golgi compartments in most eukaryotes, the polarized distribution of Golgi glycosylation enzymes, the existence of secretory cargo waves for procollagen, the apparent formation and peeling off of Golgi cisternae, or the transient nature of yeast Golgi cisternae. Moreover, this model provides no role for COPI vesicles. The assumption that secretory cargoes immediately sample the entire Golgi is troublesome, because Golgi enzyme distributions show a conserved cis-to-trans polarity that reflects the order of oligosaccharide processing reactions (Emr et al. 2009). Another serious concern is that although secretory cargo waves of VSV-G protein could be explained with a simulation involving diffusion and selective partitioning (Patterson et al. 2008), this explanation does not apply to the secretory cargo waves seen for the slowly diffusing procollagen (Bonfanti et al. 1998; Mironov et al. 2001; Trucco et al. 2004).
This model goes well beyond the experimental data. A general issue with the rapid partitioning model is that it invokes micron-scale “lipid raft” domains, yet lipid domains of this size have not been observed in cells (Eggeling et al. 2009).
MODEL 5: STABLE COMPARTMENTS AS CISTERNAL PROGENITORS
The newest attempt to explain how the Golgi works is the cisternal progenitor model (Fig. 5), although this model resembles earlier proposals (Griffiths 2000; Mironov et al. 2005). The Golgi is viewed as a set of stable compartments that are segregated into domains defined by Rab GTPases (Pfeffer 2010). This idea builds on studies indicating that in endosomes, Rab proteins can establish distinct domains within a membrane (Sonnichsen et al. 2000) and can drive the biochemical transformation of a compartment by a process known as “Rab conversion” (Rink et al. 2005; Nordmann et al. 2010; Poteryaev et al. 2010). Rab proteins can also promote the homotypic fusion of endosomes (Rink et al. 2005). By analogy, a domain in a Golgi cisterna is postulated to undergo Rab conversion followed by “homotypic” anterograde fusion with a matching Rab domain in a later cisterna from an adjacent Golgi stack. Thus, secretory cargoes could move forward through the Golgi by transferring back and forth between adjacent stacks. Alternatively, a Rab domain could pinch off from a cisterna to create a “megavesicle,” which would then fuse with a later cisterna from the same Golgi stack. The transfers of Rab domains are presumed to operate in conjunction with COPI-mediated vesicular transport (Pfeffer 2010).
Figure 5.
Stable compartments as cisternal progenitors. Secretory cargoes travel from the ER to the intermediate compartment and cis-Golgi in dissociative carriers. Golgi compartments are stable and biochemically distinct, but can segregate into domains by a Rab conversion process. A domain in a cis-cisterna undergoes Rab conversion and acquires medial character, resulting in a transient “homotypic” fusion with a medial cisterna in an adjacent stack. Small and large secretory cargoes can move forward through the resulting connection. Similar domain fusion events in later compartments enable secretory cargoes to traverse the entire stack.
Strengths
This model can potentially explain the existence of distinct Golgi compartments, the polarized distribution of Golgi glycosylation enzymes, the existence of secretory cargo waves, the transport of large secretory cargoes, the mobility of resident Golgi enzymes, the existence of heterotypic tubular connections between cisternae, the rapid intra-Golgi traffic of small secretory cargoes, and the fused Golgi network in microsporidia. Because the cisternal progenitor model encompasses a range of possible mechanisms, including heterotypic connections as well as traffic by conventional vesicles or megavesicles, this model can be viewed as consistent with multiple observations.
Studies of mammalian endosomes and yeast secretion supply precedents for the proposed mechanisms. Rab5 can promote homotypic fusion of early endosomes, and early endosomes marked by Rab5 undergo conversion into late endosomes marked by Rab7 (Rink et al. 2005; Nordmann et al. 2010; Poteryaev et al. 2010). In yeast, a Rab conversion mechanism appears to operate during TGN-to-cell surface transport (Rivera-Molina and Novick 2009).
