Abstract
Previously, we reported that oral feeding of 1% green tea polyphenols (GTPs) aggravated the dextran sulfate sodium (DSS)-induced colitis in mice. In the present study, we assessed the toxicity of 1% GTPs in several organs from normal and DSS-exposed mice. Sixty-two male ICR mice were initially divided into four groups. Non-treated group (group 1, n = 15) was given standard diet and water, GTPs (group 2, n = 15) received 1% GTPs in diet and water, DSS (group 3, n = 15) received diet and 5% DSS in water, and GTPs + DSS group (group 4, n = 17) received 1% GTPs in diet and 5% DSS in water. We found that group 4 significantly increased (P < 0.05) kidney weight, the levels of serum creatinine and thiobarbituric acid-reactive substances in both kidney and liver, as compared with those in group 3. The mRNA expression levels of antioxidant enzymes and heat-shock proteins (HSPs) in group 4 were lower than those of group 3. For instance, heme oxygenase-1 (HO-1), HSP27, and 90 mRNA in the kidney of group 4 were dramatically down-regulated as compared with those of group 3. Furthermore, 1% GTPs diet decreased the expression of HO-1, NAD(P)H:quinone oxidoreductase 1 (NQO1) and HSP90 in kidney and liver of non-treated mice. Taken together, our results indicate that high-dose GTPs diet disrupts kidney functions through the reduction of antioxidant enzymes and heat-shock protein expressions in not only colitis but also non-treated ICR mice.
Keywords: Green tea polyphenols, Nephrotoxicity, Antioxidant enzyme, Heat shock protein, Colitis
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease, is a group of chronic disorders of the intestinal tract characterized by excessive production of reactive oxygen species (ROS) and cytokines (Araki et al. 2003). The etiology of IBD is believed to involve inappropriate host responses to complex commensal microbial flora in the gut and originates from mucosal barrier dysfunction, such as an abnormal leaky mucus layer, altered tight junction protein expression, and increased epithelial apoptosis (Araki et al. 2010).
To study the mechanisms of action underlying IBD, dextran sulfate sodium (DSS)-induced colitis models have been used in many laboratories including ours (Kwon et al. 2005). DSS exhibits toxic effects toward colonic epithelium and destroys the mucosal barrier, allowing bacteria to contact lamina propria cells (Kitajima et al. 1999a, b). Excess generation of ROS caused by the gut microenvironment breaks intestinal antioxidant systems in mice with DSS-induced colitis, thereby contributing to intestinal oxidative injury and initiating pro-inflammatory signaling (Tanaka et al. 2007). In addition, several studies have demonstrated that inhibition of an antioxidant enzyme, heme oxygenase-1 (HO-1), leads to aggravation of DSS-induced colitis (Paul et al. 2005; Wang et al. 2001).
Heat shock proteins (HSPs) are a class of stress-inducible proteins that play roles as molecular chaperones and protect cells against proteotoxic damage from a variety of physiological and environmental stimuli (Musch et al. 1996; Wischmeyer et al. 1997). Interestingly, the expressions of HSP70 and HSP27 were found to be down-regulated in actively inflamed mucosa from individuals with IBD (Bhattacharrya et al. 2009; Köken et al. 2004). It is worth noting that the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin, were also found to be increased in IBD, while it down-regulated HSP70 by targeting its translation stage (Hu et al. 2007). The above background suggests that inflammatory signaling molecules aggravate colitis by down-regulating HSPs, thereby disrupting intestinal homeostasis.
Green tea is a popular and widely consumed beverage. It contains characteristic polyphenolic constituents, generally known as green tea polyphenols (GTPs), which include (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin gallate, (−)-epigallocatechin, and (−)-epicatechin. (Isemura et al. 2000). EGCG, the most abundant polyphenol, has versatile preventive effects toward several chronic diseases including cancer, inflammation, heart disease, diabetes, and neurodegenerative diseases (Chung et al. 2001; Li et al. 2010; Hosakawa et al. 2010; Suganuma et al. 1998; Paquay et al. 2000; Cai and Lin 2009; Rezai-Zadeh et al. 2008). In addition, GTPs are strong antioxidants against ROS as well as inducers of several antioxidant proteins [HO-1, NAD(P)H:quinone oxidoreductase 1 (NQO1), glutathione S-transferase pi (GSTP1), manganese superoxide dismutase] (Sahin et al. 2010; Ogborne et al. 2008; Na et al. 2008).
Recently, high-dose EGCG was reported to induce hepatotoxicity, as demonstrated by increased formation of malonyldialdehyde (MDA) and 4-hydroxynonenal (4-HNE) (Lambert et al. 2010). Along a similar line, we found that EGCG enhanced the expression of pro-matrix metalloproteinase-7 by inducing oxidative stress in HT-29 human colorectal cancer cells (Kim et al. 2007). Furthermore, a 1% GTP diet enhanced pro-inflammatory cytokines, aggravated colitis, and tended to promote colon carcinogenesis in DSS-exposed colons, while it decreased the activities of superoxide dismutase (SOD) and catalase in non-treated mice (Kim et al. 2010). In addition, several human cases of hepatotoxicity following consumption of dietary supplementation containing green tea extracts have been reported (Mazzanti et al. 2009).
