Abstract
Mucosal wound healing in adults has been reported to feature diminished scar formation compared to healing skin wounds. We sought to determine if the expression pattern of chaperonin containing T-complex polypeptide (CCT) subunits in mucosal wounds and fibroblasts is different from that observed in skin wounds and fibroblasts. We found that CCT-beta is the only subunit message to be reduced in wounded mucosa versus unwounded control, and this reduction was confirmed at the protein level. In contrast, mRNA levels of CCT-zeta, -delta, -eta, and -epsilon were significantly increased in mucosal wounds. The increase in CCT-eta was also confirmed at the protein level. Expression levels of CCT-alpha, -beta, -delta; -epsilon, and -theta mRNAs were significantly increased in adult mucosal fibroblasts in culture compared to skin-derived fibroblasts. Western blot analyses confirmed a modest increase in CCT-beta in adult mucosal fibroblasts relative to skin fibroblasts, but CCT-eta protein was unaffected. These differences may contribute to the reported difference in healing outcomes between these two tissue types.
Keywords: Chaperonin containing T-complex polypeptide, CCT, Oral mucosal wounds, qRT-PCR, Fibroblasts
Introduction
Oral mucosal tissues have been reported to heal faster with less inflammation and less scar than skin does, and the development of hypertrophic scars or keloids in the oral cavity is uncommon (Stephens et al. 1996; Lee and Eun 1999; Szpaderska et al. 2003). The healing of oral mucosal tissue has been compared to the scarless healing of skin wounds observed during early mammalian development, sharing several common characteristic features such as reduced inflammation and faster re-epithelialization (Szpaderska et al. 2003; Häkkinen et al. 2000; Ferguson and O'Kane 2004). The overlapping phases of wound healing, including hemostasis, inflammation, proliferation, and remodeling of the collagen matrix, are seen in both skin and oral mucosal tissues but these processes appear to reach completion more rapidly at the mucosal site (Sciubba et al. 1978; Walsh et al. 1996).
Some previous reports have suggested that saliva, containing abundant amounts of cytokines, growth factors, and protease inhibitors, is an important factor that may contribute to rapid oral wound healing (Zelles et al. 1995; Ashcroft et al. 2000), and sialoadenectomized mice and rats have been shown to exhibit delayed healing of oral wounds (Hutson et al. 1979; Bodner et al. 1992). On the other hand, exclusion of saliva (e.g., by means of a salivary bypass tube) is frequently employed clinically to assist in the closure of pharyngocutaneous fistulae (Sevilla García et al. 2006), indicating that the role of saliva in mucosal wound healing is still clinically uncertain. Other investigations point to differences in inflammatory infiltrates, the inherently different composition of the ECM, and phenotypically unique cells as possible contributors to rapid oral wound healing (Lee and Eun 1999; Szpaderska et al. 2003; Shannon et al. 2006; Honardoust et al. 2008). However, the key determinative factors leading to improved wound healing with less apparent scarring in oral mucosal wounds remains poorly understood.
Studies from our laboratory suggest that the chief cellular cytosolic chaperone, the chaperonin containing T-complex polypeptide (CCT), plays a significant role in differentiating scarless fetal healing from the scirrhous healing of adult skin wounds (Darden et al. 2000; Satish et al. 2008; Satish et al. 2010a, b). The CCT protein is a large (900 kD) barrel-shaped hexadecameric protein complex that has the ability to bind and engulf unfolded/misfolded proteins (Hartl and Hayer-Hartl 2002; Fenton and Horwich 2003), assisting them to achieve proper conformation. It has primarily been implicated in the folding of cytoskeletal proteins such as tubulin and actin (Willison and Kubota 1994; Kubota et al. 1995), but has been estimated to interact with up to 15% of all cellular proteins, and is an important factor in a variety of processes including embryogenesis, ciliary biogenesis, cell viability, cell proliferation, and locomotion. Alterations in CCT components, therefore, have the potential to exert pleiotropic effects on cell biology.
