Abstract
Climate warming involves not only a rise of air temperature means, but also more frequent heat waves in many regions on earth, and is predicted to intensify physiological stress especially in extremely changeable habitats like the intertidal. We investigated the heat-shock response (HSR) and enzymatic antioxidant defense levels of Patagonian shallow-water limpets, adapted to distinct tidal exposure conditions in the sub- and intertidal. Limpets were sampled in the temperate Northern Patagonia and the subpolar Magellan region. Expression levels of two Hsp70 genes and activities of the antioxidants superoxide dismutase (SOD) and catalase (CAT) were measured in submerged and 2- and 12-h air-exposed specimens. Air-exposed Patagonian limpets showed a tiered HSR increasing from South to North on the latitudinal gradient and from high to low shore levels on a tidal gradient. SOD activities in the Magellan region correlated with the tidal rhythm and were higher after 2 and 12 h when the tide was low at the experimental site compared to the 6 h value taken at high tide. This pattern was observed in intertidal and subtidal specimens, although subtidal individuals are little affected by tides. Our study shows that long-term thermal adaptation shapes the HSR in limpets, while the oxidative stress response is linked to the tidal rhythm. Close to the warm border of their distribution range, energy expenses to cope with stress might become overwhelming and represent one cause why the limpets are unable to colonize the shallow intertidal zone.
Keywords: Patagonia, Heat-shock protein, Oxidative stress, Intertidal, Nacella
Introduction
Thermal tolerance and its impact on the horizontal and vertical distribution of species have received growing interest during the past decade, as biologists have looked more intensively for observable effects of climate change. Of all factors under change, aerial warming is believed to have the most immediate effect also in marine coastal environments (Somero 2005; Harley et al. 2006). Intertidal zones are high-stress environments and further characterized by steep vertical gradients in abiotic conditions and stress levels during tidal cycles. Especially in the high intertidal, marine fauna and flora are periodically exposed to desiccation, warming or freezing depending on latitude, and also to osmotic stress caused by precipitation and evaporation (Hofmann 1999; Helmuth et al. 2006a, b; Tomanek 2002; Denny et al. 2006). Many marine animals living temporarily above the waterline have behavioral adaptations that help them avoid the most stressful conditions. Either they retreat to tidal pools during low tides, or they change their behavior to establish locally confined conditions under which they can survive, e.g. by hermetically closing their shells during aerial exposure. When conditions stray too far from the physiological optimum of the organism, this behavior can involve a state of transient hypoxia, especially if the animals are additionally warmed by solar irradiation. Organisms experiencing periodically recurring stress situations (e.g. during tidal cycles) often develop a state of heat hardening, which improves their chance of survival during severe stress, but is attained at the cost of significantly increased energetic investments into cellular protection and maintenance (Somero 2002; Hofmann 2005; Dong et al. 2008). As a consequence of the steep vertical gradients in exposure, the marine intertidal is often characterized by a pronounced faunal zonation. Distinctly adapted species typically occupy different positions on the environmental gradient, but also conspecific specimens can choose microhabitats at different shore heights of rocky intertidal zones, according to their individual tolerance towards tidal exposure (Tomanek and Sanford 2003; Weihe and Abele 2008; Weihe et al. 2010).
One important protective mechanism against a variety of stress conditions during tidal emersion such as temperature stress and oxygen deficiency is the heat-shock response (HSR). It describes the activation of so-called heat-shock proteins (Hsps) which act as chaperones stabilizing and salvaging denatured proteins and, in so doing, prevent formation of cytotoxic aggregates (Parsell and Lindquist 1993; Hartl 1996; Fink 1999). The most abundant molecular chaperones are from the Hsp70 class, which comprises several proteins of a molecular weight of 68–74 kDa (Lindquist 1986). The HSR thresholds in different marine organisms correlate with habitat temperature and the stress levels normally experienced by the organisms (Feder and Hofmann 1999), and it has been suggested that frequent expression of Hsps may be part of the physiological strategy of intertidal organisms to occupy ecological niches close to their thermal limits (Tomanek 2010).
Another important anti-stress reaction is the antioxidant defense system. Antioxidant enzymes such as superoxide dismutase and catalase I (SOD and CAT; see Abele and Puntarulo 2004) are central constituents of the inducible systems that control the detrimental effects caused by reactive oxygen species (ROS; e.g. superoxide, H2O2, OH·) produced in cells under physiological strain (Boveris and Chance 1973; Jones 2006; Murphy 2009). ROS damage DNA, proteins and lipids and thereby jeopardize cellular and organism fitness and function, forcing animals to invest more energy into cell repair. Heat stress in ectotherms generally induces mitochondrial ROS formation and, in less heat-tolerant animals, thermal inactivation of antioxidant enzymes enhances the oxidative stress condition (Abele et al. 2001, 2002; Heise et al. 2003).
We studied shallow-water limpets from the genus Nacella that inhabit different shore levels along the Patagonian coast of Southern Chile, with the objective to obtain new insight into the importance and plasticity of the HSR and the antioxidant defense in natural limpet populations. Ecologically, the study was aimed at understanding how thermal tolerance influences the distribution in our species of interest. The approach includes a large-scale comparative investigation of populations of the intertidal limpet Nacella magellanica from the northern edge (Puerto Montt, PM, ∼42°S) and from the centre (Punta Arenas, PA, ∼55°S) of its distribution range, representing two different temperature regimes. Mean summer air temperatures at PM are around 15°C compared to 8°C at PA. In a second step, we performed a small-scale comparison of the stress response between the shallow intertidal N. magellanica and the deep intertidal/shallow subtidal Nacella deaurata. Both nominal species occur in sympatry in Punta Arenas, but are differentially exposed to aerial and marine environmental conditions.
A full evaluation of the taxonomic status and genetic distinctness of N. deaurata and N. magellanica is beyond the scope of this study. Their status is controversially discussed based on morphological and genetic analyses (Powell 1973; Valdovinos and Rüth 2005; De Aranzamendi et al. 2009). Despite their clearly distinct morphologies (N. magellanica have larger shell heights and a centered apex, N. deaurata comparably flat shells and the apex shifted towards the anterior), the mitochondrial markers cyt b and COI did not distinguish both species (De Aranzamendi et al. 2009). The application of two different fast evolving molecular marker systems produced ambiguous results. An inter-simple sequence repeats analysis revealed considerable differences between N. magellanica and N. deaurata (De Aranzamendi et al. 2009), but eight microsatellite loci showed no differentiation between the two morphotypes (Pöhlmann et al., in preparation). Difficulties in determining whether morphological disparities reflect species level differentiation or are caused by phenotypic plasticity are also known for other limpets such as the Antarctic sister taxon Nacella concinna (Hoffman et al. 2010, Morley et al. 2010). Since the true genetic relationship of South American Nacella is not fully resolved, we consider them as two subpopulations between which gene flow cannot be fully ruled out.