In the present study, oral feeding of 1% GTPs caused kidney and liver dysfunctions, as revealed by increases in serum aspartate 2-oxoglutarate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine, as well as thiobarbituric acid-reactive substances (TBARS) levels in kidneys and livers, together with down-regulation of antioxidant enzymes and HSPs, in both normal and DSS-treated ICR mice.
Materials and methods
Chemicals
A GTP mixture containing 70% total catechins, 35% EGCG, and 3% caffeine was obtained from LKT laboratories, (West St. Paul, MN). DSS with a molecular weight of 36–50 kDa was purchased from MP Biomedicals, (LLC Aurora, OH). All other chemicals and kits were obtained from Wako Pure Chemical Industries (Osaka, Japan), unless specified otherwise.
Animals
Male-specific pathogen-free ICR mice (17–19 g, 4 weeks old) were purchased from Japan SLC (Shizuoka, Japan) and housed five per cage. All mice were fed rodent MF pellets (Oriental Yeast, Kyoto, Japan) and given fresh tap water ad libitum, while being kept at 22–26°C with a relative humidity of 55–65% under a 12-h (0600–1800 hours) light/dark cycle for 6 days prior to the experiment. The mice were treated in accordance with the “Guidelines for the Treatment of Experimental Animals” of Kyoto University and the experimental protocol was approved by the Experimentation Committee of the same institution (approval number 21-42).
Experimental design
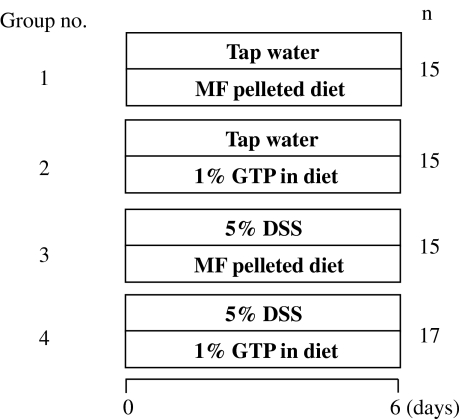
The experimental design is illustrated in Fig. 1. Mice were randomly divided into four groups: non-treated (group 1), GTPs-treated (group 2), DSS-treated (group 3), and GTPs + DSS-treated (group 4). The GTP-fed groups were given a diet containing 1% GTPs. DSS-treated groups were given 5% DSS (w/v) in water ad libitum, which induced experimental colitis. Body weights and food intake of each group were recorded each day until the end of experiment. All mice were euthanized by deep anesthesia with diethyl ether for determining the effects of dietary GTPs on DSS-induced colitis.
Fig. 1.
Experimental groups. Experimental colitis was induced in male ICR mice by administrating 5% DSS in drinking water ad libitum throughout the experimental period. The non-treated group (group 1, n = 15) was given tap water and a basal diet ad libitum, changed to fresh every day, for 6 days. The GTPs group (group 2, n = 15) was given tap water and fed a diet containing 1% GTPs for 6 days. The DSS group (group 3, n = 15) was fed a basal diet and given 5% DSS (w/v) in tap water for 6 days to induce colitis. The 1% GTPs + 5% DSS group (group 4, n = 17) was fed a diet containing 1% GTPs and given 5% DSS (w/v) in tap water for 6 days
RNA extraction and reverse transcription polymer chain reaction analysis
Total RNA was prepared using Trizol (Invitrogen, Tokyo, Japan), as described in the manufacturer's instructions. For reverse transcription (RT-PCR) analysis, 1 μg of RNA was reverse transcribed using an RNA PCR kit (TaKaRa, Shiga, Japan) with oligo dT-adaptor primer, as recommended by the supplier. PCR was done using a thermal cycler (PTC-0100; MJ Research, Cambridge, MA) with mouse hypoxanthine phosphoribosyl transferase (HPRT), HO-1, NQO-1, MnSOD, GSTP1, HSP27, HSP70, and HSP90 primers, as follows: HPRT, 5′-GTAATGATCAGTCAACGGGGAC-3′ (forward) and 5′-CCAGCAAGCTTGCAACCTTAACCA-3′ (reverse); HO-1, 5′-TCCCAGACACCGCTCCTCCAG-3′ (forward) and 5′-GGATTTGGGGCTGCTGGTTTC-3′ (reverse); NQO1, 5′-TCGGAGAACTTTCAGTACCC-3′ (forward) and 5-GCAGAGAGTACATGGAGCC-3 (reverse); GSTP1, 5-TGCCACCGTACACCATTGTGT-3 (forward) and 5′-CAGCAGGTCCAGCAAGTTGTA-3′ (reverse); MnSOD, 5′-GCACATTAACGCGCAGTCA-3′ (forward) and 5′-AGCCTCCAGCAACTCTCCTT-3′ (reverse); HSP27, 5′-TGCTTCACCCGGAAATACAC-3′ (forward) and 5′-CTCGAAAGTAACCGGAATGG-3′ (reverse); HSP70, 5′-TGGTGCTGACGAAGATGAAG-3′ (forward) and 5′-AGGTCGAAGATGAGCACGTT-3′ (reverse); and HSP90, 5′-AAAGGCAGAGGCTGACAAGA-3′ (forward) and 5′-AGGGGAGGCATTTCTTCAGT-3′ (reverse). The PCR products were subjected to electrophoresis in 3% agarose gels and stained with 0.01% SYBR Gold (Molecular Probes, Eugene, OR). Band intensities were quantified using NIH image and no PCR saturation was confirmed. HPRT was used as the internal standard (Kwon et al. 2005).
