Abstract
Aims
Peak oxygen uptake (VO2) is diminished in patients with heart failure with preserved ejection fraction (HFpEF) suggesting impaired cardiac reserve. To test this hypothesis, we assessed the haemodynamic response to exercise in HFpEF patients.
Methods and results
Eleven HFpEF patients (73 ± 7 years, 7 females/4 males) and 13 healthy controls (70 ± 4 years, 6 females/7 males) were studied during submaximal and maximal exercise. The cardiac output (Qc, acetylene rebreathing) response to exercise was determined from linear regression of Qc and VO2 (Douglas bags) at rest, ∼30% and ∼60% of peak VO2, and maximal exercise. Peak VO2 was lower in HFpEF patients than in controls (13.7 ± 3.4 vs. 21.6 ± 3.6 mL/kg/min; P < 0.001), while indices of cardiac reserve were not statistically different: peak cardiac power output [CPO = Qc × mean arterial pressure (MAP); HFpEF 1790 ± 509 vs. controls 2119 ± 581 L/mmHg/min; P = 0.20]; peak stroke work [SW = stroke volume (SV) × MAP; HFpEF 13 429 ± 2269 vs. controls 13 200 ± 3610 mL/mmHg; P = 0.80]. The ΔQc/ΔVO2 slope was abnormally elevated in HFpEF patients vs. controls (11.2 ±3.6 vs. 8.3 ± 1.5; P = 0.015).
Conclusion
Contrary to our hypothesis, cardiac reserve is not significantly impaired in well-compensated outpatients with HFpEF. The abnormal haemodynamic response to exercise (decreased peak VO2, increased ΔQc/ΔVO2 slope) is similar to that observed in patients with mitochondrial myopathies, suggesting an element of impaired skeletal muscle oxidative metabolism. This impairment may limit functional capacity by two mechanisms: (i) premature skeletal muscle fatigue and (ii) metabolic signals to increase the cardiac output response to exercise which may be poorly tolerated by a left ventricle with impaired diastolic function.
Keywords: Cardiac output response to exercise, Haemodynamic response to exercise, Heart failure with preserved ejection fraction, Exercise capacity, Myocardial contractile reserve, Oxygen consumption
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a clinical syndrome marked by limitations in functional capacity. In contrast to its counterpart, systolic heart failure, the underlying pathophysiology remains poorly understood. Previous studies have implicated decreased left ventricular chamber dimensions,1,2 impaired active relaxation during diastole,3 decreased left ventricular chamber compliance,3,4 increased levels of advanced glycation end-products,5 reduced chronotropic reserve,1 and altered ventricular–vascular coupling1,6 as contributors. These observations suggest that impairments in cardiac reserve may contribute to the clinical syndrome of HFpEF, similar to that which is seen in systolic heart failure.
In support of this concept, peak oxygen uptake (VO2), perhaps the mostly widely recognized index of cardiac reserve, is depressed in HFpEF.1,2,7 The utility of peak VO2 as a marker of cardiac reserve in heart failure is well documented;8 it was the only objective measure of cardiac reserve cited in the indications for cardiac transplantation in the 2005 ACC/AHA Guidelines for Diagnosis and Management of Chronic Heart Failure. However, using peak VO2 as a sole indicator of cardiac reserve may be misleading as this measure is dependent on age, gender, body composition, effort, and conditioning.9,10 Furthermore, as the product of cardiac output and arterial–venous oxygen content difference, a depressed peak VO2 may reflect a defect in oxygen utilization rather than a limitation in cardiac reserve, as is seen in patients with mitochondrial myopathies.11,12
Assessing the haemodynamic response to exercise allows for the measure of additional, clinically relevant indices of cardiac reserve: the cardiac output response to exercise (ΔQc/ΔVO2), peak cardiac power output [CPO =Qc × mean arterial pressure (MAP)], and peak stroke work [SW = stroke volume (SV) × MAP]. In systolic heart failure, where cardiac reserve is limited, these indices are depressed.9,10,13 To date, there are limited data regarding the ΔQc/ΔVO2, peak CPO, and peak SW in HFpEF.
Based on available evidence, we hypothesized that patients with HFpEF would have impairments in cardiac reserve with depressed peak VO2 and reduced cardiac output at peak exercise. To test this hypothesis, we assessed the haemodynamic response to exercise in a highly screened cohort of elderly well-compensated outpatients with HFpEF.