Laboratory-based heat-shock experiments under controlled conditions do not necessarily reflect the natural anti-stress response of air-exposed intertidal limpets in the field (Clark et al. 2008a, b). Therefore, the experiments in the present work were conducted in the field, to mimic the combination of naturally occurring stressors that threaten intertidal organisms when air exposed during phases of tidal uncovering and, yet, be comparable between different study sites.
The aim was to investigate how the intensities of inducible HSR and antioxidant defense levels differ between limpets adapted to differentially exposed conditions in the sub- and intertidal. The HSR and antioxidant response of animals taken from their natural habitat at different positions in the tidal zone were measured after experimental air exposure for 2, 6 and 12 h, to simulate air exposure during tidal uncovering as a natural stressor. In addition to previous studies that focused on the temperature threshold at which the HSR response is triggered in organisms with vertical zonation patterns (for reviews see Hofmann 1999, 2005 Feder and Hofmann 1999), our experimental approach also determines the timing of the onset of the HSR (Clark et al. 2008b, Dong et al. 2008). Specifically it allows us to test whether the onset of the HSR parallels previous results of the limpet antioxidant response. In the previous study, intertidal Antarctic limpets (N. concinna) had a delayed response to air exposure stress compared to their subtidal conspecifics, indicating adaptation to periodical air exposure in the intertidal specimens (Weihe et al. 2010). In addition, we performed parallel experiments in which animals were kept immersed during the whole low tidal cycle, to investigate whether either the Hsp expression or the antioxidant defense systems feature intrinsic patterns adaptive to tidal periodicity, as recently observed in a microarray study of intertidal Mytilus mussels (Gracey et al. 2008).
We hypothesize the following: (1) Limpets near their distribution edge at PM should show a more pronounced stress response to air exposure than limpets from the center at PA due to stronger temperature shifts between states of immersion and tidal uncovering at PM. (2) Limpets from the high intertidal should be better adapted to air exposure than limpets from the subtidal showing delayed onsets and less pronounced anti-stress reactions, because they endure phases of tidal uncovering more regularly.
Material and methods
Sampling sites and experimental design
Sample collection of N. magellanica and N. deaurata and experiments were carried out at two different sites in Southern Chile, in PM in Northern Patagonia and in PA in the Strait of Magellan (Fig. 1). Two to four hours is the normal time span that N. magellanica specimens experience tidal emersion twice a day at PA. By contrast, PM individuals of N. magellanica fall dry only during spring tides when the tidal range reaches its maximum of 6 m. At PA, N. deaurata suffer tidal effects when the shallow coastal waters warm up on sunny days, or when the animals become air exposed during extreme low tides. At PM, N. deaurata are absent.
Fig. 1.
Map showing the two different experimental sites at Puerto Montt, the northern distribution boundary, and Punta Arenas in the center
Samples of all subpopulations were collected by hand 1 to 2 h before low tide just before emersion of the intertidal specimens (see Table 1 for an overview of collection sites, time and conditions). For control values of gene expression and enzyme activities, control animals were dissected immediately and samples of foot muscle (gene expression) and gills (enzyme activities) collected. Foot muscle samples (100–300 mg) were stored in 1.5 ml of RNAlater (Qiagen), and gill tissues were snap frozen and kept in liquid nitrogen. Shells were kept for morphological examination.
Table 1.
Overview of experiment sites, date and time as well as water temperatures and low tide time at the beginning of the experiments
| Collection site | Coordinates | Experiment date | Water temperature | Experiment start time | Low tide time |
|---|---|---|---|---|---|
| Pta Arenas/Bahia Laredo | 52°56′56″S 70°47′44″W | 12 h: 13 February 2009 | 9.4°C | 07:30 | 09:30 |
| 2 h + 6 h: 14 February 2009 | 10.8°C | 08:20 | 10:00 | ||
| Puerto Montt/Chinquihue | 41°30′42″S 73°00′55″W | 26 February 2009 | 16.9°C | 08:30 | 09:30 |
For simulation of tidal air exposure during phases of low tides, experimental specimens were placed in three separate dry plastic tanks directly after collection, and were maintained on location over three different time spans of 2, 6 and 12 h. Each treatment group consisted of six animals of each subpopulation (two individuals of each treatment group and subpopulation per tank). Additional control experiments were conducted with submersed animals, simultaneously for each treatment group and subpopulation in plastic tanks filled with 1 l of fresh seawater. Seawater in the control was fully oxygen saturated throughout the experimental period.
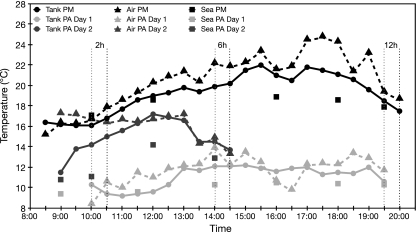
Limpets from all treatments were dissected on location as described for untreated controls. In Puerto Montt, all the experiments were conducted on the same day, but in Punta Arenas the 12-h experiment was conducted on day 1, while the 2- and 6-h experiments were conducted on day 2 to ensure accurate sample handling under difficult weather conditions. Air temperatures, water temperatures and oxygen concentrations of the seawater in the experimental tanks were recorded in 30-min intervals throughout the experiments. Temperature and oxygen concentration in the sea surface water at the experimental sites were recorded every 2 h. Figure 2 shows the temperature protocols of both experimental sites. Oxygen concentration never decreased below 90% and can therefore be regarded as stable throughout.
Fig. 2.