AST, ALT, and creatinine measurements
Blood was collected from the inferior vena cava and serum was obtained by centrifugation at 3,000×g for 10 min at 4°C for analyses of biomarkers. AST, ALT, and creatinine were quantified using commercial kits (Wako Pure Chemical Industries).
Lipid peroxidation determined by measurement of TBARS
Kidney and liver samples (each ~25 mg) were homogenized in 250 μl of RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% deoxycholic acid, 0.1% sodium dodecyl sulfate) on ice. The homogenates were centrifuged at 1,600×g for 10 min at 4°C and the supernatants were subjected to assays. Lipid peroxidation in the kidneys and livers were assessed by measuring TBARS using a TBARS Assay kit (Cayman Chemical Company, Ann Arbor, Ml).
Statistical analysis
The results are presented as the mean±standard deviation (SD) for each group. Statistical significance was assessed using one-way repeated ANOVA with a Tukey test. Differences were considered significant at p < 0.05.
Results
General observations
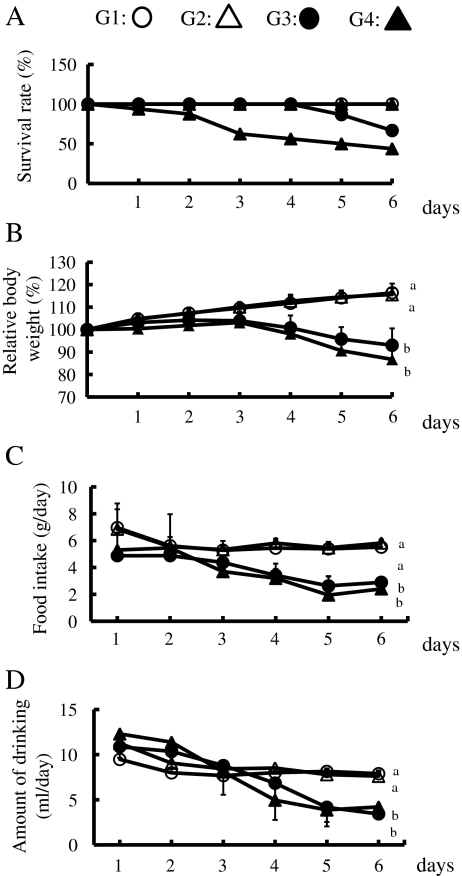
We examined the effects of a 1% GTPs diet over a 6-day observation period. None of the mice in groups 1 and 2 died during the observation period, whereas 40% of the mice in group 3 (DSS only) and 60% in group 4 (GTPs + DSS) died by day 6 (Fig. 2a). Surprisingly, half of the mice in group 4 died by day 4. Mouse body weights in groups 3 and 4 began to decrease at 4 days after DSS exposure, and were significantly lower than those in groups 1 and 2 by day 6 (Fig. 2b). Time-dependent changes of both food and water intake showed tendencies similar to those of body weight (Fig. 2c, d).
Fig. 2.
Effects of DSS and/or 1% GTPs supplementation on survival (a), relative body weight (b), food intake (c), and water intake (d). Group 1 (n = 15) (white circle), group 2 (n = 15) (white triangle), group 3 (n = 15) (black circle), group 4 (n = 17) (black triangle). Data are shown as the mean±SD. Values with different letters are significantly different, p < 0.05
Spleen, liver, and kidney weights
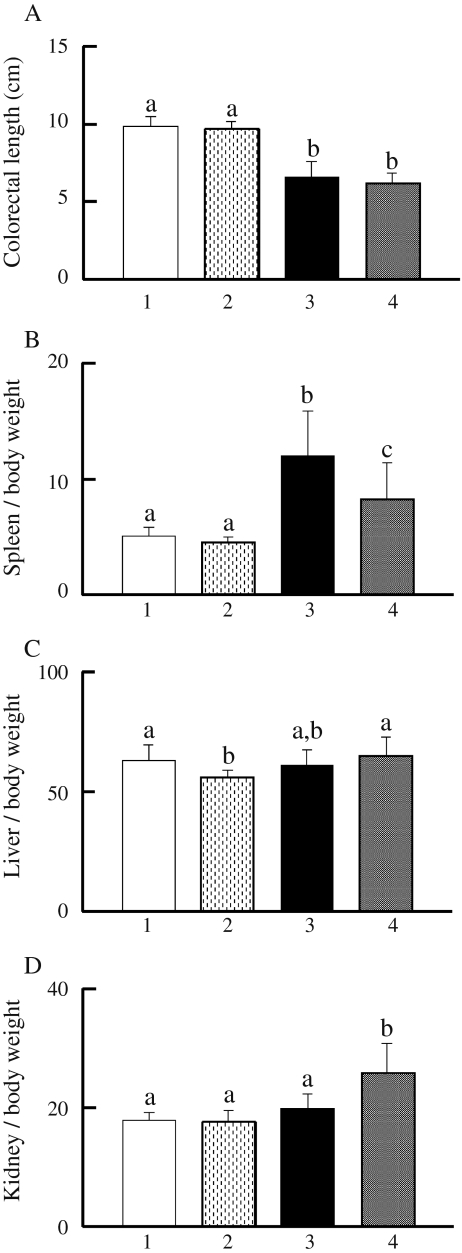
Colorectal length shortening reflects the extent of colonic damage in DSS-exposed mice (Okayasu et al. 1990). As shown in Fig. 3a, colorectal length in group 3 was shortened as compared to that in group 1, while GTPs supplementation did not have an effect (group 1 vs. 2, group 3 vs. 4). The spleen weight in group 3 was significantly greater (2.3-fold) as compared to group 1, whereas 1% GTPs in the diet suppressed that weight increase (Fig. 3b). Furthermore, liver weight in group 2 was significantly lower than that in group 1 (Fig. 3c). Notably, the kidney weight in group 4 was significantly increased (1.3-fold) as compared to group 3 (Fig. 3d).