Methods
Subjects
Eleven patients (age ≥65 years) with the diagnosis of HFpEF were recruited for the study. Additional information about the recruitment, inclusion criteria, and exclusion criteria are available elsewhere.14 In brief, stringent but simple criteria were applied for the diagnosis of HFpEF, including: the presence of Framingham criteria for the diagnosis of heart failure; an index hospitalization with evidence of pulmonary congestion, an elevated pulmonary capillary wedge pressure, or an elevated brain-type natriuretic peptide (BNP) within 6 months of enrolment; and an ejection fraction ≥50%. With the exception of the ejection fraction, echocardiographic indices were specifically not used for the diagnosis of HFpEF since the overall goal of this project was to determine the nature and extent of abnormalities of ‘diastolic function’ in patients who clearly have this complicated syndrome; this is similar to other recent work regarding HFpEF.2 Exclusion criteria included significant valvular heart disease defined as any regurgitant or stenotic lesion greater than ‘mild’ when assessed by transthoracic echocardiography; acute atrial fibrillation; congenital heart disease; coronary artery disease with provocable ischaemia as assessed by exercise stress echocardiography, an acute coronary syndrome, or a history of surgical revascularization or multivessel percutaneous revascularization; New York Heart Association functional class IV heart failure; suspicion of a restrictive or infiltrative cardiomyopathy; or any additional medical condition that might explain the subject's heart failure symptom complex. HFpEF patient co-morbidities and cardiac medications have been published previously.14
Thirteen healthy, similarly aged, sedentary individuals with no major medical conditions served as controls. Additional information about the recruitment, inclusion, and exclusion of these controls can be found elsewhere.15 All subjects signed an informed consent approved by the institutional review boards of the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas.
Cardiopulmonary stress testing
An individualized, modified Astrand–Saltin incremental treadmill protocol was used to determine peak exercise capacity. Beta-blockers were withheld for 24–48 h prior to testing, though other antihypertensive medications were continued. Measures of ventilatory gas exchange were made by use of the Douglas bag technique. Gas fractions were analysed by mass spectrometry, and ventilatory volume was measured by use of a 120 L Tissot spirometer (W.E. Collins P-1700; Braintree, MA, USA). Peak VO2 was defined as the highest oxygen uptake measured from at least a 40 s Douglas bag collection, and expressed both in absolute terms and scaled to body mass. Repeat testing of six HFpEF patients (at two points in time 3 months apart) yielded a typical error of 3% for normalized peak VO2. Resting blood pressure, assessed in the standing position, was measured in the arm by electrosphygmomanometry (Suntech Tango + ; Morrisville, NC, USA) with a microphone over the brachial artery and the detection of Korotkoff sounds gated to the electrocardiograph (ECG). Heart rate (HR) was monitored continuously via ECG (Schiller AT-10; Welch Allyn Inc., Skaneateles Falls, NY, USA).
Cardiac output was measured by use of a modification of the acetylene rebreathing technique16 at four points: standing rest, ∼5 min of steady-state exercise at ∼30% of peak VO2, ∼5 min of steady-state exercise level at ∼60% of peak VO2, and maximal exercise. The modified acetylene rebreathe technique is well validated16,17 and highly reproducible for the measurement of peak Qc;18 during maximal exercise, it has been shown to be equivalent to invasive measures.16 For peak Qc, repeat testing using this technique in six HFpEF patients (at two points in time 3 months apart) yielded a typical error of 16%. Arterial–venous oxygen difference was calculated from the ratio between cardiac output and oxygen uptake using the Fick equation.
The peak CPO was calculated by multiplying the Qc by the MAP at peak stress (CPO = Qc ×MAP). This measure was also indexed to body surface area (CPOI = CPO/BSA). Peak SW was calculated by multiplying the SV by the MAP at peak stress (SW = SV ×MAP). This measure was also indexed to BSA (SWI = SW/BSA).
31Phosphate magnetic resonance spectroscopy
Preliminary testing using 31P-magnetic resonance spectroscopy (MRS) was performed on a subset of HFpEF patients (n = 2) and healthy controls (n = 2) using a 1.5 T magnet (Siemens; Malvern, PA, USA). Patients and controls performed static leg lifts within the MRI scanner to achieve a pre-determined exercise-induced metabolic state defined as a 1:1 inorganic phosphate:phosphocreatine (Pi:PCr) ratio. Data were analysed using the jMRUI software package. The following indices were measured at rest, at end-exercise, and in recovery: workload, exercise time, PCr concentration, Pi concentration, ATP concentration, and free cytosolic ADP. Oxidative phosphorylation ATP production rate, anerobic glycolysis ATP production rate, and PCr recovery time were calculated using previously published techniques.19,20
Statistical analysis
All data are expressed as mean ± SD. SigmaStat 3.1 and SAS 9.2 were used to perform analyses. Mixed model repeated measures analysis (MMRM) was used to assess haemodynamic variable responses by modelling a between-group factor, a repeated exercise condition factor, and interaction between group and exercise condition. Where the mixed model main effects or interactions were statistically significant, comparisons within conditions were made with least squares means contrasts from the mixed models; multiple comparison results were unadjusted for multiple testing; however, the exact P-values were reported when possible. The cardiac output response to exercise (ΔQc/ΔVO2) was determined by performing a linear regression of the cardiac output over the oxygen uptake at the four previously described data points during incremental exercise. In this simple linear regression model, the mean ΔQc/ΔVO2 slope for each group was obtained by averaging the regressed slopes of the individual subjects. Two-sample t-tests were used to compare the mean ΔQc/ΔVO2 slope between the groups. A random coefficient mixed model analysis was also performed to estimate regression parameters between ΔQc and ΔVO2 for the group data. As these group data were comprised of multiple data points from individual subjects, the random coefficients mixed model analysis thereby accounted for the correlation within individual subjects. Fisher's exact test was used to compare differences in group gender. A value of P < 0.05 was considered statistically significant; all statistical analyses were two-sided. Statistical analyses were not performed on the preliminary 31P-MRS data given the small sample size.