Temperature profiles during the experiments carried out in Puerto Montt (PM, black lines) and Punta Arenas (PA, light grey lines = experimental day 1; dark grey lines = experimental day 2). Solid lines represent the water temperatures in the experiment tanks, dashed lines represent air temperature and squares describe the temperature of sea surface water at the experimental sites. Vertical dotted lines mark time points of sampling (black, Puerto Montt; grey, Punta Arenas)
Primer design
Degenerate primers reported by Clark et al. (2008a) were used to amplify fragments of the target heat-shock proteins (Hsp70A, Hsp70B, and Grp78) and of β-actin by a standard PCR in a total volume of 25 μl, containing approximately 10 ng genomic DNA, 1× HotMaster™ reaction buffer, 0.2 mM dNTPs, 0.5 μM of each primer, 0.03 U/μl HotMaster™ Taq (Eppendorf). The following PCR conditions were applied: 2 min 94°C, 35 cycles of 20 s at 94°C, 20 s at 54°C and 40 s at 65°C, and a final extension of 5 min at 65°C. PCR products were cloned with the TOPO TA Cloning® Kit (Invitrogen) according to the manufacturer’s instructions. DNA Sequences obtained from cloned fragments were aligned with ClustalW in CodonCode Aligner software V3.5.7 (CodonCode Corp.). Hsp70A, Hsp70B, Grp78 and β-actin primers published by Clark et al. (2008a) were checked for matching with the sequences obtained by our cloning process and Hsp70A, Hsp70B and Grp78 primers were used in the subsequent analysis. Actin primers were newly designed using the online program Netprimer (www.premierbiosoft.com). Primers for histone H3 were designed to fit the sequence published for N. deaurata and N. magellanica by Nakano and Ozawa (2007; GenBank accession nos. AB433688 and AB433689). Primers for HIF-1 (Hypoxia Inducible Factor 1) subunit α were designed to the sequence from N. concinna supplied by Weihe et al. (in progress).
RNA extraction and reverse transcription
For measurements of gene expression, total RNA was extracted from 50 to 100 mg foot tissue samples using TRI Reagent (Sigma) under RNase-free conditions according to the manufacturer’s instructions. Tissues were homogenized by vigorous shaking in a three-dimensional motion in a Precellys®24 Dual tissue homogenizer (Bertin Technologies) at 6,500 rpm for 25 s. To prevent possible contamination with genomic DNA, the extracted RNA solutions were digested with RNase-free DNase (1 U per μg RNA, Fermentas) in a 10 mM DTT/100 mM MgCl2 buffer. Subsequently, RNA was reverse transcribed into cDNA with Maxima™ Reverse Transcriptase (Fermentas) using oligo-(dT)18 primers, under a protocol adjusted for dissolving secondary structures, at 55°C for 40 min.
Real-time quantitative PCR
Real-time quantitative PCR (RT-qPCR) was conducted using the Type-It™ HRM PCR Master Mix with HotStarTaq® Plus DNA Polymerase and EvaGreen dye (Qiagen). Cycling was performed in a Rotor-Gene® Q 5-Plex rotary cycler (Qiagen) using the following cycling program: 5 min at 95°C, 40 cycles of 10 s at 95°C and 30 s at 55°C. Each sample was quantified in triplicate, and treatment groups were distributed evenly between runs. To confirm the specificity of the RT-qPCR amplification, melt analysis was performed directly after the cycling, by increasing temperature from 65°C to 90°C in increments of 0.3°C for 2 s each. Replicates showing by-product peaks with a height of more than 10% of the main peak were discarded. Two samples were sequenced for each product peak to confirm identity of the measured fragment. Amplification efficiency and linear range of the assay were tested by relative standard curves. As candidate reference genes for normalization of Hsp70 expression values, β-actin, histon H3, Grp78 and HIF-1α were quantified. The program GeNorm (Vandesompele et al. 2002) suggested the gene combination Grp78 + HIF as most suitable reference genes. The program NormFinder (Andersen et al. 2004) ranked Grp78 as best candidate reference, and HIF was ranked second best in terms of stability of expression over time of exposure. We therefore decided to use the combination of Grp78 and HIF as reference genes in this study (Table 2).
Table 2.
Stability ranking of candidate reference gene expression by two different algorithms
| Rank | GeNorm | NormFinder | ||
|---|---|---|---|---|
| Genes | M | Genes | Stability | |
| 1. | Grp78 + HIF | 1.1 | Grp78 | 0.426 |
| 2. | β-actin | 1.34 | HIF | 0.562 |
| 3. | Histon | 1.53 | β-actin | 0.567 |
| Best pair | Grp78 + Histon | 0.412 | ||
GeNorm calculates an average expression stability M based on the standard deviation between all genes and samples, while NormFinder returns a model-based stability value between subpopulation/treatment groups
Enzyme activity measurements of SOD and CAT
The determination of SOD activity was carried out according to Livingstone et al. (1992). Fifty to one hundred milligrammes of frozen tissue were ground in liquid nitrogen and homogenized in a buffer containing 20 mM Tris–HCl and 1 mM EDTA (pH 7.6) as a 5:1 mix. All samples were centrifuged for 3 min at 18,000×g and at 4°C. The supernatant was used to determine SOD activity photometrically at a fixed wavelength of 550 nm for 3 min with an interval time of 10 s in a potassium buffer (43 mM K2HPO4, 0.1 mM EDTA, pH 7.68, 100 μM cytochrome c, 5 mM xanthine, 0.3 mU/μl xanthine oxidase and 2 M (NH4)2SO4). The activity of SOD was determined on the basis of its inhibiting capabilities of the xanthine oxidase/xanthine reaction system, which catalyses the formation of superoxid anions (O−·2). SOD converts the formed O−·2 to hydrogen peroxide (H2O2), thus inhibiting the reduction of cytochrome c. In the applied assay, 1 U of SOD causes an inhibition of 50% of the XOD reaction.
The CAT was measured using the same extracts as for SOD. Enzyme activities were determined photometrically at 240 nm in a potassium buffer containing 50 mM K2HPO4 (pH 7.0) and 1 μM hydrogen peroxide (H2O2). CAT activity is determined via the turnover rate of a defined amount of H2O2 into water and oxygen, according to Aebi (1984).
Statistics
For quantification of gene expression, replicate CT values were transferred to Excel 2002 (Microsoft Corp.), and linear expression values were obtained using the standard curve equation output by the Rotor-Gene software. The stability of gene expression was tested by running the Excel macros GeNorm (Vandesompele et al. 2002) and NormFinder (Andersen et al. 2004) on the expression data. Relative expression values were normalized through division by the geometric mean of the most stable genes Grp78 and HIF-1, and tested for statistical significance of expression changes within subpopulations (independent variable: time of air exposure) by one-way ANOVA with Tukey’s post hoc test and among subpopulations by two-way ANOVA (independent variables location and shore height) with Bonferroni post hoc test using Graphpad Prism 5.01 (Graphpad Software Inc.).
Enzyme activity data from all treatments and subgroups were tested for normal distribution using the Kolmogorov–Smirnov test. Statistical analysis of the enzyme activities was performed using Graphpad Prism 5.01. The dependence of SOD and CAT activities on exposure time within a subpopulation was tested by one-way ANOVA with Tukey's post hoc test, and on factors subpopulation site and shore height by two-way ANOVA followed by Bonferroni post hoc test.