This model predicts the possible existence of megavesicle transport intermediates, and putative megavesicles were described in one study. Such megavesicles could transport large cargoes within a single Golgi stack (Volchuk et al. 2000).
Weaknesses
This model cannot easily explain some of the variations in Golgi structure, the apparent formation and peeling off of Golgi cisternae, or the transient nature of yeast Golgi cisternae. Moreover, this model provides no specific role for COPI vesicles. The cisternal progenitor model is most appropriate for animal cells, in which Golgi stacks can be arranged in adjacent pairs or in a laterally linked ribbon (Rambourg and Clermont 1990; Kondylis and Rabouille 2009). However, individual Golgi stacks are found in plants, algae, and fungi (Melkonian et al. 1991; Staehelin and Kang 2008; Yelinek et al. 2009), making domain transfer between stacks improbable. In S. cerevisiae, many of the Golgi compartments mature as individual structures without undergoing obvious fusion or fission (Losev et al. 2006; Matsuura-Tokita et al. 2006).
Megavesicles have not generally been observed as intra-Golgi carriers. Careful morphological studies of procollagen-secreting fibroblasts and scale-producing algae have visualized the large secretory cargoes within cisternae, but not within megavesicle-type carriers (Melkonian et al. 1991; Bonfanti et al. 1998; Trucco et al. 2004). A thin-section micrograph interpreted as showing a discontinuity in a mammalian Golgi cisterna (Pfeffer 2010) probably represented a perforation (“well”) spanning multiple cisternae (Ladinsky et al. 1999; Staehelin and Kang 2008).
The lack of specificity of the cisternal progenitor model poses a challenge for making testable predictions. For example, it is unclear how to predict the kinetics of secretory cargo exit from the Golgi region. The existence of Rab conversion in the Golgi has been proposed as a test of the cisternal progenitor model (Pfeffer 2010), but Rab conversion is an equally plausible mechanism for cisternal maturation (Glick and Nakano 2009). Indeed, Rab conversion apparently underlies the maturation of early endosomes into late endosomes (Rink et al. 2005; Nordmann et al. 2010; Poteryaev et al. 2010), suggesting that an analogous process could occur in the Golgi.
CONCLUSIONS
The models described here differ in fundamental ways, but they all offer insights into Golgi traffic. Emerging technologies will help us to distinguish between the various proposals (Rothman 2010). Meanwhile, we can offer some tentative conclusions.
A crucial point is that ideas about the Golgi are necessarily constrained by microscopy. Our models must fit with what we see in different cell types. By this criterion, cisternal progression/maturation is still the best candidate for a broadly conserved mechanism that operates in most eukaryotes. This model provides the most compelling explanation for key findings from mammalian, fungal, plant, and protist cells. An illustration is given in Figure 6, which reproduces previously published images of Golgi stacks in the alga Scherffelia dubia. Scale-forming algae can serve as a “reality check” for models of Golgi traffic (Becker et al. 1995). Figure 6A shows a thin-section electron micrograph of S. dubia (Perasso et al. 2000). Two Golgi stacks are visible, but they are separated by the nucleus, strongly suggesting that each stack operates as an independent trafficking device. Figure 6B shows a tomographic slice from a 3D reconstruction of a rapidly frozen S. dubia cell. The excellent preservation reveals that Golgi cisternae are separate compartments that are relatively smooth and uninterrupted. This Golgi stack is surrounded by vesicles, presumably of the COPI variety, but peri-Golgi vesicles in algae do not contain scales (Melkonian et al. 1991). Images of this type have long been viewed as support for cisternal progression/maturation (Becker et al. 1995), and the evidence remains persuasive.
At the same time, a basic cisternal progression/maturation model is unable to explain all of the data. There is growing evidence that heterotypic tubular connections are important for Golgi traffic in mammalian cells. Such connections may play equally important roles in other cell types, and in microsporidia, they may have evolved as the dominant mode of Golgi traffic. We therefore propose Model 3 (Cisternal Progression/Maturation with Heterotypic Tubular Transport) as a working hypothesis to guide future experimentation. Among the central issues to be explored are the contents and directionality of COPI vesicles (Rabouille and Klumperman 2005; Rothman 2010), the possible existence of a long-lived TGN or post-TGN compartment (Glick and Nakano 2009), and the role of Rab GTPases in Golgi dynamics (Pfeffer 2010).