Results
Baseline characteristics
The baseline characteristics of the 11 HFpEF outpatients and 13 healthy controls are presented in Table 1. Co-morbidities and medication use in the HFpEF patients are included in this table. Seven of 11 of the HFpEF patients and 6 of the 13 controls were female. Body mass and body mass index were greater in HFpEF patients than in controls. Baseline haematocrit was slightly lower in HFpEF patients than in controls. All other variables were similar.
Table 1.
Baseline characteristics
| Variable | HFpEF (n = 11; 4 males, 7 females) | Control (n = 13; 7 males, 6 females) | P-value |
|---|---|---|---|
| Age (years) | 73.0 ± 6.8 | 70.2 ± 3.5 | 0.21 |
| Female | 63.6% | 46.2% | 0.44 |
| Height (m) | 1.62 ± 0.10 | 1.68 ± 0.10 | 0.14 |
| Mass (kg) | 88.9 ± 21.3 | 73.1 ± 10.2 | 0.03 |
| Body fat (%) | 26.4 ± 9.7 | 27.8 ± 7.6 | 0.71 |
| Body mass index (kg/m2) | 33.6 ± 6.7 | 25.7 ± 2.3 | 0.003 |
| Body surface area (m2) | 1.99 ± 0.27 | 1.85 ± 0.17 | 0.13 |
| Haematocrit (%) | 36.5 ± 2.7 | 40.6 ± 2.7 | 0.001 |
| Co-morbid conditions | n (%) | ||
| Hypertension | 11 (100%) | ||
| Diabetes | 6 (55%) | ||
| Coronary artery disease | 0 (0%) | ||
| Chronic renal insufficiency | 1 (9%) | ||
| Hyperlipidaemia | 9 (82%) | ||
| Medications | n (%) | ||
| Diuretic | 10 (91%) | ||
| Beta-blocker | 6 (55%) | ||
| Calcium channel blocker | 5 (45%) | ||
| ACEI/ARB | 9 (82%) | ||
| HMG-CoA reductase inhibitor | 9 (82%) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HMG-CoA, 3-hydroxymethyl-glutaryl coenzyme A.
Haemodynamics at rest and during submaximal exercise
At upright rest standing on the treadmill, the absolute and normalized VO2 and the arterial–venous oxygen content difference were greater in HFpEF patients than in controls (Table 2). Other upright variables including HR, blood pressure (systolic, diastolic, mean), SV, SVI, Qc, and cardiac index were similar.
Table 2.
Rest and exercise haemodynamics
| Variable | Rest |
Steady-state 1 (∼30% of maximal exercise) |
Steady-state 2 (∼60% of maximal exercise) |
Peak exercise |
Group × condition interaction P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HFpEF | Control | P-valuea | HFpEF | Control | HFpEF | Control | HFpEF | Control | P-valuea | ||
| SBP (mmHg) | 140 ± 25 | 147 ± 22 | 0.486 | 162 ± 24 | 168 ± 20 | 179 ± 25 | 186 ± 16 | 189 ± 30 | 211 ± 26 | 0.023 | 0.160 |
| DBP (mmHg) | 75 ± 15 | 84 ± 9 | 0.190 | 79 ± 20 | 82 ± 19 | 79 ± 16 | 92 ± 24 | 85 ± 16 | 86 ± 14 | 0.901 | 0.293 |
| MAP (mmHg) | 97 ± 16 | 105 ± 13 | 0.181 | 107 ± 14 | 111 ± 17 | 112 ± 14 | 123 ± 19 | 119 ± 15 | 128 ± 15 | 0.200 | 0.678 |
| HR (b.p.m.) | 81 ± 23 | 79 ± 11 | 0.822 | 102 ± 23 | 102 ± 11 | 112 ± 25 | 112 ± 10 | 133 ± 27 | 157 ± 19 | 0.017 | 0.0004 |
| avO2 difference (%) | 5.8 ± 2.1 | 4.4 ± 0.9 | 0.002 | 6.8 ± 1.8 | 8.1 ± 1.0 | 7.7 ± 2.4 | 8.8 ± 1.3 | 8.1 ± 2.0 | 9.9 ± 1.5 | 0.002 | <0.0001 |
| CO (L/min) | 5.1 ± 1.9 | 4.7 ± 0.9 | 0.533 | 10.6 ± 2.7 | 10.3 ± 1.9 | 11.6 ± 2.8 | 11.2 ± 1.9 | 15.1 ± 4.3 | 16.4 ± 4.1 | 0.510 | 0.641 |
| CI (L/min/m2) | 2.6 ± 0.9 | 2.6 ± 0.3 | 0.943 | 5.3 ± 1.1 | 5.6 ± 0.7 | 5.8 ± 1.3 | 6.1 ± 0.7 | 7.6 ± 1.8 | 8.8 ± 1.6 | 0.112 | 0.226 |
| SV (mL) | 65 ± 20 | 61 ± 14 | 0.631 | 105 ± 21 | 102 ± 24 | 105 ± 19 | 101 ± 22 | 113 ± 18 | 102 ± 24 | 0.190 | 0.568 |