An ANCOVA model of the temperature dependence of SOD and CAT activities in gills of N. magellanica and N. deaurata at PA revealed no significant thermal effects on the activity of either enzyme when assayed at room temperature (p = 0.076, F = 2.201, n = 71). Further, the effective oxygen saturation in the experimental buckets had no modulating effect on the enzyme activities (oxygen level p = 0.985, F = 0.091, n = 71). Therefore, a slight, but statistically significant difference (p = 0.044) between CAT activities of the N. deaurata initial groups (0 h) between day 1 (n = 5) and day 2 (n = 4) was attributed to the natural intra-specific variability, and data from both days were pooled into a common 0 h exposure group (n = 9).
Results
Shell morphometry
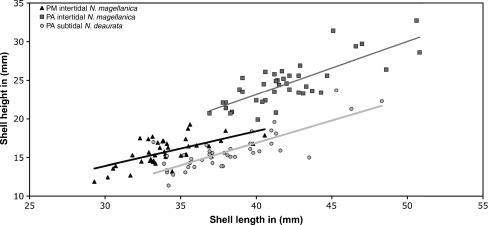
Shell lengths (SL) and heights (SH) of all experimental individuals are shown in Fig. 3. Mean shell lengths and heights were highest in intertidal N. magellanica from PA (SL = 42 ± 3.3 mm, SH = 24.6 ± 2.9 mm). The N. magellanica population from PM and the N. deaurata population from PA had the same mean SH of 15.8 mm with slightly different height variability (±1.7 mm and ±2.5 mm, respectively), but average SL was smaller in N. magellanica specimens from PM (34.1 ± 2.4 mm) compared to individuals from the N. deaurata population from PA (38.1 ± 3.4 mm).
Fig. 3.
Shell lengths to shell heights in the three investigated populations from Puerto Montt (black triangles), Punta Arenas intertidal (dark grey squares) and Punta Arenas subtidal (light grey cycles)
The strong variations in shell morphometry between the two populations of N. magellanica reflect the high phenotypic plasticity in the species complex, with bigger shells in regions where limpets are exposed more frequently to fluctuations caused by the tidal rhythm.
Heat-shock gene expression
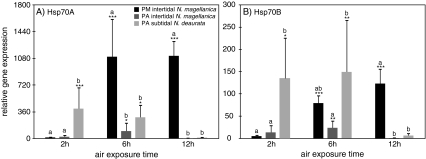
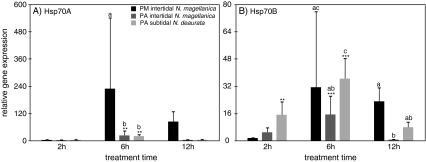
The basal, unstressed expression patterns of Hsp70A and B were similar, but up-regulation of Hsp70A was up to ten times higher when comparing the expression of both genes in each subpopulation and treatment (Table 3, Fig. 4). Temperature curves at both sites at the northern PM and the southern PA were markedly different in the course of experimental exposure. Seawater temperatures at the time of collection in PA were 11°C and at PM 15°C, and the thermal increase during the first 2 h of exposure was 6°C at PA and 3°C at PM. During these first 2 h of air exposure and in spite of 17–18°C of air temperature, no HSR was initiated in either population of N. magellanica at the border (PM) or the centre (PA) of the distribution range, indicating these limpets to be well-adapted to short periods of air exposure. Contrary, the subtidal N. deaurata from PA responded already after 2 h of air exposure with a significant up-regulation of Hsp70A (∼400-fold) and Hsp70B (∼150-fold). In the following 4 h until the end of the 6 h treatment, air temperatures at PA dropped by about 3°C, whereas air temperature in PM rose further to 22°C. This prolonged period of air exposure and desiccation elicited up-regulation of Hsp70A and Hsp70B with the response in N. magellanica at PM being an order of magnitude higher than in PA N. magellanica (PM, ∼1,100 fold; PA, ∼100-fold) and about three times higher compared to PA N. deaurata. Both subpopulations at PA responded to prolonged air exposure (12 h) with down-regulation of the HSR to control level in both Hsp genes. PM N. magellanica responded completely differently with a HSR extended until 12 h of air exposure with up-regulation about ∼1,000-fold compared to the controls for Hsp70A and ∼120-fold for Hsp70B. It is striking that inter-individual variation in N. magellanica and N. deaurata from PA is fairly high compared to less inter-individual variation in N. magellanica from PM. This further indicates that the severe temperature and desiccation stress at PM force all limpets in the experiment to increase their Hsp70 gene expression whereas temperature stress is less pronounced at PA allowing for greater variation among tested individuals.
Table 3.
Average gene expression of Hsp70A and Hsp70B in air-exposed and submerged individuals from Puerto Montt (PM), Punta Arenas (PA) intertidal and Punta Arenas subtidal, relative to untreated control animals
| Gene | Incubation | PM intertidal N. magellanica | PA intertidal N. magellanica | PA subtidal N. deaurata | |||
|---|---|---|---|---|---|---|---|
| Air exposed | Submerged | Air exposed | Submerged | Air exposed | Submerged | ||
| Hsp70A | 2 h | 14.7 ± 9.2 | 3.9 ± 3.4 | 23.3 ± 29.9 | 2.9 ± 2.7 | 402.2 ± 285.6*** | 4.3 ± 3.3 |
| 6 h | 1,101.7 ± 513.9*** | 229.9 ± 314.8 | 99.9 ± 115.9* | 23.8 ± 22.6** | 285.6 ± 172.5* | 20.8 ± 9.7** | |
| 12 h | 1,113.4 ± 200.3*** | 84.9 ± 47.5 | 2.4 ± 1 | 4.2 ± 2.1 | 11.9 ± 12.4 | 4.6 ± 5.1 | |
| Hsp70B | 2 h | 5.3 ± 2.9 | 1.7 ± 0.7 | 13.8 ± 16.6 | 5.0 ± 2.9 | 134.9 ± 92.3* | 15.3 ± 8.1** |
| 6 h | 79.5 ± 17.8*** | 31.5 ± 44.7 | 24 ± 16.5** | 15.5 ± 11.1*** | 149.5 ± 117.3** | 36.5 ± 12.2*** | |
| 12 h | 123.3 ± 34.1*** | 23.2 ± 8.2 | 0.9 ± 0.7 | 0.7 ± 0.5 | 6.6 ± 5.7 | 8.0 ± 3.5 | |
n = 6 for each treatment/population combination except n = 4 for 6 h Punta Arenas subtidal. Values in bold indicate significant up-regulation compared to the control animals (one-way ANOVA, Tukey test, *p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 4.