Fig. 3.
Effects of DSS and/or 1% GTPs supplementation on organs. Colon length (a), spleen weight (b), liver weight (c), and kidney weight (d) were determined. Group 1 (n = 15), group 2 (n = 15), group 3 (n = 15), group 4 (n = 17). Data are shown as the mean±SD. Bars with different letters show significant differences (P < 0.05)
Hepatic and renal function parameters and lipid peroxidation level
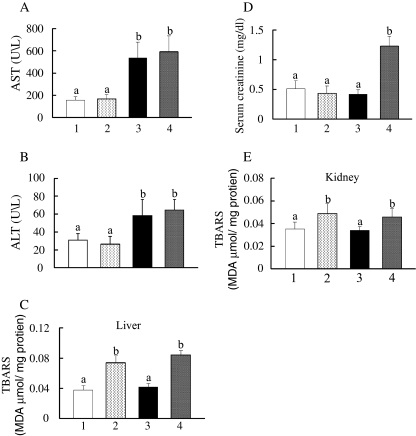
Serum AST and ALT levels, which reflect hepatic functions, were also measured. DSS (group 3) markedly increased both parameters as compared to no treatment (group 1), while 1% GTP (group 2) did not have a significant effect (Fig. 4a, b). Elevation of TBARS is a reliable indicator of lipid peroxidation, which might be closely related to tissue damage (Lambert et al. 2010). As shown in Fig. 4c, the hepatic TBARS level in group 4 was significantly greater (1.9-fold) than that in group 3. Similarly, we found a significant increase in group 2 as compared to group 1. Next, we measured serum creatinine levels as a biomarker of renal function (Nakagawa et al. 2004). Although DSS exposure did not have an effect on serum creatinine (group 1 vs. group 3), the serum creatinine level in group 4 was dramatically increased (2.9-fold) as compared to group 3 (Fig. 4d). Furthermore, TBARS levels in the kidneys of mice fed with the GTPs diet (groups 2 and 4) were markedly elevated than in those of their respective controls (groups 1 and 3; Fig. 4e).
Fig. 4.
Effects of DSS and/or 1% GTPs supplementation on hepatotoxicity and renal toxicity. Blood was collected and serum separated for determination of serum AST (a), ALT (b), and creatinine (d) levels. Obtained kidneys and livers were homogenized using RIPA buffer, then the supernatants were separated for measurement of TBARS levels in the livers (c) and kidneys (e). Data are shown as the mean±SD of seven to ten samples. Bars with different letters show significant differences (P < 0.05)
Expression levels of antioxidant and xenobiotics metabolizing enzymes
We also determined whether DSS and/or GTPs supplementation had effects on the expression levels of antioxidant and xenobiotics metabolizing enzymes, including HO-1, NQO1, MnSOD, and GSTP1, in the kidneys and liver (Fig. 5a). Both renal and hepatic HO-1 mRNA expressions in group 2 were slightly but significantly decreased (19% and 9.6%, respectively) as compared with those in group 1. More strikingly, those expressions in group 4 (GTPs + DSS) were abolished (Fig. 5b). Furthermore, renal and hepatic NQO1 mRNA expressions in groups 2 and 3 were dramatically decreased as compared to those in group 1, while those in group 4 were abolished (Fig. 5c). Similar results were seen for GSTP1 (Fig. 5d). Although renal MnSOD expression in groups 2 and 3 was significantly lower than in group 1, there was no significant difference between groups 3 and 4 (Fig. 5e). Interestingly, the expression levels of hepatic MnSOD were consistent among the four groups (Fig. 5e).
Fig. 5.