| SVI (mL/m2) | 33 ± 11 | 33 ± 6 | 0.943 | 53 ± 10 | 55 ± 10 | 53 ± 12 | 55 ± 9 | 57 ± 9 | 55 ± 10 | 0.550 | 0.584 |
| VO2 (L/min) | 0.32 ± 0.15 | 0.21 ± 0.06 | 0.027 | 0.76 ± 0.23 | 0.83 ± 0.18 | 0.98 ± 0.38 | 0.99 ± 0.23 | 1.23 ± 0.51 | 1.58 ± 0.42 | 0.066 | 0.003 |
| VO2 (mL/kg/min) | 3.5 ± 0.9 | 2.9 ± 0.5 | 0.045 | 8.7 ± 2.1 | 11.4 ± 1.8 | 11.1 ± 3.1 | 13.6 ± 1.9 | 13.7 ± 3.4 | 21.6 ± 3.6 | <0.001 | <0.0001 |
| SW (mL/mmHg) | 6437 ± 2708 | 6450 ± 1722 | 0.992 | 11 207 ± 2887 | 11 502 ± 3948 | 11 858 ± 3035 | 12 634 ± 4252 | 13 429 ± 2269 | 13 200 ± 3610 | 0.799 | 0.727 |
| SWI (mL/mmHg/m2) | 3247 ± 1248 | 3481 ± 834 | 0.683 | 5632 ± 1090 | 6168 ± 1779 | 6008 ± 1499 | 6778 ± 1891 | 6762 ± 767 | 7071 ± 1497 | 0.653 | 0.662 |
| CPO (L/mmHg/min) | 510 ± 260 | 501 ± 112 | 0.910 | 1130 ± 356 | 1155 ± 304 | 1304 ± 405 | 1392 ± 382 | 1790 ± 509 | 2119 ± 581 | 0.204 | 0.383 |
| CPOI (L/mmHg/min/m2) | 254 ± 107 | 271 ± 53 | 0.624 | 563 ± 122 | 622 ± 131 | 654 ± 156 | 749 ± 167 | 893 ± 174 | 1136 ± 240 | 0.015 | 0.055 |
The results are presented as mean and SD.
avO2difference, arterial–venous oxygen content difference; CI, cardiac index; CO, cardiac output; CPO, cardiac power output; CPOI, cardiac power output index; DBP, standing diastolic blood pressure; HR, heart rate; MAP, standing mean arterial pressure; SBP, standing systolic blood pressure; SV, stroke volume; SVI, stroke volume index; SW, stroke work; SWI, stroke work index; VO2, oxygen uptake.
aHFpEF vs. control.
bThe P-value represents the group by exercise condition interaction from mixed model repeated measures analysis.
During submaximal exercise, normalized VO2 was lower in HFpEF subjects than in controls (Table 2); other variables were similar.
Haemodynamics at peak stress
At peak stress, normalized VO2 (Figure 1) and the arterial–venous oxygen content difference were lower in HFpEF patients than in controls. Peak CPO and peak SW were not statistically different between HFpEF patients and controls (Figure 2). When indexed to BSA, peak SWI was not different between groups, though peak CPOI was lower in HFpEF patients. Peak systolic blood pressure and HR were lower in HFpEF patients than in controls. In one control subject, the peak Qc could not be measured.
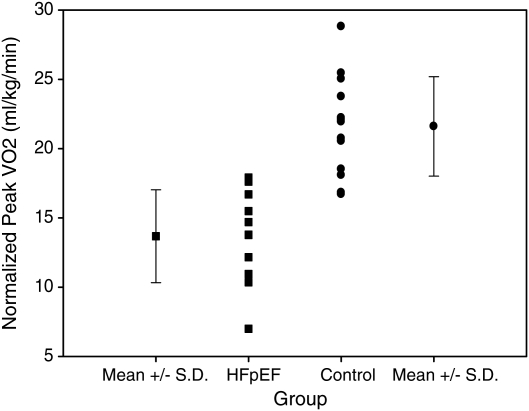
Figure 1.
Normalized peak oxygen uptake (VO2) by group. Peak VO2 was depressed in patients with heart failure with preserved ejection fraction (HFpEF), suggesting impaired cardiac reserve (P < 0.001).
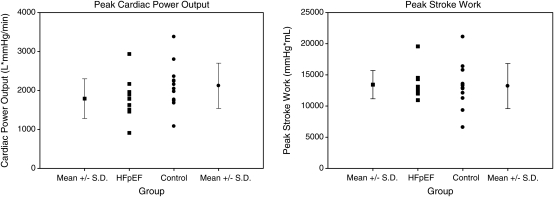
Figure 2.
Peak cardiac power output and peak stroke work. These measures of cardiac reserve were not statistically different between groups, suggesting that cardiac reserve is not impaired in heart failure with preserved ejection fraction (HFpEF).
Cardiac output response to exercise
The group ΔQc/ΔVO2 slope was markedly elevated in HFpEF patients when compared with controls (11.2 ± 3.6 vs. 8.3 ± 1.5; P = 0.015; Figure 3) based on the simple linear regression model. The random coefficient mixed model analysis yielded similar results for the ΔQc/ΔVO2 slope {10.44 [95% confidence interval (CI) 8.32–12.36] in HFpEF patients vs. 8.09 (95% CI 7.27–8.92) in controls; P = 0.021}.