ACKNOWLEDGMENTS
B.S.G. acknowledges support from NIH grant GM-61156. A.L. would like to thank Telethon, AIRC (Associazione Italiana per la Ricerca sul Cancro), the MIUR (Ministero dell’ Universita’ e della Ricerca), the EU (FP7), and the Fondazione per la Ricerca sulla Fibrosi Cistica for financial support.
Footnotes
Editors: Graham Warren and James Rothman
Additional Perspectives on The Golgi available at www.cshperspectives.org
REFERENCES
- Aoe T, Lee AJ, van Donselaar E, Peters PJ, Hsu VW 1998. Modulation of intracellular transport by transported proteins: Insight from regulation of COPI-mediated transport. Proc Natl Acad Sci 95: 1624–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Balch WE 1997. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol 138: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler ME, Doody AM, Racoosin E, Lin L, Lee KH, Brown WJ 2010. The phospholipase complex PAFAH Ib regulates the functional organization of the Golgi complex. J Cell Biol 190: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Bolinger B, Melkonian M 1995. Anterograde transport of algal scales through the Golgi complex is not mediated by vesicles. Trends Cell Biol 5: 305–307 [DOI] [PubMed] [Google Scholar]
- Bergmann JE, Singer SJ 1983. Immunoelectron microscopic studies of the intracellular transport of the membrane glycoprotein (G) of vesicular stomatitis virus in infected Chinese hamster ovary cells. J Cell Biol 97: 1777–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beznoussenko GV, Dolgikh VV, Seliverstova EV, Semenov PB, Tokarev YS, Trucco A, Micaroni M, Di Giandomenico D, Auinger P, Senderskiy IV, et al. 2007. Analogs of the Golgi complex in microsporidia: Structure and avesicular mechanisms of function. J Cell Sci 120: 1288–1298 [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AA Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A 1998. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: Evidence for cisternal maturation. Cell 95: 993–1003 [DOI] [PubMed] [Google Scholar]
- Brown RM Jr, Willison JHM 1977. Golgi apparatus and plasma membrane involvement in secretion and cell surface deposition. In International cell biology (eds Brinkley BR, Porter KR), pp. 267–283 Rockefeller University Press, New York [Google Scholar]
- Brown WJ, Chambers K, Doody A 2003. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 4: 214–221 [DOI] [PubMed] [Google Scholar]
- Clermont Y, Xia L, Rambourg A, Turner JD, Hermo L 1993. Structure of the Golgi apparatus in stimulated and nonstimulated acinar cells of mammary glands of the rat. Anat Rec 237: 308–317 [DOI] [PubMed] [Google Scholar]
- Cosson P, Amherdt M, Rothman JE, Orci L 2002. A resident Golgi protein is excluded from peri-Golgi vesicles in NRK cells. Proc Natl Acad Sci 99: 12831–12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks JB, Peden AA, Field MC 2009. Evolution of specificity in the eukaryotic endomembrane system. Int J Biochem Cell Biol 41: 330–340 [DOI] [PubMed] [Google Scholar]
- Dahan S, Ahluwalia JP, Wong L, Posner BI, Bergeron JJ 1994. Concentration of intracellular hepatic apolipoprotein E in Golgi apparatus saccular distensions and endosomes. J Cell Biol 127: 1859–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Rothman JE 1985. Compartmental organization of the Golgi stack. Cell 42: 13–21 [DOI] [PubMed] [Google Scholar]
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schonle A, et al. 2009. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457: 1159–1162 [DOI] [PubMed] [Google Scholar]
- Emr S, Glick BS, Linstedt AD, Lippincott-Schwartz J, Luini A, Malhotra V, Marsh BJ, Nakano A, Pfeffer SR, Rabouille C, et al. 2009. Journeys through the Golgi–taking stock in a new era. J Cell Biol 187: 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG 1985. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol 1: 447–488 [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE 1981. The Golgi apparatus (complex)—(1954–1981)—from artifact to center stage. J Cell Biol 91: 77s–103s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Graham TR, Emr SD 1998. COPI in ER/Golgi and intra-Golgi transport: Do yeast COPI mutants point the way? Biochim Biophys Acta 1404: 33–51 [DOI] [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, et al. 2006. Quantitative proteomics analysis of the secretory pathway. Cell 127: 1265–1281 [DOI] [PubMed] [Google Scholar]