Effects of DSS and/or 1% GTPs supplementation on antioxidant enzyme mRNA levels in mouse kidneys and livers. HO-1, NQO1, GSTP1, and MnSOD mRNA expressions were determined by RT-PCR (a), with representative findings presented. Densitometric quantification of HO-1 (b), NQO1 (c), GSTP1 (d), and MnSOD (e) was performed used NIH Image. HPRT was used as an internal control. N.D. indicates not detected. Data are shown as the mean±SD of ten samples. Bars with different letters show significant differences (P < 0.05)
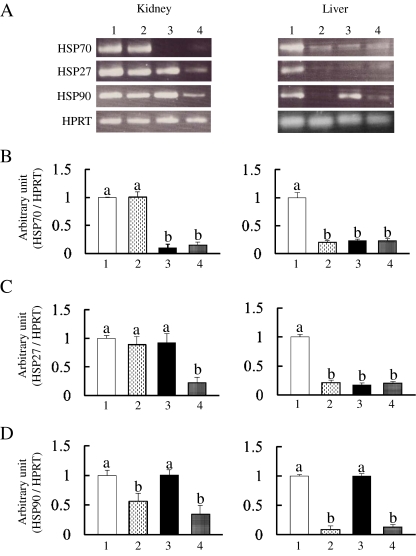
Expression levels of HSPs
HSPs are induced in response to oxidative stress as well as heat shock (Tanaka et al. 2007; Bhattacharrya et al. 2009). Thus, we investigated whether 1% GTPs and/or DSS affect the expression levels of HSP70, HSP27, and HSP90 mRNA expressions in the mouse kidneys and livers (Fig. 6a). Although renal HSP70 expression in group 2 did not change as compared with that in group 1, it was abolished in groups 3 and 4 (Fig. 6b). On the other hand, 1% GTPs dramatically decreased hepatic HSP70 and HSP27 in groups 2, 3, and 4 (Fig. 6b, c). In contrast, as compared with group 1, renal HSP27 mRNA expression in groups 2 and 3 was not significantly different (Fig. 6c). It is interesting to note that renal HSP27 in group 4 was dramatically down-regulated as compared to the other three groups (Fig. 6c). Finally, as shown in Fig. 6d, renal and hepatic HSP90 mRNA expressions were the most sensitive to the GTPs diet (group 1 vs. 2, group 3 vs. 4).
Fig. 6.
Effects of DSS and/or 1% GTPs supplementation on heat-shock protein mRNA levels in mouse kidneys and livers. HSP70, HSP27, and HSP90 mRNA expressions were determined by RT-PCR (a), with representative findings presented. Densitometric quantification of HSP70 (b), HSP27 (c), and HSP90 (d) was performed using NIH Image. HPRT was used as an internal control. Data are shown as the mean±SD of ten samples. Bars with different letters show significant differences (P < 0.05)
Discussion
GTPs have a variety of beneficial health functions, including preventive effects toward diabetes and cancer. Moreover, numerous human intervention and bioavailability studies using green tea extracts or EGCG have reported no serious adverse effects from their use (Wang et al. 2008; Abboud et al. 2008; Mochizuki and Hasegawa 2010). On the other hand, several recent studies have also noted that excess intake of green tea supplements induced hepatotoxicity in both rodents and humans (Lambert et al. 2010; Mazzanti et al. 2009; Isbrucker et al. 2006). In addition, the present study showed for the first time that high-dose GTPs caused nephrotoxicity in mice as observed by increased serum creatinine level (Fig. 4d). In support of those findings, it is well known that GTPs and EGCG function as pro-oxidants in vivo and in vitro, and exhibit genotoxicity and tumor promotional potentials (Li et al. 2010; Kim et al. 2010; Furukawa et al. 2003; Guo et al. 1996). However, the molecular mechanisms underlying their potential toxicity have not been fully elucidated.
A recent study by Lambert et al. reported that intragastric administration of high-dose EGCG (1,500 mg/kg) caused hepatotoxicity in mice (Lambert et al. 2010), while we observed in the present study that a 1% GTPs diet did not aggravate liver function, as determined by serum AST and ALT levels (Fig. 4a, b). These contrasting results may be due to differences in experimental conditions. However, we also found that hepatic HSP mRNA expression levels were substantially down-regulated by the 1% GTPs diet in mice not treated with DSS (Fig. 6a, right panel), suggesting hepatic dysfunction based on essential roles of HSPs for homeostasis. Moreover, a diet containing both GTPs and DSS dramatically increased serum creatinine (Fig. 4d), the most reliable biomarker of nephropathy (Nakagawa et al. 2004; Yamabe et al. 2006). Notably, both green tea extracts and DSS have been found to be widely distributed throughout a variety of organs in mice, including the liver and kidneys (Suganuma et al. 1998; Kitajima et al. 1999a, b). Collectively, biological interplay between GTPs and DSS may have a crucial role in the development of hepato- and nephrotoxicity.