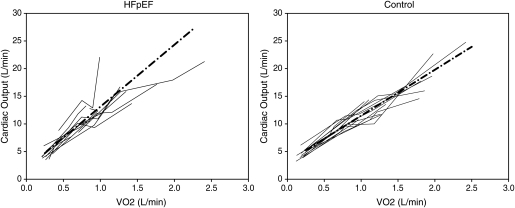
Figure 3.
Cardiac output response to exercise (Qc/VO2) by group. The ΔQc/ΔVO2 slope was markedly elevated in patients with heart failure with preserved ejection fraction (HFpEF) (11.2 ± 3.6 vs. 8.3 ± 1.5; P = 0.015). This finding, which has also been observed in patients with mitochondrial myopathies, suggests that impaired skeletal muscle oxidative metabolism in HFpEF may be the explanation for diminished oxygen uptake in this group. Excluding the HFpEF patient with a markedly elevated Qc at a low peak VO2 (the patient at the top left of the HFpEF plot), the ΔQc/ΔVO2 slope remained elevated in HFpEF patients when compared with controls (10.4 ± 2.6 vs. 8.3 ± 1.5; P = 0.023).
31Phosphate magnetic resonance spectroscopy
HFpEF patients achieved a 1:1 Pi:PCr ratio in less exercise time (37 ± 15 vs. 67 ± 6 s) while performing less work (mean area under the curve: 482 ± 218 vs. 1072 ± 163 kg/s) when compared with healthy controls. Oxidative phosphorylation ATP production rates were lower in HFpEF patients when compared with controls (0.16 ± 0.01 vs. 0.20 ± 0.02 mM/s). Anerobic glycolysis ATP production rates were greater in HFpEF patients when compared with healthy controls (0.25 ± 0.01 vs. 0.14 ± 0.08 mM/s), as was the PCr recovery time constant (62 ± 20 vs. 38 ± 2 s). Figure 4 presents PCr regeneration curves for a HFpEF patient from the current study, a healthy control from the current study, and a previously tested mitochondrial myopathy patient.
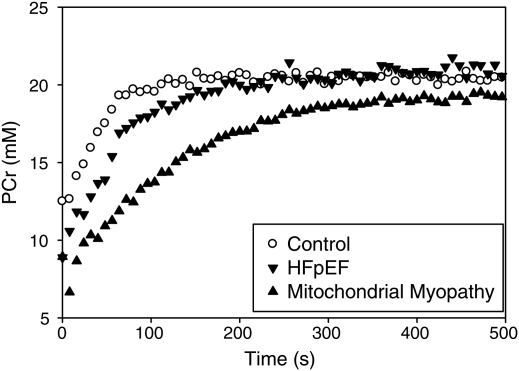
Figure 4.
31Phosphorus magnetic resonance spectroscopy (31P-MRS)-derived phosphocreatine (PCr) recovery curves in a control subject from the current study (open circles), a patient with heart failure with preserved ejection fraction (HFpEF) from the current study (inverted triangles), and a previously tested mitochondrial myopathy patient (upright triangle). Subjects were tested using a similar protocol during which the quadriceps muscle was imaged immediately after achieving a pre-defined exercise-induced metabolic state. Post-exertion, HFpEF and mitochondrial myopathy patients have diminished PCr stores with delayed regeneration, suggesting that impaired skeletal muscle oxidative metabolism in HFpEF may be the explanation for the diminished oxygen uptake in this group.
Discussion
The key findings of this study included the novel observations that: (i) in contrast to systolic heart failure, several indices of cardiac reserve were not significantly impaired in well-compensated outpatients with HFpEF and (ii) the abnormal haemodynamic response to exercise (decreased peak VO2, increased ΔQc/ΔVO2 slope) was similar to that observed in patients with mitochondrial myopathies, suggesting that impaired skeletal muscle oxidative metabolism may contribute to limitations in functional capacity observed in HFpEF. This concept was further supported by preliminary data using 31P-MRS.
Cardiac reserve is preserved in outpatients with heart failure with preserved ejection fraction
The results of cardiopulmonary stress testing in HFpEF have been reported previously;1,2,7,21,22 however, evaluations of the haemodynamic response to exercise,1,2,21,22 which necessitates measures of cardiac output with incremental stress, remain limited. The present study extends the work of Kitzman,21 Borlaug,1,22 and Maeder2 by reporting additional indices of cardiac reserve including peak CPO and peak SW. Furthermore, it presents the cardiac output response to exercise (ΔQc/ΔVO2) which has not previously been reported in HFpEF.
Impairments in cardiac reserve play a key role in the pathophysiology of systolic heart failure.23 Evidence had suggested that cardiac reserve may be impaired in HFpEF. Normalized peak VO2, perhaps the most widely recognized index of cardiac reserve, is depressed in HFpEF.1,2,7 Achieved increases in HR are also reduced, raising the question of whether cardiac reserve is impaired at higher workloads.1,2,22 Finally, shifted pressure–volume and Starling relationships due to abnormalities in active relaxation of the left ventricle (LV)3 and LV chamber compliance3,4 appear to diminish SV. Based on these observations, we hypothesized that cardiac reserve would be impaired in HFpEF. Our findings of a similar peak CPO and peak SW when compared with controls refute this hypothesis.