We have previously identified CCT subunit eta to be decreased in our rabbit model of fetal skin wound healing by differential display RT-PCR and semi-quantitative RT-PCR (Darden et al. 2000), and have recently confirmed this with quantitative real-time RT-PCR (Satish et al. 2010a, b). We noted that no other chaperonin subunits share this specific pattern of downregulation at the tissue level (Satish et al. 2008), and have further observed that fibroblasts from fetal skin tissues express substantially less CCT-eta mRNA than do fibroblasts from adult skin (Satish et al. 2010a, b). These observations led us to investigate the pattern of CCT subunit expression in mucosal tissues and fibroblasts to see if comparable patterns would emerge.
Because our model system is in rabbit, and because full genomic sequence data is unavailable, it was first necessary to design and validate primer and probe sequences that would allow quantitation of the CCT subunit messages. These quantitative assays were then used to survey the mRNA expression patterns of all eight CCT subunits in adult buccal mucosal wound and control tissues, and in fibroblasts isolated from skin and buccal mucosa (Table 1).
Table 1.
Primer and probe sequences used for quantitative real time RT-PCR
| Genes | Primer | Sequences |
|---|---|---|
| CCT-gamma | Forward | 5′-CTCATGCGGGCCAATGTC-3′ |
| Reverse | 5′-ACAGGCTCTAGCGATGCGATTA-3′ | |
| Probe | 5′-6 FAM-CAGCCATCCGCAGAGTCCGGAA-TAMRA-3′ | |
| CCT-delta | Forward | 5′-AGCGGTTGCTGATGCTATTAGAA-3′ |
| Reverse | 5′-TAATGGTCACATCGCCTTTTCC-3′ | |
| Probe | 5′-6 FAM-AGCCTTGGACCTAAAGGAATGGATAAAATGA-TAMRA-3′ | |
| CCT-epsilon | Forward | 5′-CTTGGAGCAGTACGCCATGAG-3′ |
| Reverse | 5′- GTTCATGCCACTATTTTCAGAAAGG-3′ | |
| Probe | 5′-6 FAM-TGCCCTGGAGGTCATCCCCATG-TAMRA-3′ | |
| CCT-theta | Forward | 5′-GGTGGAGCAACAGAGATCGAA-3′ |
| Reverse | 5′-ATAGCATATTGTTCAAGTCCAGGACAT-3′ | |
| Probe | 5′-6 FAM-TGGCCAAACAGATCACATCATATGGAGAGA-TAMRA-3′ |
The primer sets for rabbit CCT-gamma and CCT-theta were designed using the predicted rabbit sequences for CCT-gamma (ENSOCUT00000000137) and CCT-theta (ENSOCUT00000002642) from Ensembl (http://www.ensembl.org/). The primer set for rabbit CCT-delta was designed by identifying regions that were similar between the mouse and rat for CCT-delta (ENSMUST00000020562, ENSRNOT00000012847, respectively). For CCT-epsilon, the primer set was derived from conserved regions in mouse and rat and was confirmed with rabbit sequence from Ensembl (ENSOCUG00000021231). Primer sets to each of these four chaperonin subunits were designed within the coding regions and used in initial RT-PCR assays on 10 ng of adult mucosal control total RNA to verify that each of the respective CCT subunits were detectable in adult tissue and resulted in only a single amplicon of the expected molecular weight. Primers and probes for rabbit CCT-alpha, -beta, -eta, -zeta, and GAPDH have been previously reported (Kathju et al. 2006; Satish et al. 2008; Satish et al. 2010a, b). All Taqman primers/probes were designed using Primer Express software (Applied Biosystems, Foster City, CA). All primers and fluorocoupled Taqman probes were purchased from Integrated DNA Technologies (Coralville, IA)
Results and discussion
In this report, we examine the mRNA expression of all eight subunits of CCT, the major cytosolic chaperonin, in wounded mucosal tissues (versus unwounded control), and also examine the level of CCT message expression in fibroblasts derived from adult mucosal tissues versus adult skin. We have previously described the probes and primers used to assay for CCT subunits -alpha, -beta, -eta, and -zeta in semi-quantitative limiting dilution RT-PCR as well as quantitative real-time RT-PCR (Darden et al. 2000; Satish et al. 2008; Satish et al. 2010a, b).