- Glick BS, Malhotra V 1998. The curious status of the Golgi apparatus. Cell 95: 883–889 [DOI] [PubMed] [Google Scholar]
- Glick BS, Nakano A 2009. Membrane traffic within the Golgi apparatus. Annu Rev Cell Dev Biol 25: 113–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Elston T, Oster G 1997. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett 414: 177–181 [DOI] [PubMed] [Google Scholar]
- Grasse PP 1957. Ultrastructure, polarity and reproduction of Golgi apparatus. C R Hebd Seances Acad Sci 245: 1278–1281 [PubMed] [Google Scholar]
- Griffiths G 2000. Gut thoughts on the Golgi complex. Traffic 1: 738–745 [DOI] [PubMed] [Google Scholar]
- Harris SL, Waters MG 1996. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J Cell Biol 132: 985–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe MH, Slusarewicz P, Misteli T, Watson R, Warren G 1995. Evidence for recycling of the resident medial/trans Golgi enzyme, N-acetylglucosaminyltransferase I, in ldlD cells. J Biol Chem 270: 25057–25063 [DOI] [PubMed] [Google Scholar]
- Kleene R, Berger EG 1993. The molecular and cell biology of glycosyltransferases. Biochim Biophys Acta 1154: 283–325 [DOI] [PubMed] [Google Scholar]
- Klute MJ, Melançon P, Dacks JB 2011. Evolution and diversity of the Golgi. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a007849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis V, Rabouille C 2009. The Golgi apparatus: Lessons from Drosophila. FEBS Lett 583: 3827–3838 [DOI] [PubMed] [Google Scholar]
- Kweon HS, Beznoussenko GV, Micaroni M, Polishchuk RS, Trucco A, Martella O, Di Giandomenico D, Marra P, Fusella A, Di Pentima A, et al. 2004. Golgi enzymes are enriched in perforated zones of Golgi cisternae but are depleted in COPI vesicles. Mol Biol Cell 15: 4710–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA 1999. Golgi structure in three dimensions: Functional insights from the normal rat kidney cell. J Cell Biol 144: 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP 1989. Synthesis and secretion of collagen by cells of connective tissue, bone, and dentin. Anat Rec 224: 123–138 [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P 1994. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79: 1199–1207 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Phair RD 2010. Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu Rev Biophys 39: 559–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS 2006. Golgi maturation visualized in living yeast. Nature 441: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Malsam J, Satoh A, Pelletier L, Warren G 2005. Golgin tethers define subpopulations of COPI vesicles. Science 307: 1095–1098 [DOI] [PubMed] [Google Scholar]
- Marsh BJ, Volkmann N, McIntosh JR, Howell KE 2004. Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet β cells. Proc Natl Acad Sci 101: 5565–5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Menarguez JA, Prekeris R, Oorschot VM, Scheller R, Slot JW, Geuze HJ, Klumperman J 2001. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J Cell Biol 155: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A 2006. Live imaging of yeast Golgi cisternal maturation. Nature 441: 1007–1010 [DOI] [PubMed] [Google Scholar]
- Mavillard F, Hidalgo J, Megias D, Levitsky KL, Velasco A 2010. PKA-mediated Golgi remodeling during cAMP signal transmission. Traffic 11: 90–109 [DOI] [PubMed] [Google Scholar]
- Melkonian M, Becker B, Becker D 1991. Scale formation in algae. J Electron Microsc Tech 17: 165–178 [DOI] [PubMed] [Google Scholar]