Cardiac power output is an index of cardiac reserve which conveys the hydraulic power of the heart by relating changes in flow and afterload.13 Stroke work is a complementary measure which relates the cardiovascular system's ability to augment SV against afterload. In the present study, both indices were not statistically different between well-compensated outpatients with HFpEF and healthy controls. The individual components of these indices were generally balanced: peak SV and peak MAP were similar between groups, while peak HR was lower in HFpEF patients. These findings suggest that while abnormalities of diastolic filling and achieved heart rates may be demonstrable in HFpEF, they do not significantly diminish SV, total flow, or the cardiovascular system's ability to generate an appropriate blood pressure response to upright exercise.
The perception that cardiac reserve is impaired in HFpEF may, in some ways, be attributable to the frequent practice of normalizing haemodynamic indices for BSA or body mass. In HFpEF, BSA is increased; consequently, indices such as the cardiac index (and the resultant CPOI) or the SVI (and the resultant SWI) are diminished by ∼7–10% due to BSA alone when compared with controls. In our HFpEF patients, CPO and SW were not statistically different from those of controls; however, CPOI and SWI were depressed when compared with controls (the former being statistically significant), similar to findings reported by Borlaug et al.22 and Maeder et al.2 While increased body mass (and consequently BSA) is clearly an important factor in the pathophysiology of exercise intolerance in HFpEF, conceptually, CPO and SW are measures of work that are independent of scale just as the torque generated by a motor vehicle's engine is independent of the size of the motor vehicle in which it resides. Accordingly, while BSA was not reported in the manuscript by Borlaug et al., post-hoc calculations of peak CPOI (HFpEF = 613 vs. controls = 677 L/mmHg/min/m2) and peak SWI (5889 vs. 5547 mL/mmHg/m2) appear to be meaningfully influenced by the correction for BSA, suggesting that, if ‘un-indexed’, these measures of cardiac reserve might be more similar between HFpEF patients and controls.
Haemodynamic differences between upright exercise and supine exercise may further explain differences between the present study and previous work. Both Borlaug et al.22 and Maeder et al.2 reported diminished peak SVI in HFpEF patients (when compared with controls) when exercise was performed in the supine position. In fact, in both studies there was little change in the SVI between rest and peak exertion (Borlaug: rest SVI = 40 mL/m2, peak SVI = 47 mL/m2; Maeder: rest SVI 41 mL/m2, peak SVI = 45 mL/m2). This suggests that HFpEF patients were probably already on the flat portion of the Frank–Starling relationship due to augmented preload at baseline associated with being in the supine position. In the present study, when upright on a treadmill, SVI increased appropriately from rest to peak exertion as preload was incrementally augmented with increasing upright work.
The role of this augmented preload at baseline due to the supine position is highlighted by the notable difference in HR observed by Maeder,2 who performed separate studies in the supine (invasive) and upright (non-invasive) position. During the upright exercise phase of testing, peak HR was noted to be 124 b.p.m. while it was limited to 102 b.p.m. during peak exertion when supine.2 This observation suggests that factors which caused cessation of exercise, such as very high filling pressures, were achieved at a much lower HR while supine than while upright. This constellation of findings highlights the clinical relevance of increased preload on the haemodynamic response to exercise in HFpEF.
The method by which Qc was measured is yet another important technical consideration which differentiates the present work from previous studies. In previous studies, Qc was determined by the Fick equation22 (by measuring arterial–venous oxygen content difference and oxygen uptake) or by the thermodiluation technique.2 Both differ from the acetylene rebreathing technique used in the present study, which directly measures Qc from the effective pulmonary blood flow as determined by the disappearance of the soluble gas acetylene. The acetylene rebreathe technique has been validated in heart failure24 and is considered to be a highly reliable method to quantify Qc during exercise in individuals with normal lung function, minimizing confounders such as exercise-induced tricuspid regurgitation which may influence Qc measured by thermodilution.
Mounting evidence suggests that HFpEF is a disease process in which many divergent pathways lead to a common clinical condition; this observation is also relevant as the inclusion and exclusion criteria of studies of HFpEF often differ substantially. Borlaug et al. retrospectively studied patients who presented to the catheterization lab for the clinical assessment of exertional dyspnoea and/or fatigue. This population, described by the authors as having a ‘milder’ or ‘earlier’ stage of HFpEF, differed significantly from the present study's outpatients with adjudicated hospital admissions with both signs and symptoms of heart failure. Without longitudinally studying the natural history of a homogenous population of HFpEF patients using standardized techniques, results of haemodynamic testing may lack external validity.
The abnormal haemodynamic response to exercise: decreased peak oxygen uptake, increased ΔQc/ΔVO2 slope
In contrast to CPO and SW, normalized peak VO2 was diminished in HFpEF patients, and to an extent typical of patients with systolic heart failure. It is important to acknowledge that at least some of this reduction in peak VO2 for HFpEF patients is a function of the scaling to body mass. However, even the unscaled absolute peak VO2 was lower in the HFpEF patients, suggesting that they not only have reduced aerobic power required to move a heavier, larger body through space, but also have reduced total oxygen uptake.