In the present study, we designed and validated primer and probe sequences for rabbit CCT subunits gamma, delta, epsilon, and theta. We first tested the primer sets using 10 ng of rabbit adult control total RNA by RT-PCR. A single amplicon product of the expected molecular weight after gel electrophoresis confirmed the successful target of the correct CCT template molecule in each of the subunits. The amplicons obtained from using CCT-epsilon and -delta primers were sequenced to confirm the specificity of the primers and probe used for quantitative RT-PCR assays; recently, rabbit CCT-epsilon sequence has become available in the Ensembl database (ENSOCUG00000021231) and confirms our sequencing results. Sequences for CCT-gamma and -theta were obtained from the Ensembl database (ENSOCUT00000000137 and ENSOCUT00000002642, respectively). We also confirmed that quantitative assays displayed an appropriate range of linearity when tested across a standard curve of varying CCT subunit concentrations (data not shown).
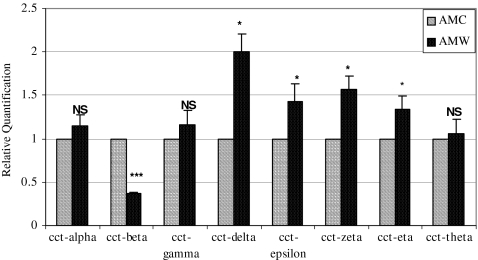
Quantitative real time RT-PCR assays were then performed on total RNA from mucosal wound tissue versus unwounded control. We determined that the only CCT subunit that was reduced in adult mucosal wound tissue was CCT-beta, while CCT-delta, -epsilon, -zeta, and -eta were modestly but significantly increased in wound tissue in comparison to unwounded controls. CCT subunits -alpha, -gamma, and -theta showed no apparent difference in mRNA levels between adult mucosal unwounded and wounded tissues (Fig. 1). These results are somewhat at variance with our observations from adult cutaneous wounds at a similar time point; in skin wounds, CCT-alpha is reduced, while CCT-beta remains invariant (Satish et al. 2008).
Fig. 1.
Quantitative RT-PCR of CCT subunits in rabbit adult wounded and unwounded oral mucosal tissues. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). Adult New Zealand white rabbits were anesthetized and bilateral 1 cm buccal mucosal incisional wounds were made. After 12 h, the animals were re-anesthetized and the wounds were excised with 1-mm margins, as well as unwounded mucosal control tissues. The samples were immediately immersed in RNAlater® (Ambion, Austin, TX) and stored at −80°C until RNA isolation. Tissues harvested from adult mucosal control (AMC) and adult mucosal wound (AMW) tissues were homogenized using a PRO 200 homogenizer (ISC Bioexpress, Kaysville, UT) and total RNA was extracted using the RNeasy Mini kit (Qiagen Inc. Valencia, CA) as per the manufacturer's instructions. This total RNA was characterized on an Agilent 2100 BioAnalyzer (Agilent Technologies Inc., Palo Alto, CA) to verify integrity (lack of degradation), and then served as the template for quantitative RT-PCR assays for the eight chaperonin subunits. Real time RT-PCR was performed to examine patterns of expression of all the eight CCT- subunits in wounded and unwounded oral mucosal tissues. Ten nanograms of total RNA from individual samples was used for reverse transcriptase (RT) reaction (using gene specific reverse primer and 11 μl of total volume); for subsequent real time PCR assays, 1.5 μL of RT reaction, 800 nM of each primer, and 160 nM of a probe (final concentrations) made to a final volume of 15 μl were used. The remaining protocol for RT reaction and real time PCR was followed as previously described (Kathju et al. 2006; Satish et al. 2008; Satish et al. 2010a, b). Using the comparative critical cycle (Ct) method and using GAPDH as the endogenous control, the expression levels of the target genes were normalized and the relative abundance was calculated. Data were analyzed using the 7900 HT SDS software version 2.1 provided by Applied Biosystems. Animal experiments were performed from RNA derived from two animals used in this study; results shown are mean ± SEM of six independent experiments done in duplicate. Statistical analyses were performed using Student's t test with p value < 0.05 considered significant. (*** represents p value < 0.001; *represents p value < 0.05). AMC adult mucosal control, AMW adult mucosal wound, NS not significant
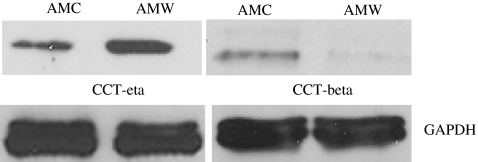
We also determined the protein expression levels of CCT subunits eta and beta. We found that CCT-eta protein was significantly elevated in adult mucosal wounds compared to unwounded mucosa, a finding that is similarly seen in wounded skin tissues. Strikingly, however, CCT-beta protein was significantly decreased in healing mucosal wounds, confirming the reduction in CCT-beta mRNA observed (Fig. 2). This is in notable contrast to healing skin wounds, where overall CCT-beta levels show essentially no variance over time.