- Mellman I, Simons K 1992. The Golgi complex: In vitro veritas? Cell 68: 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov AA, Weidman P, Luini A 1997. Variations on the intracellular transport theme: Maturing cisternae and trafficking tubules. J Cell Biol 138: 481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov AA, Beznoussenko GV, Nicoziani P, Martella O, Trucco A, Kweon HS, Di Giandomenico D, Polishchuk RS, Fusella A, Lupetti P, et al. 2001. Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae. J Cell Biol 155: 1225–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov AA, Mironov AA Jr, Beznoussenko GV, Trucco A, Lupetti P, Smith JD, Geerts WJ, Koster AJ, Burger KN, Martone ME, et al. 2003. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell 5: 583–594 [DOI] [PubMed] [Google Scholar]
- Mironov AA, Beznoussenko GV, Polishchuk RS, Trucco A 2005. Intra-Golgi transport: A way to a new paradigm? Biochim Biophys Acta 1744: 340–350 [DOI] [PubMed] [Google Scholar]
- Moelleken J, Malsam J, Betts MJ, Movafeghi A, Reckmann I, Meissner I, Hellwig A, Russell RB, Sollner T, Brugger B, et al. 2007. Differential localization of coatomer complex isoforms within the Golgi apparatus. Proc Natl Acad Sci 104: 4425–4430 [DOI] [PubMed] [Google Scholar]
- Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA 2003. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell 14: 2277–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morre DJ, Ovtracht L 1977. Dynamics of the Golgi apparatus: Membrane differentiation and membrane flow. Int Rev Cytol Suppl 5: 61–188 [PubMed] [Google Scholar]
- Nilsson T, Au CE, Bergeron JJ 2009. Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett 583: 3764–3769 [DOI] [PubMed] [Google Scholar]
- Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C 2010. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol 20: 1654–1659 [DOI] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner TH, Rothman JE 1997. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell 90: 335–349 [DOI] [PubMed] [Google Scholar]
- Orci L, Amherdt M, Ravazzola M, Perrelet A, Rothman JE 2000a. Exclusion of golgi residents from transport vesicles budding from Golgi cisternae in intact cells. J Cell Biol 150: 1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Volchuk A, Engel T, Gmachl M, Amherdt M, Perrelet A, Sollner TH, Rothman JE 2000b. Anterograde flow of cargo across the golgi stack potentially mediated via bidirectional “percolating” COPI vesicles. Proc Natl Acad Sci 97: 10400–10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman JE 1993. Stepwise assembly of functionally active transport vesicles. Cell 75: 1015–1025 [DOI] [PubMed] [Google Scholar]
- Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J 2008. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell 133: 1055–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HR 1994. About turn for the COPs? Cell 79: 1125–1127 [DOI] [PubMed] [Google Scholar]
- Pelham HR, Rothman JE 2000. The debate about transport in the Golgi–two sides of the same coin? Cell 102: 713–719 [DOI] [PubMed] [Google Scholar]
- Perasso L, Grunow A, Brüntrup IM, Bölinger B, Melkonian M, Becker B 2000. The Golgi apparatus of the scaly green flagellate Scherffelia dubia: Uncoupling of glycoprotein and polysaccharide synthesis during flagellar regeneration. Planta 210: 551–562 [DOI] [PubMed] [Google Scholar]
- Pfeffer SR 2010. How the Golgi works: A cisternal progenitor model. Proc Natl Acad Sci 107: 19614–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A 2010. Identification of the switch in early-to-late endosome transition. Cell 141: 497–508 [DOI] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D 1992. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell 3: 789–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Klumperman J 2005. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol 6: 812–817 [DOI] [PubMed] [Google Scholar]
- Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T 1995. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci 108: 1617–1627 [DOI] [PubMed] [Google Scholar]
- Rambourg A, Clermont Y 1990. Three-dimensional electron microscopy: Structure of the Golgi apparatus. Eur J Cell Biol 51: 189–200 [PubMed] [Google Scholar]