As the product of cardiac output and the arterial–venous oxygen content difference, peak VO2 is dependent on both the appropriate delivery and utilization of oxygen by skeletal muscle. In conditions in which there is a defect in oxygen utilization, such as mitochondrial myopathies, the peak VO2 is depressed despite normal cardiac function.11,12 Hence, peak VO2 may be affected by: (i) factors such as gender, age, fitness, body composition, or effort expended; (ii) impairments in cardiac reserve; and/or (iii) defects in oxygen utilization. In contrast, ΔQc/ΔVO2 slope is significantly less prone to these confounders. Reflecting the cardiovascular system's ability to meet the increasing metabolic demands of the body as workload increases, this index is highly conserved in adults and varies little with gender, mode of exercise, overall fitness, or degree of effort.25,26 Ageing, too, seems to have little effect,18,25 although small increases in this index have been reported by decade of life.27 In advanced systolic heart failure, ΔQc/ΔVO2 slope is depressed,9 offering important prognostic information; in conditions in which oxygen utilization is impaired but cardiac function is normal, such as mitochondrial myopathies, ΔQc/ΔVO2 slope is markedly elevated,11,12 providing strong evidence that metabolic signals from skeletal muscle play a role in the cardiac output response to exercise.
Previously, ΔQc/ΔVO2 has not been reported in HFpEF; however, based on a post-hoc analysis of the data reported by Kitzman et al. in a cohort of seven patients with hypertension, hypertrophic cardiomyopathy, or cardiac amyloid who were diagnosed with heart failure with normal systolic function,21 the ΔQc/ΔVO2 slope fell within the normal range.9 Notably, these subjects were also unable to augment their SVI with increasing workload, suggesting some combination of increased preload at baseline in the setting of the upright exercise used in this study and a more advanced or restrictive stage of heart failure.
In our HFpEF patients, ΔQc/ΔVO2 slope was markedly elevated when compared with controls. When coupled with the observation of a depressed peak VO2, the direction and magnitude of these abnormalities were similar to those of a cohort of 40 patients with biochemically or molecularly identified mitochondrial myopathies studied at our institution using identical methods.12 In those patients, normalized peak VO2 was depressed to 16 ± 8 mL/kg/min while ΔQc/ΔVO2 slope was elevated to 15.0 ± 13.6.
Patients with mitochondrial myopathies suffer from impaired oxidative metabolism in skeletal muscle. As a result, phosphorylation potential is abnormal and skeletal muscle relies more on substrate level phosphorylation for energy production during exercise, leading to exaggerated circulatory and ventilatory responses. These abnormalities may explain the combination of decreased VO2 and increased ΔQc/ΔVO2 slope as well as the profound exercise intolerance observed during cardiopulmonary stress testing. As a similar haemodynamic response to exercise was observed in our HFpEF outpatients, it raised the possibility of impaired skeletal muscle oxidative metabolism contributing to exercise intolerance in this condition as well.
31Phosphate magnetic resonance spectroscopy
Given the unexpected nature of the above findings, we further investigated the possibility of impaired skeletal muscle oxidative metabolism in patients with HFpEF by performing preliminary testing in a subset of HFpEF patients and controls using 31P-MRS, a validated tool to assess cellular oxidative metabolism non-invasively.28
31P-MRS of the quadriceps muscle during static leg lifts demonstrated that HFpEF patients achieved the same pre-determined exercise-induced metabolic state as controls in roughly half of the exercise time while performing half of the work. During exercise, HFpEF patients relied less on oxidative pathways, as evidenced by decreased oxidative phosphorylation ATP production rates, and more on anaerobic metabolism, as evidenced by increased anerobic glycolysis ATP production rates. Furthermore, HFpEF patients regenerated PCr at a greatly diminished rate, as indicated by the PCr recovery time constant. Similar findings have been reported in advanced systolic heart failure,28,29 supporting the notion of impaired oxidative metabolism within skeletal muscle as a factor common to all forms of chronic heart failure. While detailed investigation in systolic heart failure has demonstrated a multifactorial aetiology for impaired skeletal muscle oxidative metabolism including changes in skeletal muscle fibre subtype,29 in skeletal muscle capillary density, and mitochondrial morphology and function,30 our preliminary investigation was unable to differentiate the aetiology in HFpEF. Nonetheless, these findings support those observed on cardiopulmonary stress testing, that patients with HFpEF appear to have impaired oxidative metabolism of the skeletal muscle.
Study limitations
HFpEF is a clinical syndrome with a catchment of diverse aetiologies. Patients who present with elevated left-sided filling pressures, dyspnoea on exertion, and fluid retention may have varying contributions from hypertension, coronary disease, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, and diabetes. Hence, published studies of HFpEF differ significantly in regards to inclusion and exclusion criteria and subsequently in the characteristics and stage of heart failure of the populations studied. Stringent inclusion and exclusion criteria often necessitate screening hundreds to thousands of patients to enrol small cohorts of patients, such as the 2054 heart failure patients screened to arrive at the 13 HFpEF patients examined herein.14 Hence, as our study (with its relatively smaller sample sizes) sought to exclude other explanations for HFpEF hospitalizations, issues of internal validity and external validity must be considered when generalizing the results to broader patient populations with HFpEF. This constitutes an important avenue for future investigation. Lastly, preliminary work using 31P-MRS was not intended to be definitive, and we consider it hypothesis generating. Additional investigation into the efficiency of skeletal muscle oxidative metabolism in HFpEF is needed.