Average gene expression of A Hsp70A and B Hsp70B in air-exposed individuals from Puerto Montt, Punta Arenas intertidal and Punta Arenas subtidal, relative to untreated control animals (not shown). Asterisks indicate significant up-regulation compared to the control animals (one-way ANOVA, Tukey test, *p < 0.05, **p < 0.01, ***p < 0.001). Letters indicate significant differentiation among the populations in each treatment (two-way ANOVA, Bonferroni, p < 0.05)
Limpets maintained submerged in water during the same experimental period still produced a HSR, but the levels of heat-shock gene induction were much less pronounced than in air-exposed limpets (Fig. 5). For Hsp70A, a significant up-regulation was observed only in individuals from both PA subpopulations and only after 6 h of submersed exposure. Although on average the relative Hsp70A expression after 6 h was much higher in PM (∼240-fold) than in both PA limpet subpopulations, statistical testing failed to prove a significant difference due to high inter-individual fluctuations. Expression patterns of Hsp70B were similar to those of Hsp70A, except that in the PA subtidal limpets Hsp70B was already significantly up-regulated after 2 h.
Fig. 5.
Average gene expression of A Hsp70A and B Hsp70B in submerged individuals from Puerto Montt, Punta Arenas intertidal and Punta Arenas subtidal, relative to untreated control animals (not shown). Asterisks indicate significant up-regulation compared to the control animals (one-way ANOVA, Tukey test, *p < 0.05, **p < 0.01, ***p < 0.001). Letters indicate significant differentiation among the populations in each treatment (two-way ANOVA, Bonferroni, p < 0.05)
SOD and CAT enzyme activities in gills of submersed and air-exposed limpets
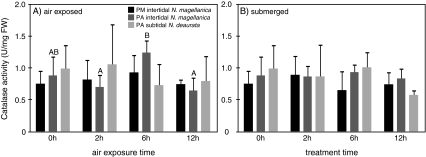
Gill CAT activities showed only little variations in air-exposed and submerged individuals of N. magellanica and N. deaurata at both locations over the time course of the experiments. A significant change in CAT activity could only be detected in gills of the PA intertidal animals after 6 h, where CAT activity was significantly elevated over the activities after 2 and 12 h of air exposure. Contrary, the difference between the 6- and the 0-h control group was not significant (see Table 4 and Fig. 6 for exact values).
Table 4.
Average enzyme activities of SOD and CAT (U/mgFW) in air-exposed and submerged individuals from Puerto Montt (PM), Punta Arenas (PA) intertidal and Punta Arenas subtidal
| Enzyme | Incubation | PM intertidal N. magellanica | PA intertidal N. magellanica | PA subtidal N. deaurata | |||
|---|---|---|---|---|---|---|---|
| Air exposed | Submerged | Air exposed | Submerged | Air exposed | Submerged | ||
| SOD | Controls | 0.414 ± 0.113 | 0.414 ± 0.113 | 0.455 ± 0.066 | 0.455 ± 0.066 | 0.485 ± 0.069 | 0.485 ± 0.069 |
| 2 h | 0.502 ± 0.166 | 0.4 ± 0.048 | 0.713 ± 0.077 | 0.681 ± 0.017 | 0.619 ± 0.135 | 1.111 ± 0.307 | |
| 6 h | 0.449 ± 0.091 | 0.4 ± 0.083 | 0.582 ± 0.031 | 0.447 ± 0.017 | 0.464 ± 0.028 | 0.441 ± 0.07 | |
| 12 h | 0.562 ± 0.103 | 0.437 ± 0.09 | 0.572 ± 0.095 | 0.419 ± 0.088 | 1.371 ± 0.407 | 1,376 ± 0,442 | |
| CAT | Controls | 0.754 ± 0.211 | 0.754 ± 0.211 | 0.883 ± 0.066 | 0.883 ± 0.066 | 0.992 ± 0.069 | 0.992 ± 0.069 |
| 2 h | 0.819 ± 0.317 | 0.893 ± 0.317 | 0.702 ± 0.077 | 0.867 ± 0.095 | 1.058 ± 0.135 | 0,868 ± 0,307 | |
| 6 h | 0.931 ± 0.317 | 0.653 ± 0.317 | 1.242 ± 0.031 | 0.935 ± 0.017 | 0.731 ± 0.028 | 1.011 ± 0.07 | |
| 12 h | 0.747 ± 0.317 | 0.744 ± 0.198 | 0.647 ± 0.095 | 0.836 ± 0.017 | 0.796 ± 0.407 | 0.578 ± 0.442 | |
Fig. 6.
CAT activities (U/mgFW) in A air-exposed and B submerged individuals from Puerto Montt, Punta Arenas intertidal and Punta Arenas subtidal. Significant changes of enzyme activities among treatments are indicated as capital alphabets (one-way ANOVA, Tukey test, p < 0.05) among each population
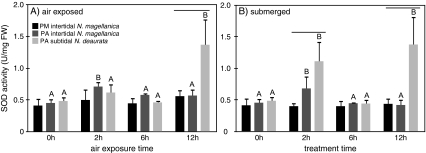
Gill SOD activities in N. magellanica limpets from the PM intertidal did not change during air exposure with respect to the control groups over time. Contrary, in PA both subpopulations responded with an increase in SOD activities at 2 h of air exposure compared to the submersed controls. While this increase was below significance in subtidal N. deaurata, it reached significance level in the intertidal N. magellanica. Indeed, SOD activities in intertidal limpets remained elevated over the submersed control groups at 6 and 12 h of air exposure. To the contrary, SOD activity in subtidal N. deaurata from PA decreased to control level at 6 h after the initial increase and finally peaked at 12 h of air exposure. Submersed N. deaurata in the control group displayed a similar fluctuating SOD pattern over time with highest activities after 2 and 12 h of experimentation (see Table 4 and Fig. 7 for exact values).
Fig. 7.