Fig. 2.
Western blot analysis of proteins isolated from rabbit adult oral mucosal wounded and unwounded tissues. Proteins were extracted from wounded and unwounded buccal mucosal tissues using Tissue Protein Extraction Reagent (T-PER) obtained from Thermo Fisher Scientific (Rockford, IL). The protein concentration was measured using Bradford assay, and equal amounts of proteins were resolved by SDS-PAGE and analyzed by immunoblotting with chaperonin-specific antibodies for CCT-beta (cat # MCA 2275) and CCT-eta (cat #MCA 2179) subunits obtained from SeroTec Inc., Raleigh, NC. Antibody against GAPDH (cat # ab8245, Abcam Inc., Cambridge, MA) was used as a loading control. Shown is a representative immunoblot of three independent studies. AMC adult mucosal control, AMW adult mucosal wound
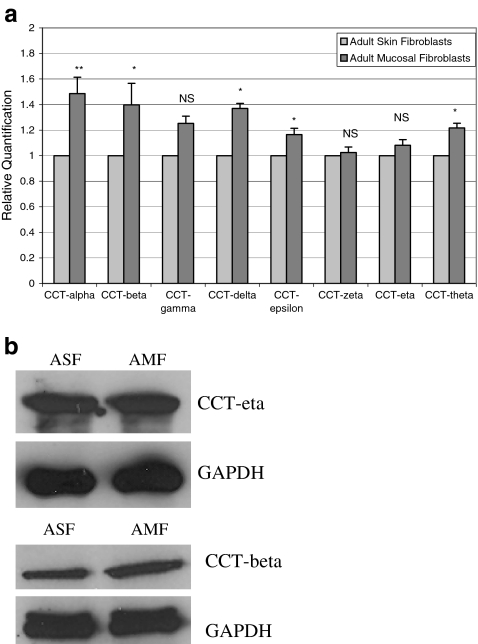
Since scar formation is widely understood to be a consequence of fibroblast activity, we next examined the relative abundance of all eight chaperonin subunits in cultured adult skin and oral mucosal fibroblasts using these same quantitative RT-PCR assays. Interestingly, we found that mRNA levels of CCT subunits alpha, beta, delta, epsilon, and theta were modestly but significantly higher in adult mucosal fibroblasts in comparison to skin-derived fibroblasts. The other three chaperonin subunits (CCT-gamma, -zeta, and -eta) did not show any significant difference in relative expression between the two cell types (Fig. 3a). Western blot analysis showed a modest increase in CCT-beta protein levels in adult mucosal fibroblasts in comparison to adult skin fibroblasts but there was no significant difference in CCT-eta between the two cell types (Fig. 3b).