Oxidative stress is accelerated by a combination of ROS generation and impaired antioxidant capacity (Kankuri et al. 2003; Keshavarzian et al. 1992; Osburn et al. 2006). Previous pharmacological studies have shown that EGCG is metabolized through methylation, glucuronidation, and sulfation under normal physiological conditions, and then subsequently excreted in urine (Okushio et al. 1999; Li et al. 2001). It is important to note that, most, if not all, metabolites are biologically inactivated and are thus much less toxic than the intact form of EGCG. On the other hand, an excess dose of EGCG drastically increased the level of TBARS in mice, a reliable indicator of lipid peroxidation for exerting toxic effects and rapid lethality (Lambert et al. 2010). In line with these observations, the present 1% GTPs diet increased TBARS levels in the kidneys and liver (Fig. 4c, e), which may have a mechanistic association with its pro-oxidative property.
As mentioned above, EGCG undergoes autoxidation to generate ROS. Oxidized EGCG in the B-ring is non-enzymatically converted to an EGCG o-quinone, which rapidly reacts with glutathione or protein thiols via covalent bindings (Mori et al. 2010). Thus, it is conceivable that administration of high-dose GTPs leads to accumulation of EGCG o-quinone, which escapes from inactivation processes by metabolism. These bioactive electrophiles may be responsible for inducing toxicity in the liver and kidneys. In fact, formation of EGCG-thiol conjugates was detected only at high doses (400 mg/kg) following intraperitoneal injection in mice (Sang et al. 2005). Meanwhile, high-dose EGCG reduced the expressions of antioxidant enzymes, including HO-1, SOD, and catalase (Kweon et al. 2006; Kim et al. 2010). Along a similar line, our present results revealed that 1% GTPs dramatically decreased the mRNA expressions of HO-1 and NQO1 in the kidneys and livers of non-treated mice (Fig. 5b, c). It is of great importance to point out that HO-1 was reported to attenuate the progression of chronic kidney disease (Desbuards et al. 2009). Therefore, we assume that high-dose GTPs act not only in a pro-oxidant manner, but also down-regulate antioxidant enzymes, leading to hepatic and renal dysfunctions.
It is interesting that down-regulation of HSP70 has been found to be associated with IBD development (Bhattacharrya et al. 2009; Köken et al. 2004; Hu et al. 2007). Although we also reported that a 1% GTPs diet aggravated colitis in DSS-exposed mice (Kim et al. 2010), it was not clear whether 1% GTPs affected the expression HSP70 in that model. In our present study, renal and hepatic HSP70 expressions were dramatically suppressed by DSS exposure, while the 1% GTPs diet did not affect those in DSS-induced colitis mice. On the other hand, it should be noted that 1% GTPs treatment decreased hepatic HSP70 by 21% as compared with the non-treated mice (Fig. 6b). Anwar et al. showed that HSP70 interacts with an unfolded form of NQO1 and thereby inhibited its degradation (Anwar et al. 2002), implying a supporting role of HSP70 in NQO1 stabilization. In support of that notion, the expression patterns of HSP70 and NQO1 in the present four groups were positively correlated, except for data from the kidneys in group 2 (Figs. 5c and 6b). Renal and hepatic HSP27 levels were also remarkably down-regulated by the combination of GTPs and DSS (Fig. 6c). Thus, the 1% GTPs diet might have induced hepatic and renal toxicity, at least in part, by attenuating the expressions of both HSP70 and HSP27. More strikingly, HSP90 in kidneys and livers of the non-treated mice was identified as a chaperone protein that is hyper-sensitive to GTPs (Fig. 6d). These results are consistent with those of Tran et al., who previously found that HSP90 was repressed by EGCG in MCF-7 human breast cancer cells (Li et al. 2009; Tran et al. 2010). Because HSP90 is the most abundant molecular chaperone and plays pivotal roles in maintaining organ homeostasis (Shi et al. 2007; Hackl et al. 2010), its down-regulation by GTPs might be associated with organ dysfunction and toxicity. In addition, it is significant to note that increased HSP70 expression by both glutamine and geranylgeranylacetone contributed to the protection against inflammation including IBD (Ohkawara et al. 2005; Xue et al. 2011).
The present 1% GTPs diet, which contained 35% EGCG, decreased survival rates (Fig. 2a) and down-regulated antioxidant and xenobiotic-metabolizing enzymes in mice with DSS-induced colitis (Fig. 5a). In contrast, we previously reported that a low-dose GTPs (0.1~0.25%) diet had a tendency to improve both ulcers and inflammation in a colitis model (Kim et al. 2010). Therefore, low or moderate doses of GTPs may exhibit beneficial effects toward DSS-induced hepatotoxicity and nephrotoxicity. This hypothesis may be supported by Calabrese and Baldwin who described the U-shaped toxicity of environmental chemicals (Calabrese and Baldwin 2002).
Taken together, our findings indicate that a high-dose GTPs diet exacerbates kidney and liver functions, presumably through down-regulation of antioxidant enzymes and HSPs, in both normal mice and those with DSS-induced colitis. The effects of low and medium dose of GTPs diets on these functions are now being investigated in our laboratory.
Acknowledgements
This work was supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry and by Research and Development Projects for Application in Promoting New Policy of Agriculture Forestry and Fisheries, Ministry of Agriculture, Forestry and Fisheries, Japan (Grant No. 23005).