SOD activities (U/mgFW) in A air-exposed and B submerged individuals from Puerto Montt, Punta Arenas intertidal and Punta Arenas subtidal. Significant changes of enzyme activities among treatments are indicated as capital alphabets (one-way ANOVA, Tukey test, p < 0.05). The vertical bars mark significantly higher SOD activities in Punta Arenas subtidal samples compared to the other two populations (two-way ANOVA, Bonferroni, p < 0.05)
Discussion
HSR on a biogeographic climate gradient along the Patagonian coast
Populations of marine ectotherms living close to the borders of their biogeographic distribution experience stress to a much greater degree than populations of the same species at the centre of the distribution range (Sorte and Hofmann 2004, Osovitz and Hofmann 2005; Tomanek 2008). This also applies to our study of the HSR of N. magellanica along the biogeographical temperature gradient from PM to PA. Short-term air exposure of 2 h did not cause a HSR in N. magellanica at either of the two sampling sites, reflecting the general capacity of these limpets to tolerate short periods of tidal uncovering. However, after prolonged air exposure and at a 10°C higher air temperature only at PM, N. magellanica at the northern distribution edge expressed Hsp genes in much greater quantities than conspecifics from PA. These results suggest that intertidal animals at PM where environmental factors, especially temperature, fluctuate with wider amplitude and produce a stronger HSR to cope with heating during tidal emersion. The higher air temperature maxima at PM appear to represent the thermal tolerance limit for N. magellanica, which would provide one explanation for the absence of limpets in the high intertidal and splash zone of this region. Structuring factors such as predation and competition need to be taking into account during future studies. However, smaller shell and body size of PM compared to PA N. magellanica specimens (Fig. 2) are a typical consequence of warmer climate and indicate higher investments into cellular stress compensation which restricts growth in ectotherms (Moore and Folt 1993; Reznick et al. 2001; Gracey et al. 2008; Daufresne et al. 2009). Laboratory acclimation studies indicate differences in Hsp induction and thermo-tolerance among intertidal congeners from thermally differing habitats to be genetically fixed (Tomanek and Somero 1999). This suggests that the physiological capability for a high-stress response is a consequence of evolutionary adaptation, which may prevent successful colonization of stressful habitats and contribute to setting the geographic boundaries for intertidal species habitat expansion.
Generally, two opposing forces determine the adaptive potential of given populations occupying different habitats. Selection, mutation and genetic drift support adaptation to local conditions, whereas gene flow usually reduces the adaptive potential (Slatkin 1987; Lenormand 2002). In the present case, ongoing gene flow between populations from PA and PM was demonstrated using fast evolving microsatellite markers (Pöhlmann and Held, in preparation). Therefore, repeated introduction of the Hsp70A and Hsp70B alleles, typical for the populations in the centre of the distribution area, might hamper the fixation of newly evolving Hsp mutants at PM and, consequently, diminish the adaptive potential of N. magellanica at its northern edge of distribution.
Interestingly, the study by Clark et al. (2008a) concerning laboratory induction temperatures of the HSR in Antarctic N. concinna showed a significant up-regulation (1,000-fold) of Hsp70A only at 18–20°C, a temperature these limpets never experience in their natural environment. Although it is questionable to what degree real-time PCR results from different studies can be compared, there is a striking similarity to the results of this study regarding the HSR in N. magellanica from PM also being 1,000-fold at temperatures of ∼20°C. In their follow-up study, Clark et al. (2008b) tested the HSR in intertidal N. concinna taken from their natural habitat at different time points during low tide and found Hsp gene up-regulation at much lower air temperatures, clearly demonstrating that the HSR is governed by a far more complex parameter scenario, and not only triggered by temperature. The in situ inducibility of Hsp70A in the study of Clark et al. (2008b) was, however, two orders of magnitude lower (5- to 25-fold) than in the laboratory experiments with heat shock at 20°C. The similarity of HSR induction temperature and response in South American and Antarctic congeners indicates that the HSR in Nacella is old and preserved, and presumably inherited from the common ancestor of today’s extant species. The strong preservation of the HSR limits migration beyond northern habitat boundaries, whereas it might be beneficial for Antarctic intertidal limpets during the ongoing climate change scenario.
Small-scale differences of the HSR in inter- and subtidal populations
At PA, onset and intensity of the HSR to air exposure differed strongly between Nacella from the inter- and the subtidal (Figs. 4 and 5). In intertidal N. magellanica, up-regulation of Hsp70A and Hsp70B occurred later during air exposure, and the intensity of up-regulation was between 2- and 20-fold lower at all time points compared to air-exposed N. deaurata from the subtidal. This again underlines that intertidal N. magellanica have developed adaptations that enable them to survive periods of air exposure during tidal uncovering, which they are prone to experience twice a day, without a necessity of inducing the gene expression of extra Hsps. Comparatively less exposed to tidal emersion than their intertidal relatives, subtidal N. deaurata did not develop these adaptations and are forced to activate the HSR earlier and to a higher extent, to survive air exposure, e.g. during spring tides. Aside from this plausible explanation for the different intensities of the HSR in limpets with different shore level distributions, the initial levels of Hsps may already have been higher in intertidal N. magellanica prior to air exposure. It is possible that shallow intertidal limpets produce Hsps routinely as a preparative defense and, therefore, showed a less pronounced HSR in the course of the experiment (Dong et al. 2008). However, since real-time qPCR represents a relative approach, we cannot make any statement about the Hsp mRNA ground levels in the control animals. Either way, the presented results clearly demonstrate the adaptations in intertidal limpets to cope with recurring stress following tidal emersion.
Most studies of the HSR in the intertidal have focused on the variation in induction temperature. Higher temperature thresholds have been found in high intertidal molluscan populations than in low- and mid-intertidal zones, even after laboratory acclimation at a common temperature, indicating intrinsic fixation of this physiological characteristic (Sanders et al. 1991; Tomanek and Somero 1999; Dong et al. 2008). Since the main trigger for activation of the HSR is the cellular amount of non-native proteins (Feder and Hofmann 1999), stress levels at defined temperatures can be assessed as temporal stress entities that suffice to produce high enough protein damage to activate a HSR. Adaptation to intertidal conditions implies higher physiological tolerance or behavioral adaptation allowing for a later and all in all weaker HSR compared to subtidal conspecifics from the same location. Similar Hsp70 expression in submersed control and air-exposed animals in the intertidal population support this view. The difference in shell morphometry between the two populations of N. magellanica from PM and PA (Fig. 2) reflects phenotypic plasticity in this species complex. Shell height seems adaptive to the habitat rather than genetically fixed. The N. magellanica population inhabiting the intertidal zone at PA features much higher SH values, adaptive to tidal air exposure twice per day, than N. magellanica from PM and N. deaurata from PA, neither of which occurs in the high intertidal. Higher shells provide more space for shell water storage, which may indeed represent an oxygen reserve during the initial period of emersion and contraction, but moreover isolates the animals thermally and prevents desiccation during air exposure (see Vermeij 1973; Hoffman et al. 2010).