Fig. 3.
a Quantitative RT-PCR of CCT subunits in adult skin and oral mucosal fibroblasts. Skin and oral mucosal tissue pieces obtained from adult rabbits were minced into small pieces within 30 min after dissection. Specimens were washed in PBS containing 1% antibiotic/antimycotic solution (Sigma, St Louis, MO) and then placed in RPMI 1640 medium (Invitrogen Corp.) containing 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA) and 1% antibiotic/antimycotic solution (Invitrogen). The cultures were left undisturbed for a week in a 37°C incubator containing 5% CO2 supplement. Fibroblast outgrowths observed after a week from primary cultures were sub-cultured immediately using 0.5% Trypsin-EDTA (Invitrogen). Once the cells reached 90% confluence, total RNA was isolated from skin and mucosal fibroblasts using the RNeasy Micro Kit (Qiagen Inc. USA, Valencia, CA). All cells were confirmed to have typical fibroblast morphology by microscopy prior to nucleic acid extraction. Real time RT-PCR analysis for all eight CCT subunits was performed as described in Figure 1, using GAPDH as internal control. Data are shown as mean ± SEM of six independent experiments performed in triplicate. Statistical analyses were performed using Student's t test with p value < 0.05 considered significant. (**represents p value < 0.01; *represents p value < 0.05; NS not significant). bWestern blot analysis of proteins isolated from adult skin and oral mucosal fibroblasts. Equal amounts of protein lysates isolated from rabbit adult skin and oral mucosal fibroblasts were subjected to SDS-PAGE and analyzed by CCT-eta and CCT-beta specific antibodies using GAPDH as controls as mentioned in Figure 2. Shown here is a representative immunoblot of three independent studies. ASF adult skin fibroblasts, AMF adult mucosal fibroblasts
Our results show that there is differential expression of CCT subunits during adult mucosal wound healing at the RNA and protein levels, but this expression is not identical to that seen in skin wounds. Notably, CCT-alpha, reduced in adult skin wounds, remains unchanged in buccal mucosal wounds, whereas CCT-beta displays the converse pattern: it is reduced in adult mucosal wounds, but unchanged in wounded adult skin. CCT-eta is increased in adult mucosal wounds, and this actually mirrors the pattern we have observed in adult skin wounds as well (Satish et al. 2010a, b). In addition the CCT-delta subunit is significantly increased to a threshold of two-fold in wounded mucosal tissue; all other subunits remain unchanged or show only modest (although statistically significant) variation. These observations show that adult mucosal wounds vary significantly from adult and fetal skin wounds, and indicate that adult mucosal wound healing is not a replica of fetal skin wound healing.
The results described here are derived from excised wound tissues, which contain a variety of cell types. It has already been noted that there are differences in inflammatory infiltrates and a likelihood of unique cellular components in mucosal versus skin tissue; these factors may be important contributors to the differential expression patterns we have noted. To tease apart specifically differential expression in particular cell types in vivo will require in situ and immunohistochemical studies for individual CCT subunits.
Apart from skin (and now mucosal) wound healing, CCT expression has only been examined in one other wound healing system. Koulikovska et al. investigated the expression of CCT-eta and alpha-smooth muscle actin in a rabbit model of corneal wound healing after a mild (not severe) ultraviolet injury (Koulikovska et al. 2005). They found that CCT-eta was increased in response to injury, tracking a similar increase seen in alpha-smooth muscle actin, before returning to essentially baseline levels after five days. One consistent observation, therefore, is that in all adult wound healing systems examined—skin, cornea, and oral mucosa—CCT-eta is elevated in response to injury.
Underlying the observation that different CCT subunits can display discrete and differential patterns of gene expression is an even more fundamental question: does differential regulation of CCT subunits exert its influence on cell behavior by modifying the activity of the CCT hexadecameric chaperonin particle, or do individual subunits have other functions without the CCT holoenzyme? There is evidence that both mechanisms may be important. The hexadecameric CCT particle has been noted to interact with different substrates through specific subunit contacts; in the case of alpha-actin, for example, interaction with CCT is thought to be mediated through subunits—delta and either beta or epsilon (Llorca et al. 1999). Since alpha-actin (smooth muscle) is known to be an important marker for fibroblasts transitioning to myofibroblasts, the cell type most directly implicated in wound contraction and scar formation, relative depletion, or excess of these subunits may well affect CCT activity and dependent cell physiology. Our own observations also point to a role for CCT-eta in this regard (Satish et al. 2010a, b). Tubulin also appears to engage with CCT through specific subunits (Llorca et al. 2000).