Conclusion
In contrast to systolic heart failure, several indices of cardiac reserve were not significantly impaired in well-compensated outpatients with HFpEF. Based on the haemodynamic response to exercise (decreased peak VO2, increased ΔQc/ΔVO2 slope), and supported by preliminary data using 31P-MRS, skeletal muscle oxidative metabolism appeared to be impaired. Impaired skeletal muscle oxidative metabolism may contribute to limitations in functional capacity by two mechanisms: premature skeletal muscle fatigue and metabolic signals to disproportionately increase the cardiac output in relation to the oxygen uptake, an increase which may be poorly tolerated by an LV with impaired diastolic function.
Funding
National Institutes of Health (grant R01 AG17479); National Institutes of Health Clinical and Translational Science Award (grant UL1 RR024982).
Conflicts of interest: none declared.
Acknowledgements
We would like to acknowledge our research team for the invaluable contributions: Mrs Diane Bedenkopt, Mr Colin Connor, Mr Daniel Creson, Mrs Peggy Fowler, Mr Cyrus Oufi, Dr Murugappan Ramanathan, and Ms Tiffany VanGundy.
References
- 1.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 2.Maeder MT, Thompson BR, Brunner-La Rocca H-P, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 4.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss H-P, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 5.Willemsen S, Hartog JWL, Hummel YM, van Ruijven MHI, van der Horst ICC, van Veldhuisen DJ, Voors AA. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail. 2011;13:76–82. doi: 10.1093/eurjhf/hfq168. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 7.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 8.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 9.Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh T-K, Pierson RN, III, Davis SF, Wilson JR. Hemodynamic exercise testing: a valuable tool in the selection of cardiac transplantation candidates. Circulation. 1996;94:3176–3183. doi: 10.1161/01.cir.94.12.3176. [DOI] [PubMed] [Google Scholar]
- 10.Lang CC, Agostoni P, Mancini DM. Prognostic significance and measurement of exercise-derived hemodynamic variables in patients with heart failure. J Card Fail. 2007;13:672–679. doi: 10.1016/j.cardfail.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Haller RG, Henriksson KG, Jorfeldt L, Hultman E, Wibom R, Sahlin K, Areskog NH, Gunder M, Ayyad K, Blomqvist CG. Deficiency of skeletal muscle succinate dehydrogenase and aconitase. Pathophysiology of exercise in a novel human muscle oxidative defect. J Clin Invest. 1991;88:1197–1206. doi: 10.1172/JCI115422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- 13.Cotter G, Williams SG, Vered Z, Tan L. Role of cardiac power in heart failure. Curr Opin Cardiol. 2003;18:215–222. doi: 10.1097/00001573-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction/clinical perspective. Circ Heart Fail. 2010;3:617–626. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol. 2007;103:867–874. doi: 10.1152/japplphysiol.01106.2006. [DOI] [PubMed] [Google Scholar]
- 17.Laszlo G. Respiratory measurements of cardiac output: from elegant idea to useful test. J Appl Physiol. 2004;96:428–437. doi: 10.1152/japplphysiol.01074.2001. [DOI] [PubMed] [Google Scholar]
- 18.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the dallas bed rest and training study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation. 2001;104:1358–1366. [PubMed] [Google Scholar]
- 19.Newcomer B, Boska M. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve. 1997;20:336–346. doi: 10.1002/(SICI)1097-4598(199703)20:3<336::AID-MUS11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 20.Larson-Meyer D, Newcomer B, Hunter G, Hetherington H, Weinsier R. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed. 2000;13:14–27. doi: 10.1002/(sici)1099-1492(200002)13:1<14::aid-nbm605>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Kitzman D, Higginbotham M, Cobb F, Sheikh K, Sullivan M. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank–Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 22.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber K, Kinasewitz G, Janicki J, Fishman A. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 24.Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G, Wasserman K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol. 2005;46:1779–1781. doi: 10.1016/j.jacc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Julius S, Amery A, Whitlock LS, Conway J. Influence of age on the hemodynamic response to exercise. Circulation. 1967;36:222–230. doi: 10.1161/01.cir.36.2.222. [DOI] [PubMed] [Google Scholar]
- 26.Astrand P-O, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- 27.Becklake MR, Frank H, Dagenais GR, Ostiguy GL, Guzman CA. Influence of age and sex on exercise cardiac output. J Appl Physiol. 1965;20:938–947. doi: 10.1152/jappl.1965.20.5.938. [DOI] [PubMed] [Google Scholar]
- 28.McCully K, Mancini D, Levine S. Nuclear magnetic resonance spectroscopy. Chest. 1999;116:1434–1441. doi: 10.1378/chest.116.5.1434. [DOI] [PubMed] [Google Scholar]
- 29.Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 30.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]