The antioxidant stress response in Patagonian Nacella during air exposure
Using the same experimental set-up and the same individuals as in the heat-shock approach, we measured the oxidative stress response in N. magellanica from PM and PA and N. deaurata from PA. The basic levels of SOD and CAT activities in gills of unstressed animals were similar in all three subpopulations. These results are in line with a study of gill tissues of N. magellanica and N. deaurata from Ushuaia, Argentina by Malanga et al. (2005). To the contrary, SOD and CAT activities in digestive gland varied between intertidal and sublitoral limpets at Ushuaia (Malanga et al. 2004) and in the Antarctic (Weihe et al. 2010), and we conclude that antioxidant enzyme activities in South American limpets are a tissue specific phenomenon which, in gills, reflects the oxygenation levels the animals encounter in their respective habitats. The antioxidant activities in gills of the Antarctic congener N. concinna (Weihe et al. 2010), however, revealed considerably higher levels of both antioxidants. Especially SOD was higher in intertidal compared to subtidal Antarctic N. concinna. The subtidal N. concinna had antioxidant levels similar to the South American limpet populations in our study. SOD activities twice as high in Antarctic intertidal N. concinna than in South American intertidal N. magellanica may represent an adaptation to the harsh environment in the Antarctic high intertidal, where extremely low temperatures, thermal fluctuation and fresh water run-off may call for special metabolic adaptations (see Weihe and Abele 2008).
An oxidative stress response in limpet gills from PM was not detectable throughout the whole experiment. Both SOD and CAT activities in gill tissues of air-exposed animals remained stable. Either it is not necessary to increase SOD levels as respiration is controlled on low levels in stress exposed limpets, or available energy is invested into the HSR under conditions of severe warming, rather than into antioxidant defense. It has been reported that the intensive synthesis of Hsps during severe thermal stress can block synthesis of other non-Hsp stress proteins (Lindquist 1980, 1981; Storti et al. 1980).
By contrast, both subpopulations of PA showed elevated SOD activities after 2 h and the subtidal N. deaurata featured their highest SOD activity of all groups at 12 h of air exposure. These patterns of up- and down-regulation of antioxidant enzyme activities can be seen even in the control treatments where animals were kept submerged for the whole experimental period. Strikingly, SOD activity levels seem to correlate with the tidal rhythm in the field, with high SOD activity during low tide and vice versa (Fig. 7). Coupling of gene expression to tidal rhythms could be shown in a large-scale microarray study with the intertidal mussel Mytilus californianus where distinct sets of genes link to different tidal periods (Gracey et al. 2008). As submerged and immersed limpets from the Punta Arenas region showed the same response, there might even be an internal trigger, which regulates SOD activity to match the tidal cycle. This mechanism seems superior even for N. deaurata, which do not regularly fall dry, but presumably feature higher metabolic activity during low tide periods, when animals are exposed to warmer surface waters. In colder environments, investing into antioxidant defense seems to be important for survival in the intertidal and in the shallow subtidal to minimize the risk of oxidative damage during emersion.
Conclusions
Our study provides new insight into the biogeography, and locally into the time resolution pattern, of the anti-stress response in tidally emerged limpets in Patagonia. The stress response includes the heat-shock protein gene expression and enzymatic antioxidant defense. Patagonian Nacella have developed markedly different physiological strategies to survive thermal and air exposure stress upon emersion depending on their climatic and shore level positioning and adaptation. In the Northern Patagonian region at Puerto Montt, we are dealing with a population that, once air exposed on the beach under experimental conditions, is stressed beyond levels normally experienced in their deep intertidal environment. These animals exhibit a pronounced HSR and apparently the heat stress is so high that the animals are unable to increase antioxidant activities for protection. A failure of the antioxidant response has been shown to occur in animals stressed beyond tolerance limits (Abele 2011 in press). In Punta Arenas, thermal stress is very limited and only the subtidal animals show a pronounced HSR when experimentally exposed to air and to warming. Here, the dynamics of antioxidant defense system correlate to the tidal rhythm, with higher activities during times of tidal air exposure. Apparently, antioxidants are important anti-stress proteins that prepare limpets in their biogeographical optimum range for short periods of regularly occurring tidal emersion. It follows that fluctuations of antioxidants with tidal cycles, together with a mild HSR during emersion indicate optimal adaptation in these intertidal mollusks. Contrary, an extreme HSR and no antioxidant response at all seem to indicate extreme, presumably eventually lethal stress condition at the border of the thermal tolerance range at the northern (warm) edge of geographic distribution.
Acknowledgements
We would like to thank Kurt Paschke from the UACh, Puerto Montt and Erika Mutschke, Carlos Rios and Rodrigo Mancilla from the Universidad de Magallanes, Punta Arenas for their great support during the experimental field work, and two anonymous reviewers for the time they invested and their help in improving the manuscript. The study was funded by the German Academic Exchange Service (DAAD) grant number D/08/46637.
References
- Abele D. Temperature adaptation in changing climate marine fish and invertebrates. In: Storey KB, Tannino K, editors. Temperature Adaptation in a Changing Climate. Wallingford, UK: CABI Publishers; 2011. [Google Scholar]
- Abele D, Puntarulo S. Formation of reactive species and induction of antioxidant defense systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A. 2004;138:405–415. doi: 10.1016/j.cbpb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Abele D, Tesch C, Wencke P, Pörtner HO. How oxidative stress parameters relate to thermal tolerance in the Antarctic bivalve Yoldia eightsi? Antarct Sci. 2001;13:111–118. doi: 10.1017/S0954102001000189. [DOI] [Google Scholar]
- Abele D, Heise K, Pörtner HO, Puntarulo S. Temperature dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol. 2002;205:1831–1841. doi: 10.1242/jeb.205.13.1831. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Fraser KPP, Peck LS. Antarctic marine molluscs do have an hsp70 heat-shock response. Cell Stress Chaperon. 2008;13:39–49. doi: 10.1007/s12192-008-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Geissler P, Waller C, Fraser KPP, Barnes DKA, Peck LS. Low heat-shock thresholds in wild Antarctic inter-tidal limpets (Nacella concinna) Cell Stress Chaperon. 2008;13:51–58. doi: 10.1007/s12192-008-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci USA. 2009;106:12788–12793. doi: 10.1073/pnas.0902080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranzamendi MC, Gardenal CN, Martin JP, Bastida R. Limpets of the genus Nacella (Patellogastropoda) from the Southwestern Atlantic: species identification based on molecular data. J Molluscan Stud. 2009;75:241–251. doi: 10.1093/mollus/eyp025. [DOI] [Google Scholar]