References
- Abboud PA, Hake PW, Burroughs TJ, Odoms K, O'Connor M, Mangeshkar P, Wong HR, Zingarelli B. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Clin Pharmacol. 2008;579(1–3):411. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Anwar A, Siegel D, Kepa JK, Ross D. Interaction of the molecular chaperone Hsp70 with human NAD(P)H:quinone oxidoreductase 1. J Biol Chem. 2002;277(16):14060. doi: 10.1074/jbc.M111576200. [DOI] [PubMed] [Google Scholar]
- Araki Y, Andoh A, Fujiyama Y. The free radical scavenger edaravone suppresses experimental dextran sulfate sodium-induced colitis in rats. Int J Mol Med. 2003;12(1):125. [PubMed] [Google Scholar]
- Araki Y, Mukaisyo K, Sugihara H, Fujiyama Y, Httori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol Rep. 2010;24(4):869. doi: 10.3892/or.2010.869. [DOI] [PubMed] [Google Scholar]
- Bhattacharrya S, Dudeja PK, Tobacman JK. ROS, HSP27, and IKKbeta mediate dextran sodium sulfate (DSS) activation of Ikappaba, NFKappaB, and IL-8. Inflamm Bowel Dis. 2009;15(5):673. doi: 10.1002/ibd.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai EP, Lin JK. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J Agric Food Chem. 2009;57(20):9817. doi: 10.1021/jf902618v. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Applications of hormesis in toxicology, risk assessment and chemotherapeutics. Trends Pharmacol Sci. 2002;23(7):331. doi: 10.1016/S0165-6147(02)02034-5. [DOI] [PubMed] [Google Scholar]
- Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chan ZY, Kwok TT. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. 2001;68(10):1207. doi: 10.1016/S0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- Desbuards N, Hyvelin JM, Machet MC, Eder V, Garrigue MA, Halimi JM, Antier D. Heme oxygenase-1 inducer hemin attenuates the progression of remnant kidney model. Nephron Exp Nephrol. 2009;113(1):e35. doi: 10.1159/000228081. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S. (−)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem Pharmacol. 2003;66(9):1769. doi: 10.1016/S0006-2952(03)00541-0. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996;1304(3):210. doi: 10.1016/s0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Hackl C, Mori A, Moser C, Lang SA, Dayoub R, Weiss TS, Schlitt HJ, Geissler EK, Hellerbrand C, Stoeltzing O. Effect of heat-shock protein-90 (HSP90) inhibition on human hepatocytes and on liver regeneration in experimental models. Surgery. 2010;147(5):704. doi: 10.1016/j.surg.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Hosakawa Y, Hosakawa I, Ozaki K, Nakanishi T, Nakane H, Matsuo T. Tea polyphenols inhibit IL-6 production in tumor necrosis factor superfamily 14-stimulated human gingival fibroblasts. Mol Nutr Food Res. 2010;54(2):S151. doi: 10.1002/mnfr.200900549. [DOI] [PubMed] [Google Scholar]
- Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, Chang EB. Translation inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133(6):1893. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44(5):636. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Isemura M, Saeki K, Kimura T, Hayakawa S, Minami T, Sazuka M. Tea catechins and related polyphenols as anti-cancer agents. Biofactors. 2000;13(1–4):81. doi: 10.1002/biof.5520130114. [DOI] [PubMed] [Google Scholar]
- Kankuri E, Hämäläinen M, Hukkanen M, Salmenperä P, Kivilaakso E, Vapaatalo H, Moilanen E. Suppression of pro-inflammatory cytokine release by selective inhibition of inducible nitric oxide synthase in mucosal explants from patients with ulcerative colitis. Scand J Gastroenterol. 2003;38(2):186. doi: 10.1080/00365520310000681. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Sedghi S, Kanofsky J, List T, Robinson C, Ibrahim C, Winship D. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology. 1992;103(1):177. doi: 10.1016/0016-5085(92)91111-g. [DOI] [PubMed] [Google Scholar]
- Kim M, Murakami A, Ohigashi H. Modifying effects of dietary factors on (−)-epigallate-induced pro-matrix metalloproteinase-7 production in HT-29 human colorectal cancer cells. Biosc Biotechnol Biochem. 2007;71(10):2442. doi: 10.1271/bbb.70213. [DOI] [PubMed] [Google Scholar]
- Kim M, Murakami A, Miyamoto S, Tanaka T, Ohigashi H. The modifying effects of green tea polyphenols on acute colitis and inflammation-association colon carcinogenesis in male ICR mice. Biofactors. 2010;36(1):43. doi: 10.1002/biof.69. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Takuma S, Morimoto M. Changes in colon mucosal permeability in mouse colitis induced dextran sulfate sodium. Exp Anim. 1999;48(3):137. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Takuma S, Morimoto M. Tissue distribution of dextran sulfate sodium (DSS) in the acute phase of murine DSS-induced colitis. J Vet Med Sci. 1999;61(1):67. doi: 10.1292/jvms.61.67. [DOI] [PubMed] [Google Scholar]