Alternatively, there is an increasing body of evidence that CCT subunits may have discrete functions separate from their involvement with the hexadecameric CCT holoenzyme. CCT-delta, for example, has been found to directly stimulate the binding of the trans-acting factor TRP-185 to its target RNA sequence (Wu-Baer et al. 1996). CCT-eta has been found to be a direct biological partner for the soluble guanylyl cyclase (sGC), the chief intracellular second messenger for nitric oxide signaling (Hanafy et al. 2004). CCT-eta inhibits the nitric oxide-dependent activation of sGC both in vitro and when overexpressed in cells. Since nitric oxide is known to play an important role in controlling the wound healing response, this may also prove a relevant mechanism by which CCT can affect tissue repair and scar formation. Future investigation will hopefully clarify which of these (or other) possibilities are most pertinent to the biology of both mucosal and skin wound healing.
Acknowledgements
This study was supported by the Allegheny-Singer Research Institute, Allegheny General Hospital, and Pittsburgh Tissue Engineering Institute (PTEI). This work was funded from grants awarded to S.K. (DE014780; Triological Society Career Development Award), and L.S. (3M Fellowship).
References
- Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Bodner L, Dayan D, Oberman M, Hirshberg A, Tal H. Healing of experimental wounds in sialadenectomized rat. J Clin Periodontol. 1992;19:345–347. doi: 10.1111/j.1600-051X.1992.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Darden DL, Hu FZ, Ehrlich MD, Gorry MC, Dressman D, Li HS, Whitcomb DC, Hebda PA, Dohar JE, Ehrlich GD. RNA differential display of scarless wound healing in fetal rabbit indicates downregulation of a CCT chaperonin subunit and upregulation of a glycophorin-like gene transcript. J Pediatr Surg. 2000;35:406–419. doi: 10.1016/S0022-3468(00)90204-5. [DOI] [PubMed] [Google Scholar]
- Fenton WA, Horwich AL. Chaperonin-mediated protein folding: Fate of substrate polypeptide. Q Rev Biophys. 2003;36:229–256. doi: 10.1017/S0033583503003883. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24:127–152. doi: 10.1034/j.1600-0757.2000.024001127.x. [DOI] [PubMed] [Google Scholar]
- Hanafy KA, Martin E, Murad F. CCTeta, a novel soluble guanylyl cyclase-interacting protein. J Biol Chem. 2004;279:46946–46953. doi: 10.1074/jbc.M404134200. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Honardoust D, Eslami A, Larjava H, Häkkinen L. Localization of small leucine-rich proteoglycans and transforming growth factor-beta in human oral mucosal wound healing. Wound Repair Regen. 2008;16:814–823. doi: 10.1111/j.1524-475X.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Niall M, Evans D, Fowler R. Effect of salivary glands on wound contraction in mice. Nature. 1979;279:793–795. doi: 10.1038/279793a0. [DOI] [PubMed] [Google Scholar]
- Kathju S, Satish L, Rabik C, Rupert T, Oswald D, Johnson S, Hu FZ, Post JC. Identification of differentially expressed genes in scarless wound healing utilizing polymerase chain reaction-suppression subtractive hybridization. Wound Repair Regen. 2006;14:413–420. doi: 10.1111/j.1743-6109.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Koulikovska M, Podskochy A, Fagerholm P. The expression pattern of the subunit of chaperonin containing T-complex polypeptide 1 and its substrate, alpha-smooth muscle actin, during corneal wound healing. Acta Opthalmol Scand. 2005;83:543–548. doi: 10.1111/j.1600-0420.2005.00482.x. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Willison K. The chaperonin containing t-complex polypeptide 1 (TCP-1) Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem. 1995;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- Lee HG, Eun HC. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J Dermatol Sci. 1999;21:176–182. doi: 10.1016/S0923-1811(99)00037-7. [DOI] [PubMed] [Google Scholar]