- Denny MW, Miller LP, Harley CDG. Thermal stress on intertidal limpets: long-term hindcasts and lethal limits. J Ex Biol. 2006;209:2420–2431. doi: 10.1242/jeb.02258. [DOI] [PubMed] [Google Scholar]
- Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in-situ exposure to heat stress. Biol Bull. 2008;215:173–181. doi: 10.2307/25470698. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, Somero GN. Rhythms of gene expression in a fluctuating intertidal environment. Curr Biol. 2008;18:1501–1507. doi: 10.1016/j.cub.2008.08.049. [DOI] [PubMed] [Google Scholar]
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. The impacts of climate change in coastal marine systems. Ecol Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Heise K, Puntarulo S, Pörtner HO, Abele D. Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternula elliptica (King and Broderip) under heat stress. Comp Biochem Physiol C. 2003;134:79–90. doi: 10.1016/S1096-4959(02)00231-2. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Harley CDG, Halpin PM, O’Donnell MJ, Hofmann GE, Blanchette CA. Climate change and latitudinal patterns of intertidal thermal stress. Science. 2006;298:1015–1017. doi: 10.1126/science.1076814. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Broitman BR, Blanchette CA, Gilman S, Halpin PM, Harley CDG, O’Donnell MJ, Hofmann GE, Menge B, Strickland D. Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr. 2006;76:461–479. doi: 10.1890/0012-9615(2006)076[0461:MPOTSI]2.0.CO;2. [DOI] [Google Scholar]
- Hoffman JI, Peck LS, Hillyard G, Zieritz A, Clark MS. No evidence for genetic differentiation between Antarctic limpet Nacella concinna morphotypes. Mar Biol. 2010;157:765–778. doi: 10.1007/s00227-009-1360-5. [DOI] [Google Scholar]
- Hofmann GE. Ecologically relevant variation in induction and function of heat-shock proteins in marine organisms. Am Zool. 1999;39:889–900. [Google Scholar]
- Hofmann GE. Patterns of Hsp gene expression in ectothermic marine organisms on small to large biogeographic scales. Integr Comp Biol. 2005;45:247–255. doi: 10.1093/icb/45.2.247. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17:183–189. doi: 10.1016/S0169-5347(02)02497-7. [DOI] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat-shock: implications for regulations. Dev Biol. 1980;77:463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat-shock. Nature. 1981;293:311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Livingstone DR, Lips F, Garcia Martinez P, Pipe RK. Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Biol. 1992;112:265–276. doi: 10.1007/BF00702471. [DOI] [Google Scholar]
- Malanga G, Estevez MS, Calvo J, Puntarulo S. Oxidative stress in limpets exposed to different environmental conditions in the Beagle Channel. Aquat Toxicol. 2004;69:299–309. doi: 10.1016/j.aquatox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Malanga G, Estevez MS, Calvo J, Abele D, Puntarulo S. Oxidative stress in gills of limpets from the Beagle Channel: comparison with limpets from the Antarctic. Sci Mar. 2005;69:297–304. [Google Scholar]
- Moore M, Folt C. Zooplankton body size and community structure: effects of thermal and toxicant stress. Trends Ecol Evol. 1993;8:178–183. doi: 10.1016/0169-5347(93)90144-E. [DOI] [PubMed] [Google Scholar]
- Morley SA, Clark MS, Peck LS. Depth gradients in shell morphology correlate with thermal limits for activity and ice disturbance in Antarctic limpets. J Exp Mar Biol Ecol. 2010;390:1–5. doi: 10.1016/j.jembe.2010.04.040. [DOI] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ozawa T. Worldwide phylogeography of limpets of the order Patellogastropoda: molecular, morphological and palaeontological evidence. J Molluscan Stud. 2007;73:79–99. doi: 10.1093/mollus/eym001. [DOI] [Google Scholar]
- Osovitz CJ, Hofmann GE. Thermal history-dependent expression of the hsp70 gene in purple sea urchins: biogeographic patterns and the effect of temperature acclimation. J Exp Mar Biol Ecol. 2005;327:134–143. doi: 10.1016/j.jembe.2005.06.011. [DOI] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance—degradation and reactivation of damaged proteins. Ann Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Powell AWB. The patellid limpets of the world (Patellidae) Indo-Pacific Mollusca. 1973;3:75–206. [Google Scholar]
- Reznick D, Butler MJ, Rodd H. Life-history in guppies. VII. The comparative ecology of high- and low-predation environments. Am Nat. 2001;157:12–26. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- Sanders BM, Hope C, Pascoe VM, Martin LS. Characterization of the stress protein response in two species of Collisella limpets with different temperature tolerances. Physiol Zool. 1991;64:1471–1489. [Google Scholar]
- Slatkin Gene flow and the geographic structure of natural populations. Science. 1987;236:787–792. doi: 10.1126/science.3576198. [DOI] [PubMed] [Google Scholar]
- Somero GN. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol. 2002;42:780–789. doi: 10.1093/icb/42.4.780. [DOI] [PubMed] [Google Scholar]
- Somero GN. Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front Zool. 2005;2:1. doi: 10.1186/1742-9994-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorte CJB, Hofmann GE. Changes in latitudes, changes in aptitudes: Nucella canaliculata (Mollusca: Gastropoda) is more stressed at its range edge. Mar Ecol Prog Ser. 2004;274:263–268. doi: 10.3354/meps274263. [DOI] [Google Scholar]
- Storti RV, Scott MP, Rich A, Pardue ML. Translational control of protein synthesis in response to heat-shock in D. melanogaster cells. Cell. 1980;22:825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tomanek L. The heat-shock response: its variation, regulation and ecological importance in intertidal gastropods (genus Tegula) Integr Comp Biol. 2002;42:797–807. doi: 10.1093/icb/42.4.797. [DOI] [PubMed] [Google Scholar]
- Tomanek L. The importance of physiological limits in determining biogeographical range shifts due to global climate change: the heat-shock response. Physiol Biochem Zool. 2008;81:709–717. doi: 10.1086/590163. [DOI] [PubMed] [Google Scholar]
- Tomanek L. Variation in the heat-shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J Exp Biol. 2010;213:971–979. doi: 10.1242/jeb.038034. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Sanford E. Heat-shock protein 70 (hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula) Biol Bull. 2003;205:276–284. doi: 10.2307/1543291. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero G. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Valdovinos C, Rüth M. Nacellidae limpets of the southern end of South America: taxonomy and distribution. Rev Chil Hist Nat. 2005;78:497–517. doi: 10.4067/S0716-078X2005000300011. [DOI] [Google Scholar]
- Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034–research0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij Morphological patterns in high-intertidal gastropods: adaptive strategies and their limitations. Mar Biol. 1973;20:319–346. doi: 10.1007/BF00354275. [DOI] [Google Scholar]
- Weihe E, Abele D. Differences in the physiological response of inter- and subtidal Antarctic limpets Nacella concinna to aerial exposure. Aquat Biol. 2008;4:155–166. doi: 10.3354/ab00103. [DOI] [Google Scholar]
- Weihe E, Kriews M, Abele D. Differences in heavy metal concentrations and in the response of the antioxidant system to hypoxia and air exposure in the Antarctic limpet Nacella concinna. Mar Environ Res. 2010;69:127–135. doi: 10.1016/j.marenvres.2009.09.003. [DOI] [PubMed] [Google Scholar]