- Köken T, Serteser M, Kahraman A, Akbulut G, Dilek ON. Which is more effective in the prevention of renal ischemia-reperfusion-induced oxidative injury in the early period in mice: interleukin (IL)-10 or anti-IL-12? Clin Biochem. 2004;37(1):50. doi: 10.1016/j.clinbiochem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem. 2006;281(44):33761. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- Kwon KH, Murakami A, Hayashi R, Ohigashi H. Interleukin-1 beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005;337(2):647. doi: 10.1016/j.bbrc.2005.09.107. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Yang, C., Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48(1):409. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li C, Meng X, Winnik B, Lee MJ, Lu H, Sheng S, Buckley B, Yang CS. Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. Chem Res Toxicol. 2001;14(6):702. doi: 10.1021/tx0002536. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Jiang Y, Lee HF, Schwartz SJ, Sun D. (−)-Epigallocatechin-3-gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line Mia Paca-2. Mol Pharm. 2009;6(4):1152. doi: 10.1021/mp900037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, Lee MJ, Liu B, Guan F, Yang Z, Yang CS. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31(5):902. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65(4):331. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Hasegawa N. (−)-epigallocatechin 3-gallate reduces experimental colon injury in rats by regulating macrophage and mast cell. Phytother Res. 2010;24(S1):S120. doi: 10.1002/ptr.2862. [DOI] [PubMed] [Google Scholar]
- Mori T, Ishii T, Akagawa M, Nakamura Y, Nakayama T. Covalent binding of tea catechins to protein thiols: the relationship between stability and electrophilic reactivity. Biosc Biotechnol Biochem. 2010;74(12):2451. doi: 10.1271/bbb.100509. [DOI] [PubMed] [Google Scholar]
- Musch MW, Ciancio MJ, Sarge K, Chang EB. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am J Physiol. 1996;270(2 Pt 1):C429. doi: 10.1152/ajpcell.1996.270.2.C429. [DOI] [PubMed] [Google Scholar]
- Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476(2):171. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yokozawa T, Sano M, Takeuchi S, Kim M, Minamoto S. Activity of (−)-epigallocatechin 3-O- gallate against oxidative stress in rats with adenine-induced renal failure. J Agric Food Chem. 2004;52(7):2103. doi: 10.1021/jf030258j. [DOI] [PubMed] [Google Scholar]
- Ogborne RM, Rushworth SA, O'Connell MA. Epigallocatechin activates haem oxygenase-1 expression via protein kinase Cδ and Nrf2. Biochem Biophys Res Commun. 2008;373(4):584. doi: 10.1016/j.bbrc.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara T, Nishihira J, Takeda H, Miyashita K, Kato K, Kato M, Sugiyama T, Asaka M. Geranylgeranylacetone protects mice from dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2005;40(9):1049. doi: 10.1080/00365520510023161. [DOI] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Okushio K, Suzuki M, Matsumoto N, Nanjo F, Hara Y. Methylation of tea catechins by rat liver homogenates. Biosc Biotechnol Biochem. 1999;63(2):430. doi: 10.1271/bbb.63.430. [DOI] [PubMed] [Google Scholar]
- Osburn WO, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush MA, Kensler TW. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454(1):7. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquay JB, Haenen GR, Stender G, Wiseman SA, Tijburg LB, Bast A. Protection against nitric oxide toxicity by tea. J Agric Food Chem. 2000;48(11):5768. doi: 10.1021/jf981316h. [DOI] [PubMed] [Google Scholar]
- Paul G, Battaille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rümmele P, Farkas S, Schölmerich J, Fleck M, Rogler G, Herfarth H. Analysis of intestinal haem oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol. 2005;140(3):547. doi: 10.1111/j.1365-2249.2005.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Aredash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214(12):177. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- Sahin K, Tuzuc M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, Kucuk O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010;87(7–8):240. doi: 10.1016/j.lfs.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (−)-epigallocatechin gallate and their urinary levels in mice. Chem Res Toxicol. 2005;18(11):1762. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- Shi Q, Domg Z, Wei H. The involvement of heat shock proteins in murine liver regeneration. Cell Mol Immunol. 2007;4(1):53. [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H] (−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19(10):1771. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, Takeuchi K, Nakai A, Mizushima T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem. 2007;282(32):23240. doi: 10.1074/jbc.M704081200. [DOI] [PubMed] [Google Scholar]
- Tran PL, Kim SA, Choi HS, Yoon JH, Ahn SG. Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer. 2010;10:276. doi: 10.1186/1471-2407-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G586. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- Wang JS, Luo H, Wang P, Tang L, Yu J, Huang T, Cox S, Gao W. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46(1):232. doi: 10.1016/j.fct.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol Gastrointest Liver Physiol. 1997;272(4 Pt 1):G879. doi: 10.1152/ajpgi.1997.272.4.G879. [DOI] [PubMed] [Google Scholar]
- Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experiment colitis models. JPEN J Parenter Enteral Nutr. 2011;35(2):188. doi: 10.1177/0148607110381407. [DOI] [PubMed] [Google Scholar]
- Yamabe N, Yokozawa T, Oya T, Kim M. Therapeutic potential of (−)-epigallocatechin 3-O- gallate on renal damage in diabetic nephropathy model rats. J Pharmacol Exp Ther. 2006;319(1):228. doi: 10.1124/jpet.106.107029. [DOI] [PubMed] [Google Scholar]