- Rambourg A, Clermont Y, Chretien M, Olivier L 1993. Modulation of the Golgi apparatus in stimulated and nonstimulated prolactin cells of female rats. Anat Rec 235: 353–362 [DOI] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M 2005. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122: 735–749 [DOI] [PubMed] [Google Scholar]
- Rivera-Molina FE, Novick PJ 2009. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci 106: 14408–14413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE 1981. The golgi apparatus: Two organelles in tandem. Science 213: 1212–1219 [DOI] [PubMed] [Google Scholar]
- Rothman JE 1994. Mechanisms of intracellular protein transport. Nature 372: 55–63 [DOI] [PubMed] [Google Scholar]
- Rothman JE 2010. The future of Golgi research. Mol Biol Cell 21: 3776–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT 1996. Protein sorting by transport vesicles. Science 272: 227–234 [DOI] [PubMed] [Google Scholar]
- San Pietro E, Capestrano M, Polishchuk EV, DiPentima A, Trucco A, Zizza P, Mariggio S, Pulvirenti T, Sallese M, Tete S, et al. 2009. Group IV phospholipase A(2)α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol 7: e1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L 1996. Coat proteins and vesicle budding. Science 271: 1526–1533 [DOI] [PubMed] [Google Scholar]
- Schmidt JA, Brown WJ 2009. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J Cell Biol 186: 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf E 1993. Golgi apparatus and slime secretion in plants: The early implications and recent models of membrane traffic. Protoplasma 172: 3–11 [Google Scholar]
- Schoberer J, Runions J, Steinkellner H, Strasser R, Hawes C, Osterrieder A 2010. Sequential depletion and acquisition of proteins during Golgi stack disassembly and reformation. Traffic 11: 1429–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B, Lowe M, Levine T, Jamsa E, Dirac-Svejstrup B, Warren G 1998. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol 140: 1013–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149: 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Kang BH 2008. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol 147: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, San Pietro E, Beznoussenko GV, Polishchuk EV, Baldassarre M, et al. 2004. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol 6: 1071–1081 [DOI] [PubMed] [Google Scholar]
- Vivero-Salmeron G, Ballesta J, Martinez-Menarguez JA 2008. Heterotypic tubular connections at the endoplasmic reticulum-Golgi complex interface. Histochem Cell Biol 130: 709–717 [DOI] [PubMed] [Google Scholar]
- Volchuk A, Amherdt M, Ravazzola M, Brugger B, Rivera VM, Clackson T, Perrelet A, Sollner TH, Rothman JE, Orci L 2000. Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell 102: 335–348 [DOI] [PubMed] [Google Scholar]
- Volchuk A, Ravazzola M, Perrelet A, Eng WS, Di Liberto M, Varlamov O, Fukasawa M, Engel T, Sollner TH, Rothman JE, et al. 2004. Countercurrent distribution of two distinct SNARE complexes mediating transport within the Golgi stack. Mol Biol Cell 15: 1506–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman PJ 1995. Anterograde transport through the Golgi complex: Do Golgi tubules hold the key? Trends Cell Biol 5: 302–305 [DOI] [PubMed] [Google Scholar]
- Weigert R, Silletta MG, Spano S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, et al. 1999. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402: 429–433 [DOI] [PubMed] [Google Scholar]
- Weiss M, Nilsson T 2000. Protein sorting in the Golgi apparatus: A consequence of maturation and triggered sorting. FEBS Lett 486: 2–9 [DOI] [PubMed] [Google Scholar]
- Wooding S, Pelham HR 1998. The dynamics of golgi protein traffic visualized in living yeast cells. Mol Biol Cell 9: 2667–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelinek JT, He CY, Warren G 2009. Ultrastructural study of Golgi duplication in Trypanosoma brucei. Traffic 10: 300–306 [DOI] [PubMed] [Google Scholar]