- Llorca O, McCormack EA, Hynes G, Grantham J, Cordell J, Carrascosa JL, Willison KR, Fernandez JJ, Valpuesta JM. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature. 1999;402:693–696. doi: 10.1038/45294. [DOI] [PubMed] [Google Scholar]
- Llorca O, Martín-Benito J, Ritco-Vonsovici M, Grantham J, Hynes GM, Willison KR, Carrascosa JL, Valpuesta JM. Eukaryotic chaperonin CCT stabilizes actin and tubulin folding intermediates in open quasi-native conformations. EMBO J. 2000;19:5971–5979. doi: 10.1093/emboj/19.22.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish L, Abdulally A, Oswald D, Johnson S, Hu FZ, Post JC, Ehrlich GD, Kathju S. Differential expression of chaperonin containing T-complex polypeptide (CCT) subunits during fetal and adult skin wound healing. Cell Stress Chaperones. 2008;13:527–533. doi: 10.1007/s12192-008-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish L, Johnson S, Wang JH, Post JC, Ehrlich GD, Kathju S. Chaperonin containing T-complex polypeptide subunit eta (CCT-eta) is a specific regulator of fibroblast motility and contractility. PLoS One. 2010;5:e10063. doi: 10.1371/journal.pone.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish L, Johnson S, Abdulally A, Post JC, Ehrlich GD, Kathju S. Cloning and expression of rabbit CCT subunits eta and beta in healing cutaneous wounds. Cell Stress Chaperones. 2010;15:819–826. doi: 10.1007/s12192-010-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciubba JJ, Waterhouse JP, Meyer J. A fine structural comparison of the healing of incisional wounds of mucosa and skin. J Oral Pathol. 1978;7:214–227. doi: 10.1111/j.1600-0714.1978.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Sevilla García MA, Suárez Fente V, Rodrigo Tapia JP, Llorente Pendás JL. Montogomery salivary bypass tube: a simple solution for pharyngocutaneous fistulas. Acta Otorrinolaringol Esp. 2006;57:467–470. doi: 10.1016/s0001-6519(06)78750-7. [DOI] [PubMed] [Google Scholar]
- Shannon DB, McKeown ST, Lundy FT, Irwin CR. Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen. 2006;14:172–178. doi: 10.1111/j.1743-6109.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- Stephens P, Davies KJ, Al-Khateeb T, Shepherd JP, Thomas DW. A comparison of the ability of intra-oral and extra-oral fibroblasts to stimulate extracellular matrix reorganization in a model of wound contraction. J Dent Res. 1996;75:1358–1364. doi: 10.1177/00220345960750060601. [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- Walsh LJ, L'Estrange PR, Seymour GJ. High magnification in situ viewing of wound healing in oral mucosa. Aust Dent J. 1996;41:75–79. doi: 10.1111/j.1834-7819.1996.tb05916.x. [DOI] [PubMed] [Google Scholar]
- Willison KR, Kubota H. The structure, function, and genetics of the chaperonin containing TCP-1 (CCT) in eukaryotic cytosol. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994. pp. 299–312. [Google Scholar]
- Wu-Baer F, Lane WS, Gaynor RB. Identification of a group of cellular cofactors that stimulate the binding of RNA polymerase II and TRP-185 to human immunodeficiency virus 1 TAR RNA. J Biol Chem. 1996;271:4201–4208. doi: 10.1074/jbc.271.8.4201. [DOI] [PubMed] [Google Scholar]
- Zelles T, Purushotham KR, Macauley SP, Oxford GE, Humphreys-Beher MG. Saliva and growth factors: the fountain of youth resides in us all. J Dent Res. 1995;74:1826–1832. doi: 10.1177/00220345950740120301. [DOI] [PubMed] [Google Scholar]